
Mitral regurgitation mechanisms related to systolic anterior motion in hypertrophic cardiomyopathy
Highlight box
Key findings
• In patients with obstructive hypertrophic cardiomyopathy (HCM), the size of the tenting area was significantly associated with mitral regurgitation (MR) severity.
What is known and what is new?
• Systolic anterior motion (SAM) can result in significant MR and adverse outcomes in patients with obstructive HCM.
• Chordal cutting with septal myectomy was associated with improvement of tenting area, thereby contributing to the MR severity.
What is the implication, and what should change now?
• When performing septal myectomy, chordal cutting procedure may be a particularly valuable intervention for patients with SAM and significant MR.
Introduction
Background
Hypertrophic cardiomyopathy (HCM) is a common genetic heart disease characterized by the presence of increased left ventricular wall thickening, cardiomyocyte hypertrophy, disarrayed myofibers, and interstitial fibrosis. These manifestations occur in the absence of hemodynamic stresses adequate to account for the observed hypertrophy and systemic diseases (1,2). Left ventricular outflow tract (LVOT) obstruction is a frequent cause of symptoms and is associated with worse clinical outcomes (1,3).
Rationale and knowledge gap
Mitral regurgitation (MR) is an additional contributor to symptoms in HCM (4). In this context, MR may be mediated by systolic anterior motion (SAM) of the mitral valve, even in the absence of intrinsic mitral valve disease (4,5). Despite advances in technology, such as vector flow mapping, the variable degree and clinical impact of SAM remain unresolved (4,6-9). Echocardiography provides a high spatial resolution to assess morphology, structural abnormalities, and hemodynamic disturbances in HCM, especially in mitral valve assessment (10).
Objective
We have adopted cardiac three-dimensional computed tomography (3DCT) as an adjunctive tool for the preoperative assessment of morphology and to perform virtual septal myectomy, transforming our operative approach (11-13). We hypothesized that specific mitral anatomical anomalies in obstructive HCM were associated with SAM-related MR and its severity. This study aimed to (I) examine the impact of SAM-related MR and (II) investigate the association between MR severity and clinical factors and mitral anatomical geometries by using comprehensive imaging and intraoperative quantitative data. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1206/rc).
Methods
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of the Columbia University Medical Center (AAAT2539, approval date 9/8/2022), and individual consent for this retrospective analysis was waived.
Patients and data collection
In this study, we retrospectively reviewed the records of consecutive patients who underwent septal myectomy for obstructive HCM at Columbia University Medical Center between January 2017 and April 2022. We excluded patients with missing images or insufficient image quality. Clinical, pre- and postoperative transthoracic echocardiogram (TTE), preoperative 3DCT, laboratory, and surgical data were collected by reviewing electronic medical records. Intrinsic mitral valve disease was identified on TTE and during surgical procedures.
TTE examination
All TTE examinations were performed by trained cardiac sonographers and interpreted by attending physicians at our institution. If a patient underwent multiple TTE examinations during the study period, the examinations performed closest to the date of septal myectomy and most recently after the procedure were used for analysis (14). LVOT peak velocity was measured using continuous wave Doppler echocardiography at rest and with provocative measures using the Valsalva maneuver, and MR severity was graded on a scale of 0–3 (0, no or trivial; 1, mild; 2, moderate; 3, severe) according to previously published guidelines (15). Moderate or more MR was defined as significant MR. MR jet direction was assessed as posterior or non-posterior (central or anterior) in patients with significant MR (4).
The anatomical variables of the mitral valve were measured in a parasternal long-axis view of TTE (14). The tenting area was measured as the area enclosed between the annular plane and the mitral leaflets at the onset of systole (at mitral valve coaptation, Figure 1). The anterior mitral leaflet (AML)-ratio was calculated as the ratio of the AML projection from the mitral annulus plane and the annulus diameter (14,16). The C-Sept distance was measured between the coaptation point of the leaflets and the septum at the onset of systole in the same view (16,17). The lengths of the mitral leaflets were measured during diastole (5,18), and the aortomitral angle was measured as the intersection angle of the planes of the mitral annulus and aortic valve at end-diastole (19). SAM severity was graded on a scale of 0–3 depending on the mitral-septal distance (grade 0, no SAM; grade 3, severe SAM—prolonged contact between the mitral valve and septum during ≥30% of systole) (20). The interobserver reproducibility and intraobserver variability were assessed with an intraclass correlation coefficient >0.80. All other TTE parameters were measured according to guidelines, evaluated on at least three different beats, and averaged (21,22).

