
Benign tumors of the chest wall
Introduction
Benign tumors of the chest wall are uncommon entities arising from vessels, nerves, bones, cartilage, and soft tissues of the thoracic wall. Primary chest wall tumors are around 2% in the entire population, with a 50% percentage of benign histology (1). Benign chest wall tumors often appear as palpable and indolent masses in the chest wall. Still, there are not any apparent clinical features that can support physicians in distinguishing malignant from benign cancer. Given these tumors’ rarity and benign nature, prospective data or large studies exploring the best treatments are lacking.
The majority of benign chest wall tumors might benefit from a radical R0 surgical resection, which sometimes might require extended procedures leading to functional or aesthetical impairment (2,3).
Conservative treatment or radiotherapy can be considered a first-line approach according to the histology. Only in selected cases systemic medical treatment may have a role (Table 1).
Table 1
| Tumor | Origin | Frequent location | Imaging | Treatment |
|---|---|---|---|---|
| Osteoblastoma | Bone | Vertebrae, ribs, sternum | CT | Surgery |
| Chondroblastoma | Cartilage | Ribs | MRI | Surgery (alternative: radiotherapy) |
| Aneurysmal bone cyst | Bone | Ribs, sternum | CT/MRI | Surgery; chemical cauterization; cryotherapy; sclerotherapy |
| Giant cell tumor | Bone | Vertebrae, ribs, sternum | CT | Surgery; neo-adjuvant or adjuvant, zoledronic acid or Denosumab |
| Mesenchymal hamartoma | Bone | Ribs | MRI | Surgery; watch and wait |
| Osteochondroma | Bone/cartilage | Ribs, scapula | MRI | Surgery |
| Chondroma | Cartilage | Ribs, sternum | CT/MRI | Surgery |
| Fibrous dysplasia | Bone | Ribs | CT | Watch and wait; surgery; bisphosphonate therapy |
| Eosinophilic granuloma | Bone | Vertebrae, ribs, sternum | CT/MRI | Surgery; radiotherapy; methylprednisolone injection; vinblastine and prednisone or cytarabine |
| Desmoid tumor | Soft tissue | Chest wall | MRI | Watch and wait; surgery; chemotherapy; tyrosine kinase inhibitors |
| Leiomyoma | Soft tissue (muscle) | Pleura, chest wall | MRI | Surgery |
| Schwannoma/neurofibroma | Soft tissue (nerve sheath) | Intercostal spaces | CT/MRI | Surgery |
| Lymphangioma | Soft tissue (lymphatic system) | Chest wall | MRI | Surgery (alternative: chemotherapy) |
| Lipoma | Soft tissue | Chest wall | CT/MRI | Surgery; watch and wait |
| Hemangioma | Soft tissue (vascular tissue) | Intercostal space | MRI | Surgery (with eventually pre-op embolization) |
| Elastofibroma dorsi | Soft tissue | Infrascapular region | MRI | Surgery; watch and wait |
CT, computed tomography; MRI, magnetic resonance imaging.
Diagnostic imaging
The first diagnostic step is always collecting an accurate patient’s clinical history, focusing on previous cancers or radiation exposure and symptoms possibly deriving from the mass. A meticulous physical examination should be carried out before proceeding with diagnostic imaging modalities.
Chest radiographs can provide basic information mainly in the setting of osseous tumors. Ultrasound imaging can help characterize superficial lesions and even perform ultrasound-guided biopsies.
Computed tomography (CT) and magnetic resonance (MR) are the gold standards for delineating the location and extent of the tumor and to differentiate tumor tissues and types. Despite the frequent overlap of the radiologic features of benign and malignant chest wall tumors, differences in characteristic location and appearance often allow a differential diagnosis.
Biopsy
In most chest wall benign tumors, radiological features and clinical signs and symptoms might predict the behavior so that surgical resection can guarantee a diagnostic and curative treatment.
Preoperative biopsy might be required in those cases with unclear radiological features with a possible malignant component or if surgical resection is not feasible or not indica (4-6).
In particular, bone tumors might have borderline characteristics that do not allow a clear differential diagnosis between benign or malignant behaviour. For instance, chondroblastoma and aneurysmal bone cyst have non-specific radiological features that might simulate an infiltrating growth (7). In a series of 121 patients with chest wall swelling, Kaplan and colleagues suggest performing an early excisional biopsy in order to avoid useless resections or misdiagnosed of malignant tumors (8).
Biopsy for chest wall tumors should be a multidisciplinary decision based on patients features, neoplasms’ characteristics and further therapeutical option.
