Coronary stent thrombosis: what have we learned?
“Not everything that can be counted counts, and not everything that counts can be counted.” ——William Bruce Cameron
Introduction
The advent of drug eluting stents (DES) produced a remarkable improvement in outcomes after percutaneous coronary interventions (PCI) (1,2). Drug elution suppressed smooth muscle cell migration, reduced the amount of neointimal hyperplasia and the rate of clinical driven target lesion revascularization (3). Nevertheless, the effectiveness of DES technology came at the cost of a greater risk of late and very late stent thrombosis (ST) (4). To overcome this limitation, refinements in stent geometry with thinner struts, more biocompatible polymers, lower drug dose eluted, PCI techniques (i.e., intravascular imaging guided procedures) in conjunction with potent adjunct pharmacological therapy (i.e., dual antiplatelet therapy) have further enhanced late DES safety profile (4-6). However, despite the reduction in thrombotic events with newer-generation DES to rates lower than 1%, ST still remains a catastrophic complication of PCI with high mortality rate (7,8).
Intravascular coronary imaging has emerged as a clinical tool to aid in the understanding of the pathophysiology of ST. Using intravascular ultrasound (IVUS), stent underexpansion, edge dissections and residual lesions in the outflow or inflow of the treated region have been recognized as causes of ST with both bare-metal stents (BMS) and DES (9-11). Recently, optical coherence tomography (OCT), which has 10 times higher resolution than IVUS, has been introduced into clinical practice and used to further investigate ST (12). Because of its high resolution (10–20 µm) OCT has become the optimal modality to assess in vivo stent failure. Nonetheless, due to the low incidence of ST with contemporary DES, very large prospective trials with serial imaging will be necessary to prospectively identify the causes of ST. Therefore, our current knowledge of the potential causes of ST relies on the analysis of thrombotic events.
The larger series of patients presenting with ST investigated by OCT is the PESTO French registry. This multicenter study included 120 patients presenting with acute, subacute, late and very-late ST after implantation of mainly BMS or DES. The majority of these patients (75%) presented with very late ST (more than 365 days after PCI) and 59% of cases were DES related ST. Notably, the use of OCT during the acute event allowed to identify the underlying morphological abnormality in 97% of cases. Overall, the most common findings in this series were strut malapposition (34%), neoatherosclerotic lesions (22%), stent underexpansion (11%), coronary evagination (8%), isolated uncovered struts (8%) and edge-related disease progression (8%) (Figure 1). In early ST (<30 days) malapposition and underexpansion were the predominant findings whereas in late ST cases (>30 days) malapposition and neoatherosclerosis were more prevalent (13). Although the observational design of this registry precludes concluding a causal relationship between these findings and ST, it further provides important information of the underlying factors associated with ST.
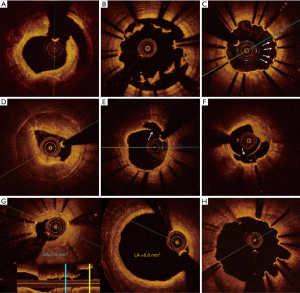
Pathophysiology
Multiple and overlapping causes may lead to the occurrence of ST. Factors related to the patient, lesion, procedure and post-procedure have been described (8). The interaction between blood and pro-thrombotic sub-endothelial elements, stents struts and polymers might lead to the activation of the extrinsic pathway of the coagulation cascade (14). Moreover, thrombogenicity and vascular healing may differ among metallic alloys and polymeric stent surfaces (15). Newer stents with thinner struts and more biocompatible polymers have shown to elicit less inflammation and reduced platelet activation (16). Nevertheless, platelet suppression and/or anticoagulation are essential to avoid adverse thrombotic events particularly in the early phase post-implantation.