3DCT
Before surgical septal myectomy, a “virtual myectomy” based on 3DCT images was performed as previously described (11). In brief, a 320-detector low volumetric scanner (Aquilion One; Toshiba America Medical System, Ohtawara, Japan) was used to perform a 350-msec continuous electrocardiogram-gated volume scan of the heart, focusing on the late diastolic phase (90% of the R-R interval). The resulting images were analyzed with Vitrea software (Vital Images, Minnetonka, MN, USA). Three-dimensionally reconstructed images provided hypertrophy measurements and predicted the volume of myocardial resection. Left ventricular (LV) wall thickness was measured in a 17-segment model (21,23). The definition of papillary muscles and their heads were determined according to a previous study (24). We categorized the location of the distal-most portion of the papillary muscle within the LV cavity as basal, mid-ventricular, or apical (12,24).
Septal myectomy
Our septal myectomy procedure, previously described (25), involved a transaortic approach thorough sternotomy. In brief, septal myectomy was performed thus: (I) resection of the septal myocardium, similar to the original Morrow procedure, with extension toward the LV apex; (II) left-sided excision toward the left trigone; and (III) right-sided excision toward the base of the posterolateral papillary muscle. The actual resected myocardial volume was measured with volume displacement using a syringe. Concomitant procedures were performed as indicated. During septal myectomy, the mitral subvalvular apparatus was examined, and abnormal secondary chordae were identified and resected (16,25). Abnormal secondary chordae were defined as fibrotic (thickened and agglutinated) chordae attached to the mid-basal portion of the AML.
Statistical analysis
Continuous variables are presented as medians with interquartile ranges or means with standard deviations, and categorical variables are expressed as numbers and percentages. Normality was evaluated using the D’Agostino-Pearson test. The data were compared using the Student’s t-test or Mann-Whitney U test for continuous variables and chi-squared or Fisher’s exact tests for categorical variables. The paired t-test or Wilcoxon signed-rank test was used to detect any significant differences between pre- and postoperative parameters, as appropriate. The relationships between MR severity and significant variables were examined using bivariate Spearman’s correlation coefficients. The preoperative TTE and 3DCT parameters with a P value <0.05 on univariate analyses were entered into a multivariate linear regression model to determine the independent importance of each parameter.
All statistical analyses were performed using MedCalc (version 15.8; MedCalc Software, Ostend, Belgium) and SAS (version 9.4; SAS Institute, Cary, NC, USA).
Results
Patient characteristics and postoperative outcomes
In total, 67 patients with preoperative 3DCT underwent septal myectomy for obstructive HCM during the 5-year study period. A flowchart of the study population is shown in Figure 2. We excluded 19 patients with TTEs missing or with poor image quality. Of the remaining 48 patients, 14 with intrinsic mitral valve pathology were excluded; thus, 34 patients were included in the study. The mean age of the cohort was 58±13 years, 16 patients (47%) were male, and the median follow-up duration was 21 months (range, 4–39 months).
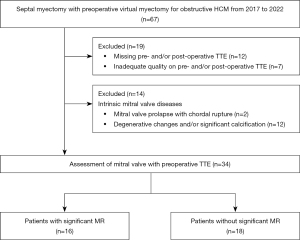
Mitral valve repair was performed through chordal cutting, as previously described. Notably, no patients required mitral valve replacement, and there were no instances of in-hospital mortality, excluding patients with intrinsic mitral valve pathology. Additionally, there were no occurrences of ventricular septal defects or stroke. However, post-surgical septal myectomy, 2 patients [significant MR (n=1, 6%) and no significant MR (n=1, 6%)] required pacemaker implantation. After septal myectomy, MR severity improved by three, two, and one grade in 5, 7, and 12 patients, respectively (Figure 3).

On preoperative TTE, all 34 patients had SAM, and 16 (47%) had significant MR. Patients were divided into two groups: patients with significant MR (n=16) and those without significant MR (n=18). The characteristics of each group are shown in Table 1. Patients with MR had a higher preoperative New York Heart Association (NYHA) functional class (III or IV, P=0.02) and a higher incidence of abnormal secondary chordae (P=0.01) (Table 1 and Figure 3) than those without MR. Otherwise, there were no significant differences between both groups after septal myectomy.