Tumor originating from bone tissue
Osteoblastoma
Osteoblastoma is an uncommon bone tumor, accounting for roughly 1% of all bone tumors (9). Histologically, there is anastomosing trabeculae of woven bone, embedded in a loose edematous fibrovascular stroma. Although the most common involved areas are the vertebral column and long bone, ribs and even sternum can be anecdotally involved (10). Osteoblastoma typically involves young patients in second or third decade, with a predilection for the male gender. Symptoms and signs are variable, but pain is the most characteristic symptom. It is in general localized and dull, with a typical night exacerbation.
Treatment
Due to the potentially aggressive growth, osteoblastoma benefits from radical surgical resection. Both curettage and en bloc resection can be proposed, but intralesional curettage is associated with a higher incidence of recurrence (11). In case of sternal involvement resection and reconstruction are mandatory (12).
Radiotherapy and chemotherapy did not show any significant effects (10).
In case of recurrence repeated resections are possible and embolization has been described too.
Chondroblastoma
Chondroblastoma is a rare tumor arising from cartilages. Histologically, round, or polyhedral chondroblasts are seen. They show abundant eosinophilic cytoplasm and well defined cell borders. Its growth is often destructive. Males are affected twice as often as females, and it more commonly appears in the second decade.
Although epiphysis of a long bone are the most common sites of origin, ribs have been anecdotally involved (13,14). Despite its benign nature, chondroblastoma can recur locally, and rarely metastasize.
Treatment
Surgery is usually the treatment of choice. Rib resection might be performed with an open or minimally invasive approach (14). Also, in case of radical resection, up to 20% of patients can recur and surgery can be offered if recurrence is technically resectable. Lung metastasis have been described, and surgery might play a role also in lung metastasectomy.
In case of non resectable lesions or patients that are not fit for surgery radiotherapy can be used as alternative treatment (15). The role of chemotherapy and immunotherapy is still to be defined: recently a combined therapy of doxorubicin and pembrolizumab showed promising results in case of lung metastasis concurrently (16). Denosumab has been used in metastatic patients (17).
Aneurysmal bone cyst
Aneurysmal bone cyst is an unusual benign cystic neoplasm that is composed of vascular channel that can grow and expand and might bring to bone destruction (11). The differential diagnosis should include telangiectatic osteosarcoma.
In the chest both ribs and sternum might be involved, even though these sites are rare (18,19).
Treatment
Resection or curettage have been considered for long the treatment of choice, associated to preoperative embolization to reduce intraoperative blood loss. A relatively high recurrence rate of up to 20% is found especially after curettage. Recently, more alternatives to surgery have been proposed such as chemical cauterization, cryotherapy, and, in particular, sclerotherapy with polidocanol which seems to reach similar long terms outcomes compared to surgery with a lower risk of deformity after treatment (20-23). Radiotherapy is seldom used due to the risk of malignant transformation.
Giant cell tumor
Giant cell tumors are a relatively frequent benign bone neoplasm, accounting for 15–20% of benign bone neoplasms in the United States (24). It is more frequently diagnosed in young adult in their third decade. Despite its benign nature, metastases to the lung or local aggressive behavior can occur (25). Histologically, the tumor discloses numerous non-neoplastic osteoclast-like giant cells with embedded mononuclear neoplastic cells.
Chest wall is an atypical localization for this kind of tumor (1% of all cases) and might involve ribs and sternum (26).
Treatment
Surgery and curettage are usually indicated for the treatment of this tumor. In the majority of reported cases of chest wall involvement an en bloc resection has been carried out with no signs of recurrences afterwards (26,27). Curettage has been described to be related to higher incidence of recurrence, but it allows a functional preservation of joints especially in the long bones of the limbs.
Zoledronic acid and denosumab have a role in the treatment of giant cell tumor. Zoledronic acid has been used both pre- and post-operatively with better intraoperative results and long-term outcomes respectively (28,29). Also, denosumab used pre-operatively can allow safer outcomes, but it is related to a significant higher incidence of disease recurrence (30,31).
Mesenchymal hamartoma
Mesenchymal hamartoma of the chest wall is a tumor that arises from one or more ribs and affects infant in the perinatal period or in their first years (32,33). Although the most frequent presentation is unilateral and solitary, multiple or bilateral masses have been reported (34). Histologically, there is abnormally organized arrangement of primitive mesenchyme, admixed with loose myxoid stroma, bland spindled cells and collagen.
Treatment
Surgery or conservative treatment are suggested in these tumors (35). Surgery encompasses radical resection of the rib or the involved bone portion with safe margins and it is proposed to symptomatic patients. In case of non-radical resection recurrence is possible.