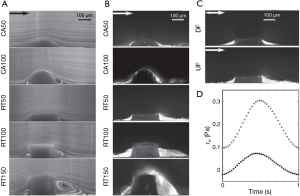
Furthermore, malapposition, under-deployment, device-vessel mismatch, incomplete coverage of lesion and overlapping stents produces disturbance of laminar blood flow. Low endothelial shear stress attenuates the endothelial expression of protective factors such as nitric oxide, prostacyclin I2, and tissue plasminogen activator (tPA), shifting toward a prothrombotic state (17). Additionally, low endothelial shear stress may promote ST by inhibiting endothelial cell proliferation and retarding re-endothelialization of the arterial and strut surface (18). Endothelial shear stress peaks over the strut surface and activates platelets that release thromboxane A2 and adenosine diphosphate, two potent mediators of platelet activation. Activated platelets enter flow separation zones downstream to the struts and reach high concentrations due to slow flow in conjunction with low endothelial shear stress, resulting in activation of the coagulation cascade (19,20) Furthermore, the thrombogenicity of coronary stent has been related to strut thickness. As areas of recirculation are created behind thick struts which promotes deposition of fibrin and thrombus in the microenvironment around the struts (Figure 2) (21).
Another important factor is the presence of a systemic prothrombotic state. An increased risk of ST in observed in the setting of acute coronary syndromes (ACS) (22). During ACS, stent implantation is associated with a higher incidence of irregular tissue protrusion and thrombi, which have been prospectively identified as a predictor of cardiovascular events (23,24). Moreover, stenting in denuded regions without protection factors such as tPA, prostacyclin, and nitric oxide may predispose to ST (19). Pathologic and in vivo studies have shown that DES implantation in lesions with abundant necrotic core might result in delayed or absent healing and endothelialization supporting the mechanistic pathophysiology of increased risk of ST after ACS (25-27).
Mechanical factors of stent thrombosis
Acute and subacute ST
Sub-optimal procedure results (i.e., underexpansion, malapposition and edge dissections) are the main causes of early ST (10,24). These mechanisms commonly coexist increasing the potential to trigger ST in the acute phase after stent implantation.
Underexpansion
Inadequate stent expansion has been consistently recognized as an important predictor of ST (10,24). This results from restricted expansion due to plaque-related factors (e.g., calcified plaque), implantation of an undersized stent for the vessel or under-deployment. Intravascular imaging guided PCI adequately assesses stent expansion and has demonstrated to reduce the occurrence of ST (5). In addition, recognition of underexpansion as the cause of ST using IVUS or OCT may aid in the selection of a treatment strategy (i.e., non-compliance balloon high-pressure dilatation). In the PESTO registry, major stent underexpansion was the underlying mechanism in 11% of patients and was more prevalent in acute and sub-acute ST cases (13).
Dissections
Stent edge dissections expose endothelial content to the blood and have been associated with acute and sub-acute ST when detected by angiography or IVUS (10,24). A major advantage of OCT is the capacity to detect edge dissections. However, during OCT-guided stent implantation, edge dissections are found in 30% to 40% of cases (28). The majority of these dissections healed completely during follow-up. Therefore, the clinical repercussion of edges dissections detected by OCT is not completely clear; however, it seems to be related with the length, width and circumferential extension of the dissection (29). For example, a non-flow-limiting, short and superficial dissections does not generally require additional treatment; whereas flow-limiting, long and deep dissection should be cover with an additional stent to restore adequate flow.
Incomplete coverage of lesion (geographical miss)
Incomplete lesion coverage in a frequent finding in patients undergoing angiography guided PCI (30). It is defined as a residual stenosis at the edge with a lumen cross-sectional area of <4.0 mm2 and >70% plaque burden, 5 to 10 mm from stent edges (9). Lumen compromised at the edges may predispose to flow disturbance and ST. Using IVUS, inflow and/or outflow obstructions have been shown to be predictors of early ST in the stable angina and ACS (9,10). The PESTO registry found edge related disease progression, assessed by OCT, as the underlying abnormality in 8% of ST cases (13).