Table 1
| Characteristics | Significant MR (n=16) | No significant MR (n=18) | P value |
|---|---|---|---|
| Patient characteristics | |||
| Age, years | 59±13 | 56±13 | 0.49 |
| Sex, male | 9 (56%) | 7 (39%) | 0.32 |
| BSA, m2 | 1.9±0.3 | 2.0±0.2 | 0.28 |
| Hypertension | 12 (75%) | 14 (78%) | 0.85 |
| Diabetes mellitus | 3 (19%) | 5 (28%) | 0.54 |
| Dyslipidemia | 11 (69%) | 8 (44%) | 0.16 |
| COPD | 1 (6%) | 0 | 0.29 |
| CAD | 0 | 3 (17%) | 0.09 |
| Atrial fibrillation | 4 (25%) | 6 (33%) | 0.60 |
| Family history of sudden cardiac death | 2 (13%) | 5 (28%) | 0.28 |
| NYHA functional class | 0.05 | ||
| I | 0 | 0 | |
| II | 2 (13%) | 9 (50%) | |
| III | 13 (81%) | 9 (50%) | |
| IV | 1 (6%) | 0 | |
| NYHA functional class III or IV | 14 (88%) | 9 (50%) | 0.02 |
| Presence of abnormal secondary chordae | 13 (81%) | 7 (39%) | 0.01 |
| Prior AICD implantation | 2 (13%) | 2 (11%) | 0.90 |
| Laboratory parameters | |||
| Hemoglobin, g/dL | 12.7±1.9 | 13.8±2.0 | 0.09 |
| Creatinine, mg/dL | 0.9±0.2 | 1.0±0.2 | 0.19 |
| Albumin, g/dL | 4.5±0.3 | 4.4±0.4 | 0.66 |
| Total bilirubin, mg/dL | 0.6±0.3 | 0.6±0.3 | 0.90 |
| Concomitant surgery | |||
| Abnormal chordal cutting | 13 (81%) | 7 (39%) | 0.01 |
| Mitral valve leaflet plication | 1 (6%) | 0 | 0.29 |
| Maze procedure | 3 (19%) | 5 (28%) | 0.54 |
| Aortic valve procedure | 1 (6%) | 1 (6%) | 0.93 |
| Left atrial appendage closure | 6 (38%) | 7 (39%) | 0.69 |
| CPB time, min | 137±35 | 117±23 | 0.07 |
| ACC time, min | 89±20 | 87±20 | 0.75 |
| Actual volume of myocardial resection, mL | 6.4±2.6 | 7.2±2.2 | 0.33 |
Values are expressed as mean ± standard deviation or numbers (%). MR, mitral regurgitation; BSA, body surface area; COPD, chronic obstructive pulmonary disease; CAD, coronary artery disease (defined as ≥1 coronary arteries with ≥50% stenosis); NYHA, New York Heart Association; AICD, automatic implantable cardioverter-defibrillator; CPB, cardiopulmonary bypass; ACC, aortic cross-clamp.
TTE parameters and 3DCT
The preoperative and follow-up TTE parameters are summarized in Table 2. TTE was performed at a median of 20 days (range, 5–48 days) preoperatively and 195 days (range, 27–1,012 days) postoperatively.
Table 2
| TTE parameters | Preoperative | Postoperative | |||||
|---|---|---|---|---|---|---|---|
| Significant MR (n=16) | No significant MR (n=18) | P value | Significant MR (n=16) | No significant MR (n=18) | P value | ||
| LVEF, % | 67 [63–72] | 63 [63–68] | 0.14 | 58 [50–63] | 61 [55–63] | 0.48 | |
| LVDD, mm | 42 [38–45] | 42 [38–45] | 0.82 | 43 [39–47] | 45 [40–47] | 0.60 | |
| LVDS, mm | 24 [20–26] | 23 [20–28] | 0.81 | 28 [23–34] | 29 [26–32] | 0.77 | |
| IVS, mm | 18 [16–23] | 18 [16–20] | 0.85 | 14 [12–15] | 14 [13–16] | 0.35 | |
| PW, mm | 13 [11–15] | 12 [10–14] | 0.37 | 12 [10–14] | 12 [10–12] | 0.61 | |
| LVMI, g/m2 | 133 [119–183] | 127 [97–150] | 0.30 | 98 [90–112] | 104 [87–129] | 0.65 | |
| LA volume index, mL/m2 | 47 [39–63] | 36 [31–55] | 0.04 | 36 [32–48] | 34 [25–48] | 0.89 | |
| E wave velocity, cm/s | 89 [81–101] | 67 [61–86] | 0.004 | 81 [60–95] | 73 [60–90] | 0.57 | |
| A wave velocity, cm/s | 80 [59–104] | 82 [58–101] | 0.58 | 69 [50–122] | 78 [61–105] | 0.77 | |
| e' (medial) | 5 [4–6] | 5 [3–7] | 0.81 | 5 [3–7] | 5 [4–6] | 0.50 | |
| e' (lateral) | 8 [6–9] | 7 [6–9] | 0.77 | 7 [5–11] | 8 [6–9] | 0.84 | |
| E/e' (average) | 16 [12–20] | 12 [9–16] | 0.10 | 13 [10–16] | 12 [10–14] | 0.58 | |