On the other hand, asymptomatic patients, might benefit from a close follow-up.
Osteochondroma
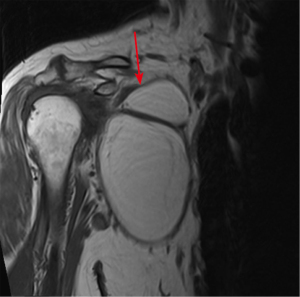
It is a benign bone surface tumor (Figure 1). It is made up of mature bone with a cartilage cap, which usually exhibits a growth plate-like morphology (Figure 2). It may be solitary or occur as multiple hereditary exostoses and is more often found in children and adolescents. Both sporadic solitary tumors and multiple hereditary exostoses are linked with loss of function (LoF) mutations in the genes EXT1 (8q24) and EXT2 (11p11) (36). However, in solitary lesions, EXT gene inactivation is substantially restricted to the cartilage cap and is somatic. On the other hand, the multiple exostoses evidence an autosomal dominant inheritance (37). If a cartilaginous cap is identified grossly, the microscopic examination of these tumors reveals a cap composed of mature hyaline cartilage with an overlying fibrous perichondrium. The transition between bone and cartilage cap in children and adolescents looks like a growth plate. It is easy to demonstrate endochondral ossification into mature bone. Cartilage cap diminishes and may basically be lacking later in life.

Treatment
Bakhshi et al. reported pain, uncertain diagnosis, and recurrent pneumothorax as the most frequent indications for surgical excision in patients affected by osteochondroma (38). Ribs are the most frequent site of chest involvement, but a few cases of osteochondroma involving the scapula are described in the literature (39,40). According to Thomas and Shen, resection of osteochondroma should not be indicated in asymptomatic prepubescent patients except in cases of enlarging tumors or of uncharacteristic radiological findings (41). However, when detected after puberty, osteochondromas are indicated for complete surgical resection because of the possible risk of pathologic fractures and malignant transformation. Complete resection encompasses excision of the interested bone with grossly negative margins.
Recurrence may occur if the cartilaginous cap is not completely resected, and it was reported to be approximately 2% in a series from the Mayo Clinic (41).
Chondroma
It is a tumor, which is benign and cartilaginous. It occurs in adults, mainly in the hands or feet. Grossly, the tumor is lobulated, hyaline, and calcified, while the microscopic examination discloses the lobulation at low power and plump neoplastic cells with fine punctate calcification at high magnification. Despite being benign, this tumor may disclose a nuclear hyperchromasia, which may be worrying in some cases. Fibrosis may be encountered. Other features may include osteoclast-like giant cells, vacuoles resembling lipoblasts, and histiocyte-like cells. The differential diagnosis should include chondrosarcoma, which usually exhibits more worrying features, and calcifying aponeurotic fibroma (41).
Treatment
Due to its histological and radiological similarity to chondrosarcoma, all chondromas should be considered malignant and excised surgically with wide local resection and negative margins. Pre-operative biopsy of the lesion is not recommended since it cannot precisely assess the presence of a malignant component (41). Recurrences are usually not reported after radical resection (42,43).
Fibrous dysplasia
It is a benign lesion with a fibro-osseous aspect (Figure 3). The lesion may involve one (monostotic) or multiple (polyostotic) bones. It is considered a developmental disorder of bone. It fails to form mature lamellar bone. There is a developmental defect, including woven bone and fibroblast-like spindle cells. There is a failure in bone maturation. Evidently, these lesions exhibit an arrest as woven bone, and it has been associated with clinical or syndromic manifestations. It can affect any bone and soft tissue, but the involvement of the spine is considered very rare (44). Grossly, there is often a well-circumscribed lesion with a sclerotic rim, which remains centered within the cortex. The cortex itself may be thinned as lesion expands. There is the eventuality that the lesion may undergo cartilaginous metaplasia or even aneurysmal bone cyst-like changes (45). The microscopic examination discloses branching and anastomosing irregular trabeculae of woven bone. They are often called “Chinese alphabet” characters or often “C” and “S” shapes. There is no noticeable osteoblastic rimming. The intervening fibrous stroma shows cytologically bland spindle cells. There is no conspicuous cytologic atypia, and mitotic activity is rare. Stromal changes occur at places, including myxoid change and fatty metaplasia (46). On the molecular biology aspect, there is a gain of function mutations in GNAS (guanine nucleotide-binding protein/α-subunit), located in 20q13.2-3, leading to overexpression of Gsα protein and increased downstream adenyl cyclase activity, activation of c-fos, c-jun, and Wnt/β-catenin are associated with activation of Gsα protein (47). It has been suggested that the inconstant expression of GNAS mutations may explain the variability in clinical manifestations. This lesion can be seen with McCune-Albright syndrome (endocrine abnormalities, café au lait spots) or Mazabraud syndrome (soft tissue myxomas). There is the extreme rare transformation into sarcoma, which usually occurs decades after initial diagnosis (48). It has been recommended that this lesion should be examined in toto and with multiple levels. By immunohistochemistry, there are positive stains, which include SATB2, which is expressed in bone-forming lesions (49). There is the absence of keratins. On the molecular side, postzygotic somatic mutations in GNAS are seen. They usually harbor gain of function single nucleotide substitutions (50,51). The differential diagnosis should include parosteal and low grade central osteosarcoma (destructive pattern), osteofibrous dysplasia (prominent osteoblastic rimming), and liposclerosing myxofibrous tumor (LSMFT), which typically exhibits activation of Protein G as well.