Incomplete stent apposition (malapposition)
Stent malapposition is defined as the lack of contact between the stent struts and the underlying intimal surface of the vessel wall in a segment not overlying a sidebranch (31). Acute stent malapposition has been reported in up to 40% of patients undergoing OCT guided-PCI (23). Small malapposition areas have shown to resolved during follow-up. However, persistent protruding and malapposed struts disturbed blood flow and produced flow separation areas with eddy, which are known modulators of platelet activation and thrombosis (32). Nakano et al. evaluating autopsies of patients with early ST identified incomplete stent apposition and plaque morphology (i.e., necrotic core prolapse and thrombus burden) as triggers of early ST (33). Nonetheless, data coming from the CLI-THRO study do not support this hypothesis. In this OCT study, underexpansion and edge dissection were the only factors associated with early ST (24). In contrast, the PESTO registry found incomplete stent apposition in nearly half of the cases presenting with early ST (13). Despite the fact that stent malapposition is the most frequent finding in ST cases no causal relationship between stent malapposition and ST has been proven. Furthermore, the coexistence of malapposition with other mechanisms adds confounders into the analysis of the real impact of this entity as a cause of early ST.
Late and very late stent thrombosis
Uncovered strut
Delayed endothelial coverage resulting in uncovered struts is a frequent finding in very late ST patients. Finn et al., with pathological data suggested that the proportion of uncovered struts is one of the primary risk factors associated with ST. In this study, more than 30% of uncovered struts were associated with nine-fold increase in the odds ratio for thrombosis compared to covered stent struts (34). Guagliumi et al. identified uncoverage struts as the most common OCT correlate of very late ST (12). Interestingly, Taniwaki et al. comparing thrombus vs. control regions within the same stent observed a direct association between thrombus and uncovered struts or malapposition length (35). Additionally, strut coverage does not necessarily portend a functional endothelium, as OCT cannot differentiate material deposition (e.g., fibrin) over the struts from endothelial coverage (36).
Neoatherosclerosis
Neoatherosclerosis is characterized by accumulation of lipid-laden foamy macrophages within the neointimal, with or without calcification or necrotic core. The development of neoatherosclerosis may occur in month of years following stent implantation (37). Kang et al. using OCT found that neointimal plaque rupture in up to 70% of patients with very late ST (38). In the PESTO registry in-stent neointimal plaque rupture was the second most frequent finding of late and very late ST (28%; 14% of LST and 29% VLST) (13). In concordance with pathological studies, the onset of neoatherosclerotic lesions were later with BMS [10.6 (8.8–14.8) vs. 5.3 (2.4–7.0) years, P=0.001] compared to DES. This phenomenon has important clinical implications since it has been correlated with late restenosis and very late ST and has shown to have a similar incidence between first and second-generation DES (37). Futures studies should assess whether secondary prevention measures or new devices mitigate the risk of neoatherosclerosis.
Coronary evagination
A coronary evagination is defined as the presence of an outward bulge in the luminal vessel contour between apposed struts with a maximum depth of the bulge exceeding that of the actual strut thickness. Evagination could potentially produce flow stagnation and promote thrombus formation. Evagination within the stented segment were first reported in patients with sirolimus-eluting stent (SES) implantation and peri-stent contrast staining and have been found to be less frequent in second generation DES (39,40).
Stent fracture
Stent fracture is defined as a gap between struts and classified according to the shape and degree of displacement of the resulting segments (41). The prevalence of stent fracture ranges from 1.3% to 22% and has been associated with PCI in the right coronary artery (particularly in proximal and mid segment), lesion complexity, stent length and is more frequent with stainless steel stents compared with cobalt-chromium stents (41,42). Historically, angiography has been used for detection of stent fractures, however any intravascular imaging modality or non-invasive multislice computed tomography could be used (43). Stent fractures have been associated with a higher rate of stent restenosis, target lesion revascularization, coronary aneurysm development and definite ST (42). In recent reports of ST investigated by OCT, stent fractures has been an infrequent finding (13).
Malapposition
Incomplete stent apposition (ISA) may results acutely after the procedure or might be acquired during follow-up. Small areas of ISA might resolve spontaneously in nearly half of the patients, whereas larger ISA might persist over time. Late-acquired stent malapposition could results from thrombus dissolution (behind the struts) after primary PCI, positive vessel remodeling with detachment of the stent from the vessel wall and rarely from chronic stent recoil (31). Histological studies have been instrumental to enable a better understanding of the mechanisms of ISA. Virmani et al. was the first who linked hypersensitivity reactions and inflammation to the occurrence of malapposition and ST with sirolimus-eluting stent (Cypher, Cordis, New Jersey, USA) (44). In the clinical setting, Cook et al. using IVUS demonstrated that ISA was highly prevalent in patients presenting with very late ST (45). In addition, OCT studies have consistently shown a correlation between malapposition and very late ST (35,46). In the PESTO registry strut malapposition was the most frequent abnormality in patients with late and very late ST (13).