| SPAP, mmHg | 26 [14–37] | 19 [14–25] | 0.17 | 23 [19–31] | 22 [14–29] | 0.31 | |
| LVOT gradient (at rest), mmHg | 73 [39–88] | 47 [33–65] | 0.07 | 14 [11–18] | 8 [6–14] | 0.03 | |
| LVOT gradient (with provocation), mmHg | 89 [61–121] | 69 [63–82] | 0.26 | 16 [11–20] | 9 [6–14] | 0.04 | |
| Severity of MR | <0.001 | 0.30 | |||||
| No | 0 | 4 (22%) | 9 (56%) | 14 (78%) | |||
| Mild | 0 | 14 (78%) | 6 (38%) | 4 (22%) | |||
| Moderate | 8 (50%) | 0 | 1 (6%) | 0 | |||
| Severe | 8 (50%) | 0 | 0 | 0 | |||
| Significant MR | 16 (100%) | 0 | <0.001 | 1 (6%) | 0 | 0.29 | |
| MR jet direction of posterior | 9 (56%) | – | – | 0 | – | – | |
| Significant AS and/or AR | 1 (6%) | 1 (6%) | 0.93 | 0 | 0 | 0.73 | |
| Significant TR | 1 (6%) | 0 | 0.29 | 0 | 0 | 0.73 | |
| Mitral geometry | |||||||
| AML length, mm | 32 [30–33] | 28 [27–31] | 0.05 | 31 [28–32] | 28 [27–31] | 0.25 | |
| PML length, mm | 21 [18–22] | 20 [18–23] | 0.85 | 20 [18–23] | 21 [19–23] | 0.65 | |
| Tenting area, cm2 | 2.2 [2.1–2.8] | 1.7 [1.3–1.8] | <0.001 | 1.6 [1.3–1.7] | 1.4 [1.0–1.6] | 0.37 | |
| Aortomitral angle, ° | 128 [122–130] | 126 [116–130] | 0.76 | 128 [119–135] | 129 [120–135] | 0.86 | |
| C-Sept distance, mm | 20 [18–22] | 20 [18–21] | 0.79 | 24 [22–27] | 26 [24–30] | 0.09 | |
| AML-ratio | 0.48 [0.45–0.51] | 0.51 [0.46–0.55] | 0.20 | 0.57 [0.54–0.65] | 0.54 [0.50–0.59] | 0.05 | |
| Severity of SAM | 0.19 | 0.29 | |||||
| No | 0 | 0 | 15 (94%) | 18 (100%) | |||
| Mild | 0 | 0 | 0 | 0 | |||
| Moderate | 7 (44%) | 12 (67%) | 1 (6%) | 0 | |||
| Severe | 9 (56%) | 6 (33%) | 0 | 0 | |||
| Systolic BP, mmHg | 125 [120–143] | 129 [114–142] | 0.81 | 122 [111–142] | 130 [111–148] | 0.55 | |
| Diastolic BP, mmHg | 72 [62–75] | 75 [70–80] | 0.13 | 72 [62–75] | 75 [70–80] | 0.13 | |
Values are expressed as mean ± standard deviation, median [interquartile range], or numbers (%). Significant valvular heart diseases were defined as moderate or severe. The AML-ratio was calculated as the ratio between the AML projection on the mitral annulus plane and annulus length. TTE, transthoracic echocardiogram; MR, mitral regurgitation; LVEF, left ventricular ejection fraction; LVDD, left ventricular end-diastolic dimension; LVDS, left ventricular end-systolic dimension; IVS, interventricular septum; PW, posterior wall; LVMI, left ventricular mass index; LA, left atrium; SPAP, systolic pulmonary arterial pressure; LVOT, left ventricular outflow tract; AS, aortic stenosis; AR, aortic regurgitation; TR, tricuspid regurgitation; AML, anterior mitral leaflet; PML, posterior mitral leaflet; C-Sept distance, coaptation point to the septum; SAM, systolic anterior motion; BP, blood pressure.
Following septal myectomy, there were significant reductions in LA volume index (P<0.001), LVOT peak gradient (P<0.001), MR severity (P<0.001), and tenting area (P<0.001). There were also significant increases in C-Sept distance (P<0.001) and AML-ratio (P<0.001). Moreover, on preoperative TTE, patients with MR had a significantly larger LA volume index (P=0.04), tenting area (P<0.001), and E wave velocity (P=0.004) than those without MR.
In contrast, on follow-up TTE, patients with MR had significantly higher residual LVOT peak gradients at rest (P=0.03) and with provocative measures (P=0.04) than those without MR (Table 2 and Figure 3). Preoperative 3DCT was performed at a median of 6 days (range, 3–9 days); there were no significant differences between the groups (Table S1).