Treatment
In the chest wall, fibrous dysplasia (FD) is more common in the ribs and seldom is reported to involve the sternum. Stable and asymptomatic lesions can be monitored, while surgery can be proposed in case of symptomatic lesions or if a histological confirmation is needed or for aesthetical reasons (52-55). In patients who cannot be exposed to surgery, bisphosphonate therapy might help in pain control, especially in polyostotic forms.
Traibi and colleagues (56) reported six cases, all treated with surgical resections; the authors acknowledge one case requiring a posterior disarticulation, while the remaining five has a simple rib resection with a 1-cm free margin. No postoperative complications or disease recurrences were reported. Although open surgery is the most frequently reported, robotic resection of the first and second rib for cases of FD has been reported (57,58) with excellent postoperative outcomes.
Eosinophilic granuloma
It is one manifestation of Langerhans cell histiocytosis (LCH), a hematological neoplasm exhibiting a granulomatous disorder affecting the mononuclear phagocytic cell system. Patients with LCH of the thorax or the lung are cigarette smokers with very few complaints. The microscopic examination discloses multiple infiltrates in the interstitium, sometimes encircling small bronchioles. The Langerhans cells harbor a pale eosinophilic cytoplasm and an irregularly folded nucleus, which can reveal a small nucleolus at places. These findings should not be considered reactive inflammatory changes of endothelial cells, type II pneumocytes, and alveolar macrophages. The immunohistochemistry shows positivity for S-100, CD1a, and Langerin and the characteristic racquet-shaped Birbeck granules ultrastructurally only in the event of LCH, but not of the interstitial lung disease-like pattern seen in smokers.
Treatment
Chest wall eosinophilic granuloma represented a very rare entity, and only a few case reports are available reporting cases of eosinophilic granuloma involving the ribs or the sternum (59,60). On the other hand, vertebral bodies are frequently involved.
Treatment may differ according to the location and the age of the patient. Non-operative techniques account for the observation, immobilization, low-dose radiotherapy (6 to 12 Gy) and methylprednisolone injection; a 12-month treatment with vinblastine and prednisone or cytarabine might be used in highly selected patients, usually with polyostotic diffusion (61). In the chest wall, rib and sternal resections have been described, but the diagnosis was usually not known before surgery. Surgical resection is often done with no diagnosis, as the disease might present as a spontaneous rib fracture. Complete resection with a negative margin usually allows a safe, disease-free survival (61-64).
Tumor originating from soft tissue
Desmoid tumor
It is a locally aggressive, never metastasizing fibroblastic or myofibroblastic tumor. It arises in deep soft tissues with no metastatic potential, but it may be associated with familial adenomatous polyposis (Gardner syndrome). Grossly, the fibromatosis is typically 5–10 cm in diameter. It may be substantially poorly defined or discloses well circumscription. On the cut surface, it is firm, shining white, gritty, and indelicately trabeculated approaching scar tissue. The microscopic examination discloses long, sweeping fascicles with delicate walled vessels and the occurrence of microhemorrhages (Figure 4). The tumor cells are bland cells with mild to moderate cellularity. No or minimal atypia is common. The differential diagnosis includes scar tissue, nodular fasciitis, proliferative fasciitis/myositis, myofibroma/myofibromatosis, calcifying fibrous pseudotumor, solitary fibrous tumor, metastasizing gastrointestinal stromal tumor (GIST), leiomyoma, nerve sheath tumors, and fibrosarcoma.

Treatment
In the chest, desmoid tumor might occur both growing in the chest wall (most commonly) or from the chest wall into the pleural space. Data on the best management of desmoid tumors are based on small series and small prospective studies, but compared to other rare benign diseases of the chest wall, the body of evidence is less limited.