Despite the outstanding contribution of intracoronary imaging (i.e., IVUS and OCT) to the understanding of the mechanism leading to ST these data should be carefully interpreted because it was found in a highly selected population (i.e., patients who had ST). Moreover, due to the high resolution of OCT, abnormalities at the strut level can be visualized and quantitatively evaluated; however, not everything that can be counted counts, and not everything that counts can be counted. Nevertheless, these findings are patho-mechanistically plausible causes of ST and have shown to be consistent within studies and coherent with the theoretical knowledge. For that reason, and in the absence of direct evidence they should be considered as putative causes of ST. Therefore, optimization of stent implantation with the systematic use of intravascular imaging to avoid underexpansion and areas of ISA in conjunction with refinements of the stent technology to promote arterial healing and adequate pharmacological platelet suppression should be encouraged to further improve the outcomes of PCI and to further reduce the rate of ST.
Acknowledgements
None.
Footnote
Provenance: This is an invited Editorial commissioned by the Section Editor Feng Zhang (Department of Cardiology, Zhongshan Hospital of Fudan University, Shanghai, China).
Conflicts of Interest: Dr. Y. Sotomi is a consultant of GOODMAN and has received a grant from Fukuda Memorial Foundation for Medical Research and SUNRISE lab. Dr. P. W. Serruys and Y. Onuma are a members of the Advisory Board for Abbott Vascular. The other authors have no conflicts of interest to declare.
References
- Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 2003;349:1315-23. [Crossref] [PubMed]
- Stone GW, Ellis SG, Cox DA, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med 2004;350:221-31. [Crossref] [PubMed]
- Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Clinical outcomes with bioabsorbable polymer- versus durable polymer-based drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol 2014;63:299-307. [Crossref] [PubMed]
- Palmerini T, Benedetto U, Biondi-Zoccai G, et al. Long-Term Safety of Drug-Eluting and Bare-Metal Stents: Evidence From a Comprehensive Network Meta-Analysis. J Am Coll Cardiol 2015;65:2496-507. [Crossref] [PubMed]
- Ahn JM, Kang SJ, Yoon SH, et al. Meta-analysis of outcomes after intravascular ultrasound-guided versus angiography-guided drug-eluting stent implantation in 26,503 patients enrolled in three randomized trials and 14 observational studies. Am J Cardiol 2014;113:1338-47. [Crossref] [PubMed]
- Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045-57. [Crossref] [PubMed]
- Kereiakes DJ, Meredith IT, Windecker S, et al. Efficacy and safety of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent: the EVOLVE II Randomized Trial. Circ Cardiovasc Interv 2015.8. [PubMed]
- Claessen BE, Henriques JP, Jaffer FA, et al. Stent thrombosis: a clinical perspective. JACC Cardiovasc Interv 2014;7:1081-92. [Crossref] [PubMed]
- Fujii K, Carlier SG, Mintz GS, et al. Stent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimus-eluting stent implantation: an intravascular ultrasound study. J Am Coll Cardiol 2005;45:995-8. [Crossref] [PubMed]
- Choi SY, Witzenbichler B, Maehara A, et al. Intravascular ultrasound findings of early stent thrombosis after primary percutaneous intervention in acute myocardial infarction: a Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) substudy. Circ Cardiovasc Interv 2011;4:239-47. [Crossref] [PubMed]
- Doi H, Maehara A, Mintz GS, et al. Impact of post-intervention minimal stent area on 9-month follow-up patency of paclitaxel-eluting stents: an integrated intravascular ultrasound analysis from the TAXUS IV, V, and VI and TAXUS ATLAS Workhorse, Long Lesion, and Direct Stent Trials. JACC Cardiovasc Interv 2009;2:1269-75. [Crossref] [PubMed]