Correlations between MR severity and other parameters
Correlations between the severity of preoperative MR and other parameters were assessed (Table 3). The tenting area was strongly associated with MR severity (r=0.84, P<0.001), and the presence of abnormal secondary chordae and E wave velocity were also significantly associated with MR severity (r=0.41, P=0.02 and r=0.40, P=0.02, respectively). Multiple linear regression analysis revealed that the tenting area was an independent determinant of MR severity among preoperative TTE and 3DCT parameters (Table S2).
Table 3
| Variables | r | 95% CI | P value |
|---|---|---|---|
| Patient characteristics | |||
| Age, years | 0.004 | −0.33 to 0.34 | 0.98 |
| Sex, male | −0.19 | −0.50 to 0.16 | 0.28 |
| BSA, m2 | −0.17 | −0.48 to 0.18 | 0.35 |
| Hypertension | 0.02 | −0.32 to 0.36 | 0.90 |
| Diabetes mellitus | −0.17 | −0.48 to 0.18 | 0.33 |
| Dyslipidemia | 0.31 | −0.03 to 0.59 | 0.08 |
| COPD | 0.09 | −0.25 to 0.42 | 0.60 |
| CAD | −0.28 | −0.57 to 0.06 | 0.11 |
| Atrial fibrillation | 0.05 | −0.30 to 0.38 | 0.79 |
| Family history of sudden cardiac death | −0.21 | −0.51 to 0.14 | 0.23 |
| NYHA functional class | 0.40 | 0.07 to 0.65 | 0.02 |
| Presence of abnormal secondary chordae | 0.41 | 0.08 to 0.66 | 0.02 |
| Preoperative TTE parameters | |||
| LVEF, % | 0.21 | −0.14 to 0.51 | 0.23 |
| LVDD, mm | −0.08 | −0.41 to 0.27 | 0.66 |
| LVDS, mm | −0.04 | −0.37 to 0.31 | 0.84 |
| IVS, mm | 0.09 | −0.26 to 0.41 | 0.62 |
| LVMI, g/m2 | 0.21 | −0.14 to 0.51 | 0.23 |
| LA volume index, mL/m2 | 0.36 | −0.02 to 0.62 | 0.04 |
| E wave velocity, cm/s | 0.40 | 0.07 to 0.65 | 0.02 |
| E/e' (average) | 0.26 | −0.09 to 0.55 | 0.14 |
| SPAP, mmHg | 0.002 | −0.37 to 0.37 | 0.99 |
| LVOT gradient (at rest), mmHg | 0.33 | −0.01 to 0.60 | 0.06 |
| LVOT gradient (with provocation), mmHg | 0.16 | −0.22 to 0.50 | 0.41 |
| Mitral geometry | |||
| AML length, mm | 0.41 | 0.08 to 0.66 | 0.02 |
| PML length, mm | 0.03 | −0.31 to 0.36 | 0.87 |
| Tenting area, cm2 | 0.84 | 0.70 to 0.92 | <0.001 |
| Aortomitral angle, ° | −0.02 | −0.37 to 0.32 | 0.93 |
| C-Sept distance, mm | 0.03 | −0.34 to 0.36 | 0.87 |
| AML-ratio | −0.20 | −0.50 to 0.15 | 0.26 |
| 3DCT parameters | |||
| LV wall thickness, mm | |||
| Basal | |||
| Anterior | −0.02 | −0.36 to 0.32 | 0.90 |
| Anteroseptal | 0.02 | −0.33 to 0.36 | 0.92 |
| Inferoseptum | 0.13 | −0.22 to 0.46 | 0.46 |
| Inferior | −0.02 | −0.36 to 0.32 | 0.90 |
| Inferolateral | −0.14 | −0.46 to 0.22 | 0.45 |
| Anterolateral | −0.18 | −0.50 to 0.17 | 0.31 |
| Mid | |||
| Anterior | −0.26 | −0.55 to 0.09 | 0.15 |
| Anteroseptal | −0.05 | −0.38 to 0.30 | 0.80 |
| Inferoseptum | −0.001 | −0.34 to 0.34 | 1.00 |
| Inferior | −0.11 | −0.43 to 0.25 | 0.56 |
| Inferolateral | −0.17 | −0.48 to 0.19 | 0.35 |
| Anterolateral | −0.25 | −0.55 to 0.11 | 0.17 |
| Apical | |||
| Anterior | −0.14 | −0.46 to 0.21 | 0.43 |
| Septal | −0.27 | −0.56 to 0.08 | 0.13 |
| Inferior | −0.002 | −0.35 to 0.34 | 0.99 |
| Lateral | −0.002 | −0.35 to 0.34 | 0.99 |
| Apex | −0.15 | −0.47 to 0.21 | 0.41 |
| Number of PM heads | |||
| Anterolateral | 0.11 | −0.24 to 0.44 | 0.53 |
| Posteromedial | −0.15 | −0.47 to 0.21 | 0.42 |
| Distal-most location of PMs in LV | 0.11 | −0.24 to 0.43 | 0.55 |
| Predicted volume of myocardial resection, mL | 0.11 | −0.30 to 0.48 | 0.61 |
The AML-ratio was calculated as the ratio between the AML projection on the mitral annulus plane and annulus length. MR, mitral regurgitation; BSA, body surface area; COPD, chronic obstructive pulmonary disease; CAD, coronary artery disease; NYHA, New York Heart Association; TTE, transthoracic echocardiogram; LVEF, left ventricular ejection fraction; LVDD, left ventricular end-diastolic dimension; LVDS, left ventricular end-systolic dimension; IVS, interventricular septum; LVMI, left ventricular mass index; LA, left atrium; SPAP, systolic pulmonary arterial pressure; LVOT, left ventricular outflow tract; AML, anterior mitral leaflet; PML, posterior mitral leaflet; C-Sept, coaptation point to the septum; 3DCT, three-dimensional computed tomography; LV, left ventricule; PM, papillary muscle.