- Guagliumi G, Sirbu V, Musumeci G, et al. Examination of the in vivo mechanisms of late drug-eluting stent thrombosis: findings from optical coherence tomography and intravascular ultrasound imaging. JACC Cardiovasc Interv 2012;5:12-20. [Crossref] [PubMed]
- Souteyrand G, Amabile N, Mangin L, et al. Mechanisms of stent thrombosis analysed by optical coherence tomography: insights from the national PESTO French registry. Eur Heart J 2016;37:1208-16. [Crossref] [PubMed]
- Palmaz JC. Intravascular stenting: from basic research to clinical application. Cardiovasc Intervent Radiol 1992;15:279-84. [Crossref] [PubMed]
- Eppihimer MJ, Sushkova N, Grimsby JL, et al. Impact of stent surface on thrombogenicity and vascular healing: a comparative analysis of metallic and polymeric surfaces. Circ Cardiovasc Interv 2013;6:370-7. [Crossref] [PubMed]
- Otsuka F, Cheng Q, Yahagi K, et al. Acute Thrombogenicity of a Durable Polymer Everolimus-Eluting Stent Relative to Contemporary Drug-Eluting Stents With Biodegradable Polymer Coatings Assessed Ex Vivo in a Swine Shunt Model. JACC Cardiovasc Interv 2015;8:1248-60. [Crossref] [PubMed]
- Chatzizisis YS, Coskun AU, Jonas M, et al. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol 2007;49:2379-93. [Crossref] [PubMed]
- Akagawa E, Ookawa K, Ohshima N. Endovascular stent configuration affects intraluminal flow dynamics and in vitro endothelialization. Biorheology 2004;41:665-80. [PubMed]
- Jiménez JM, Davies PF. Hemodynamically driven stent strut design. Ann Biomed Eng 2009;37:1483-94. [Crossref] [PubMed]
- Koskinas KC, Chatzizisis YS, Antoniadis AP, et al. Role of endothelial shear stress in stent restenosis and thrombosis: pathophysiologic mechanisms and implications for clinical translation. J Am Coll Cardiol 2012;59:1337-49. [Crossref] [PubMed]
- Jiménez JM, Prasad V, Yu MD, et al. Macro- and microscale variables regulate stent haemodynamics, fibrin deposition and thrombomodulin expression. J R Soc Interface 2014;11:20131079. [Crossref] [PubMed]
- Bauer T, Möllmann H, Weidinger F, et al. Predictors of hospital mortality in the elderly undergoing percutaneous coronary intervention for acute coronary syndromes and stable angina. Int J Cardiol 2011;151:164-9. [Crossref] [PubMed]
- Soeda T, Uemura S, Park SJ, et al. Incidence and Clinical Significance of Poststent Optical Coherence Tomography Findings: One-Year Follow-Up Study From a Multicenter Registry. Circulation 2015;132:1020-9. [Crossref] [PubMed]
- Prati F, Kodama T, Romagnoli E, et al. Suboptimal stent deployment is associated with subacute stent thrombosis: optical coherence tomography insights from a multicenter matched study. From the CLI Foundation investigators: the CLI-THRO study. Am Heart J 2015;169:249-56. [Crossref] [PubMed]
- Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 2006;48:193-202. [Crossref] [PubMed]
- Lüscher TF, Steffel J, Eberli FR, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation 2007;115:1051-8. [Crossref] [PubMed]
- Räber L, Zanchin T, Baumgartner S, et al. Differential healing response attributed to culprit lesions of patients with acute coronary syndromes and stable coronary artery after implantation of drug-eluting stents: an optical coherence tomography study. Int J Cardiol 2014;173:259-67. [Crossref] [PubMed]
- Chamié D, Bezerra HG, Attizzani GF, et al. Incidence, predictors, morphological characteristics, and clinical outcomes of stent edge dissections detected by optical coherence tomography. JACC Cardiovasc Interv 2013;6:800-13. [Crossref] [PubMed]