Impact of the chordal cutting procedure
We evaluated the impact chordal cutting on mitral valve geometry (Table S3). We found that the tenting area and AML-ratio (all P<0.001) only changed significantly when septal myectomy was accompanied by chordal cutting (n=20) and not when chordal cutting was not performed (n=14) in patients both with and without significant MR (Tables S3,S4 and Figure 4).

Discussion
Key findings
HCM can be characterized by increased LV wall thickness, abnormal cardiomyocyte hypertrophy, disarrayed myofibers and interstitial fibrosis, anomalies in mitral valve apparatus, papillary muscle orientation, and narrowing of the LVOT (8). Combined with SAM, these anomalies lead to dynamic LVOT obstruction. MR is sometimes present even without intrinsic mitral valve disease, but its mechanism is poorly understood.
The associations demonstrated between preoperative MR severity, tenting area implies a novel mechanism of SAM-related MR. Abnormal secondary chordae may induce tethering of the AML, leading to shallow coaptation of the leaflets compromised by SAM, and MR subsequently occurs. Chordal cutting ameliorated MR severity and the tenting area in patients with and without MR, consistent with prior studies (14).
Strengths and limitations
This was a retrospective, single-center, observational study with a small sample size. We excluded 19 patients due to poor image quality or missing data. Furthermore, the quantitative assessment of MR, using the proximal isovelocity surface area and volumetric methods, is yet to be established in patients with obstructive HCM (5,11). In this study, the severity of MR was evaluated using expert, careful semi-quantitative assessments. Abnormal chordal cutting could potentially alter LV spatial geometry and impair LV function. However, in patients with obstructive HCM, these potentially unfavorable effects are avoidable by the preserved or hyperdynamic systolic function and small LV cavity, contributing to SAM (26). The judgement of abnormal chordal presence was at the surgeons’ discretion, while the mitral subvalvular apparatus was directly and carefully examined. Additionally, the assessment via a combination of 3DCT and TTE revealed the relationship between mitral valve geometry and MR severity in patients with obstructive HCM.
Comparison with similar researches
In functional MR, a large tenting area is associated with MR severity. In these cases, leaflet tethering by pathological papillary muscle displacement due to LV dysfunction or LV dilatation leads to a larger tenting area (27). Previous studies show that LV deformation and papillary muscle displacement in HCM may contribute to leaflet tethering. However, we found no significant differences in LV wall thickness and papillary muscle morphology between patients with and without MR. Patients with HCM more often have double bifid papillary muscles and anteroapical displacement of the papillary muscle within the LV that could account for severe LVOT obstruction (24,28). However, the risk factors of MR severity may differ from those of LVOT obstruction; we found that MR severity was not significantly correlated with SAM severity.
The clinical impact and mechanisms of MR severity are not well known. Contrary to our results, Yu et al. demonstrated that MR severity was directly related to the magnitude of the LVOT gradient (7). Our study focused on MR mediated by SAM, and current guidelines have defined eligible patients for septal myectomy as those with LVOT gradients at rest or with physiologic provocation with an approximate peak gradient of ≥50 mmHg (10). All patients in this study had moderate or more SAM on preoperative TTE. Previous studies revealed that the mechanism underlying SAM involves drag forces caused by the impact of early systolic flow on the PML rather than the Venturi effect caused by the narrowing of the LVOT and high flow velocity (6). Future prospective studies with larger cohorts should address these issues.
Explanations of findings
Understanding the mechanisms of SAM-related MR has clinical implications. While some studies have proposed that a sufficient myectomy alone will improve MR, this study demonstrated that an improvement in MR severity was associated with chordal cutting procedure.
Implications and actions needed
SAM-related MR may require the identification of abnormal secondary chords to be addressed with chordal cutting at the time of septal reduction therapy. Thus, a surgical approach with adequate septal myectomy and chordal cutting may be more appropriate than alcohol septal ablation alone.
Patients with MR had improved yet higher residual LVOT gradients after myectomy than those without. This suggests that preoperative MR is a high-risk factor for a difficult myectomy, and may explain a recent analysis showing an association between a program’s myectomy volume and the frequency of mitral valve replacement (29). We acknowledge the scope to modify our surgical strategy in patients with MR attempting to minimize residual gradients. Song et al. demonstrated that a spiral pattern of LV hypertrophy and increased AML length were independent predictors of LVOT obstruction (12), consistent with this study. Therefore, intervention regarding the AML might be necessary.
Conclusions
The tenting areas were significantly associated with MR severity in patients with obstructive HCM. Moreover, the combination of septal myectomy and chordal cutting procedures seemed effective in eliminating MR while concurrently reducing LVOT gradients. These unique preliminary findings generate a novel hypothesis that this procedure might be an effective therapy for patients with SAM and significant MR, thereby contributing to MR associated with SAM.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1206/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1206/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1206/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1206/coif). H.T. serves as an unpaid editorial board member of Journal of Thoracic Disease from October 2022 to January 2025. R.T.H. reports receiving speaker fees from Abbott Structural, Baylis Medical, Edwards Lifesciences, and Philips Healthcare. Additionally, she has institutional consulting contracts for which she receives no direct compensation with Abbott Structural, Boston Scientific, Edwards Lifesciences, Medtronic, and Novartis. R.T.H. also has stock options with Navigate and is Chief Scientific Officer for the Echocardiography Core Laboratory at the Cardiovascular Research Foundation for multiple industry-sponsored trials, for which she receives no direct industry compensation. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of the Columbia University Medical Center (AAAT2539, approval date 9/8/2022), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Veselka J, Anavekar NS, Charron P. Hypertrophic obstructive cardiomyopathy. Lancet 2017;389:1253-67. [Crossref] [PubMed]
- Nishimura RA, Holmes DR Jr. Clinical practice. Hypertrophic obstructive cardiomyopathy. N Engl J Med 2004;350:1320-7. [Crossref] [PubMed]
- Maron BJ, Maron MS, Wigle ED, et al. The 50-year history, controversy, and clinical implications of left ventricular outflow tract obstruction in hypertrophic cardiomyopathy from idiopathic hypertrophic subaortic stenosis to hypertrophic cardiomyopathy: from idiopathic hypertrophic subaortic stenosis to hypertrophic cardiomyopathy. J Am Coll Cardiol 2009;54:191-200. [Crossref] [PubMed]
- Hang D, Schaff HV, Nishimura RA, et al. Accuracy of Jet Direction on Doppler Echocardiography in Identifying the Etiology of Mitral Regurgitation in Obstructive Hypertrophic Cardiomyopathy. J Am Soc Echocardiogr 2019;32:333-40. [Crossref] [PubMed]
- Groarke JD, Galazka PZ, Cirino AL, et al. Intrinsic mitral valve alterations in hypertrophic cardiomyopathy sarcomere mutation carriers. Eur Heart J Cardiovasc Imaging 2018;19:1109-16. [Crossref] [PubMed]
- Ro R, Halpern D, Sahn DJ, et al. Vector flow mapping in obstructive hypertrophic cardiomyopathy to assess the relationship of early systolic left ventricular flow and the mitral valve. J Am Coll Cardiol 2014;64:1984-95. [Crossref] [PubMed]
- Yu EH, Omran AS, Wigle ED, et al. Mitral regurgitation in hypertrophic obstructive cardiomyopathy: relationship to obstruction and relief with myectomy. J Am Coll Cardiol 2000;36:2219-25. [Crossref] [PubMed]