- Radu MD, Räber L, Heo J, et al. Natural history of optical coherence tomography-detected non-flow-limiting edge dissections following drug-eluting stent implantation. EuroIntervention 2014;9:1085-94. [Crossref] [PubMed]
- Sarno G, Garg S, Gomez-Lara J, et al. Intravascular ultrasound radiofrequency analysis after optimal coronary stenting with initial quantitative coronary angiography guidance: an ATHEROREMO sub-study. EuroIntervention 2011;6:977-84. [Crossref] [PubMed]
- Attizzani GF, Capodanno D, Ohno Y, et al. Mechanisms, pathophysiology, and clinical aspects of incomplete stent apposition. J Am Coll Cardiol 2014;63:1355-67. [Crossref] [PubMed]
- Foin N, Gutiérrez-Chico JL, Nakatani S, et al. Incomplete stent apposition causes high shear flow disturbances and delay in neointimal coverage as a function of strut to wall detachment distance: implications for the management of incomplete stent apposition. Circ Cardiovasc Interv 2014;7:180-9. [Crossref] [PubMed]
- Nakano M, Yahagi K, Otsuka F, et al. Causes of early stent thrombosis in patients presenting with acute coronary syndrome: an ex vivo human autopsy study. J Am Coll Cardiol 2014;63:2510-20. [Crossref] [PubMed]
- Finn AV, Joner M, Nakazawa G, et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation 2007;115:2435-41. [Crossref] [PubMed]
- Taniwaki M, Radu MD, Zaugg S, et al. Mechanisms of Very Late Drug-Eluting Stent Thrombosis Assessed by Optical Coherence Tomography. Circulation 2016;133:650-60. [Crossref] [PubMed]
- Onuma Y, van Beusekom HM, Sorop O, et al. The paradigm of endothelium and stent thrombosis in DES. EuroIntervention 2008;4 Suppl C:C17-21.
- Otsuka F, Byrne RA, Yahagi K, et al. Neoatherosclerosis: overview of histopathologic findings and implications for intravascular imaging assessment. Eur Heart J 2015;36:2147-59. [Crossref] [PubMed]
- Kang SJ, Lee CW, Song H, et al. OCT analysis in patients with very late stent thrombosis. JACC Cardiovasc Imaging 2013;6:695-703. [Crossref] [PubMed]
- Radu MD, Pfenniger A, Räber L, et al. Flow disturbances in stent-related coronary evaginations: a computational fluid-dynamic simulation study. EuroIntervention 2014;10:113-23. [Crossref] [PubMed]
- Radu MD, Räber L, Kalesan B, et al. Coronary evaginations are associated with positive vessel remodelling and are nearly absent following implantation of newer-generation drug-eluting stents: an optical coherence tomography and intravascular ultrasound study. Eur Heart J 2014;35:795-807. [Crossref] [PubMed]
- Popma JJ, Tiroch K, Almonacid A, et al. A qualitative and quantitative angiographic analysis of stent fracture late following sirolimus-eluting stent implantation. Am J Cardiol 2009;103:923-9. [Crossref] [PubMed]
- Kan J, Ge Z, Zhang JJ, et al. Incidence and Clinical Outcomes of Stent Fractures on the Basis of 6,555 Patients and 16,482 Drug-Eluting Stents From 4 Centers. JACC Cardiovasc Interv 2016;9:1115-23. [Crossref] [PubMed]
- Ito T, Kimura M, Ehara M, et al. Impact of sirolimus-eluting stent fractures without early cardiac events on long-term clinical outcomes: a multislice computed tomography study. Eur Radiol 2014;24:1006-12. [Crossref] [PubMed]
- Virmani R, Guagliumi G, Farb A, et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation 2004;109:701-5. [Crossref] [PubMed]
- Cook S, Wenaweser P, Togni M, et al. Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implantation. Circulation 2007;115:2426-34. [Crossref] [PubMed]
- Parodi G, La Manna A, Di Vito L, et al. Stent-related defects in patients presenting with stent thrombosis: differences at optical coherence tomography between subacute and late/very late thrombosis in the Mechanism Of Stent Thrombosis (MOST) study. EuroIntervention 2013;9:936-44. [Crossref] [PubMed]