- Feneon D, Schnell F, Galli E, et al. Impact of exercise-induced mitral regurgitation on hypertrophic cardiomyopathy outcomes. Eur Heart J Cardiovasc Imaging 2016;17:1110-7. [Crossref] [PubMed]
- Guigui SA, Torres C, Escolar E, et al. Systolic anterior motion of the mitral valve in hypertrophic cardiomyopathy: a narrative review. J Thorac Dis 2022;14:2309-25. [Crossref] [PubMed]
- Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2020;142:e533-57. [Crossref] [PubMed]
- Takayama H, Yu SN, Sorabella R, et al. Virtual septal myectomy for preoperative planning in hypertrophic cardiomyopathy. J Thorac Cardiovasc Surg 2019;158:455-63. [Crossref] [PubMed]
- Song Y, Yang DH, Ó Hartaigh B, et al. Geometric predictors of left ventricular outflow tract obstruction in patients with hypertrophic cardiomyopathy: a 3D computed tomography analysis. Eur Heart J Cardiovasc Imaging 2018;19:1149-56. [Crossref] [PubMed]
- Andrushchuk U, Adzintsou V, Nevyglas A, et al. Virtual and real septal myectomy using 3-dimensional printed models. Interact Cardiovasc Thorac Surg 2018;26:881-2. [Crossref] [PubMed]
- Ferrazzi P, Spirito P, Iacovoni A, et al. Transaortic Chordal Cutting: Mitral Valve Repair for Obstructive Hypertrophic Cardiomyopathy With Mild Septal Hypertrophy. J Am Coll Cardiol 2015;66:1687-96. [Crossref] [PubMed]
- Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303-71. [Crossref] [PubMed]
- Delling FN, Sanborn DY, Levine RA, et al. Frequency and mechanism of persistent systolic anterior motion and mitral regurgitation after septal ablation in obstructive hypertrophic cardiomyopathy. Am J Cardiol 2007;100:1691-5. [Crossref] [PubMed]
- Maslow AD, Regan MM, Haering JM, et al. Echocardiographic predictors of left ventricular outflow tract obstruction and systolic anterior motion of the mitral valve after mitral valve reconstruction for myxomatous valve disease. J Am Coll Cardiol 1999;34:2096-104. [Crossref] [PubMed]
- Klues HG, Proschan MA, Dollar AL, et al. Echocardiographic assessment of mitral valve size in obstructive hypertrophic cardiomyopathy. Anatomic validation from mitral valve specimen. Circulation 1993;88:548-55. [Crossref] [PubMed]
- Nicoara A, Skubas N, Ad N, et al. Guidelines for the Use of Transesophageal Echocardiography to Assist with Surgical Decision-Making in the Operating Room: A Surgery-Based Approach: From the American Society of Echocardiography in Collaboration with the Society of Cardiovascular Anesthesiologists and the Society of Thoracic Surgeons. J Am Soc Echocardiogr 2020;33:692-734. [Crossref] [PubMed]
- Gilbert BW, Pollick C, Adelman AG, et al. Hypertrophic cardiomyopathy: subclassification by m mode echocardiography. Am J Cardiol 1980;45:861-72. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233-70. [Crossref] [PubMed]
- Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2009;10:165-93. [Crossref] [PubMed]
- Yamabe T, Ginns J, Vedula V, et al. Left ventricular remodeling following septal myectomy in hypertrophic obstructive cardiomyopathy. JTCVS Open 2022;11:105-15. [Crossref] [PubMed]
- Patel P, Dhillon A, Popovic ZB, et al. Left Ventricular Outflow Tract Obstruction in Hypertrophic Cardiomyopathy Patients Without Severe Septal Hypertrophy: Implications of Mitral Valve and Papillary Muscle Abnormalities Assessed Using Cardiac Magnetic Resonance and Echocardiography. Circ Cardiovasc Imaging 2015;8:e003132. [Crossref] [PubMed]
- Nguyen SN, Blitzer D, Weiner S, et al. Septal Myectomy: How I Teach It. Ann Thorac Surg 2020;110:764-7. [Crossref] [PubMed]
- Rodriguez F, Langer F, Harrington KB, et al. Importance of mitral valve second-order chordae for left ventricular geometry, wall thickening mechanics, and global systolic function. Circulation 2004;110:II115-22. [Crossref] [PubMed]
- Otsuji Y, Handschumacher MD, Schwammenthal E, et al. Insights from three-dimensional echocardiography into the mechanism of functional mitral regurgitation: direct in vivo demonstration of altered leaflet tethering geometry. Circulation 1997;96:1999-2008. [Crossref] [PubMed]
- Kwon DH, Setser RM, Thamilarasan M, et al. Abnormal papillary muscle morphology is independently associated with increased left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. Heart 2008;94:1295-301. [Crossref] [PubMed]
- Holst KA, Schaff HV, Smedira NG, et al. Impact of Hospital Volume on Outcomes of Septal Myectomy for Hypertrophic Cardiomyopathy. Ann Thorac Surg 2022;114:2131-8. [Crossref] [PubMed]