Surgery has been widely described as the treatment of choice in the past (65,66). Nevertheless, according to the results of a recent consensus on desmoid tumors, conservative treatment, namely active surveillance for 1 or 2 years, is the standard of care at the moment of diagnosis, as it showed similar outcomes compared to surgical resection; more in detail, in patients with non-abdominal desmoid tumor, 2-year event free survival was significantly better in those patients managed non-surgically (52%) compared to those who underwent initial surgery (25%; P=0.001) (67,68). In a cohort of 216 patients, only 5% switched to surgical therapy after initial conservative treatment, 51% switched to medical treatment, and 20% of patients experienced spontaneous regression.
Surgery is indicated in case of a rapidly growing tumor, and the target is an R0 resection; R1 resection may be acceptable in case of functional severe, or cosmetic issues. According to the position and the growth of the desmoid tumor, resection might involve not only the chest wall but also the lung (65). The role of adjuvant radiotherapy is debated as it does not guarantee significantly better recurrence-free outcomes compared to surgery alone. On the other hand, moderate doses of radiotherapy might be considered as an alternative to surgery in case of non-resectable tumors (67).
Concurrently, medical treatments also have a role in the treatment of desmoid tumors. Systemic treatment options for desmoid tumor comprise antihormonal therapies (tamoxifen or toremifene) with or without non-steroidal anti-inflammatory drugs (NSAIDs), tyrosine kinase inhibitors (TKIs; namely sorafenib, pazopanib and imatinib), and “low-dose” or conventional chemotherapeutic regimens (methotrexate plus vinorelbine or vinblastine; anthracyclines regimens) and liposomal doxorubicin. With the apparent bias of the lack of high-quality, prospective data, a recent study questioned the real benefit of hormonal therapy that is now not recommended (69). On the other hand, both sorafenib and pazopanib showed interesting results at low dosages; in details in a phase II trial comparing sorafenib versus placebo, patients treated with sorafenib had a significantly better progression-free survival; nevertheless, the overall response rate was 31% in the sorafenib group and 20% in the placebo group, due the spontaneous regression. Similar results were obtained for pazopanib in phase II randomized study (70,71). Low doses chemotherapy regimens using methotrexate associated to vinblastine or vinorelbine have been evaluated in retrospective and prospective studies (65,72,73) with a disease control obtained in up to 70% of cases. Oral vinorelbine also showed interesting long-term results with a good toxicity profile (74). Lastly, anthracycline-based regimens using conventional doses for 6 to 8 cycles gave a response rate of up to 54% (75,76).
To conclude, the current treatment algorithm for chest wall desmoid tumor requires histological proof by fine needle biopsy and active surveillance. In case of progression, medical treatment is suggested and, in case of disease relapse, additional medical treatment, surgery, or radiotherapy should be considered. In the case of intrathoracic desmoid tumor, surgery and/or radiotherapy might be proposed as a first-step treatment after evidence of progression.
Leiomyoma
Only 15 cases of chest wall leiomyoma have been described in literature (77). Histologically, the tumor shows fascicles or bundles of spindled cells exhibiting eosinophilic and focally fibrillary cytoplasm.
Treatment
The majority, 13 out of 15, underwent radical surgical excision with further recurrences. Although there are no standardized guidelines, surgical option is usually the treatment of choice as it allows to prevent the rare possibility of malignant degeneration and to relief to symptoms such as chest pain or dyspnea (78).
Schwannoma/neurofibroma
Schwannoma represents a large majority of neurogenic tumors, while neurofibromas have been seldom reported in the chest wall. Histologically, there are compact hypercellular Antoni A areas admixed with myxoid hypocellular Antoni B areas. Verocay bodies can be found. They are nuclei showing palisading around fibrillary process.
Treatment
Type of surgical approach depends on the location of the mass and its dimensions: minimally invasive surgery, such as video-assisted thoracic surgery (VATS) or robotic-assisted thoracic surgery (RATS), can be offered if the mass is small and it develops towards the pleural cavity (Figure 5), while thoracotomy is indicated for larger masses or for those tumors which develops in the subcutaneous chest wall or into the intercostal space. The aim is an en-bloc excision of the mass which may require important tissue demolition (i.e., rib resection or even laminectomy if the mass involves the neural root) (79-83).
Lymphangioma
The tumor is constituted by thin walled, dilated lymphatic channels. They may disclose or not intraluminal proteinaceous material and lymphocytes. D2-40/podoplanin is the critical immunohistochemical marker. It is consistently expressed in small vessels but may be lost in large vessels (84).
Treatment
Alkwai et al. reported that for patients affected by lymphangioma the correct choice of treatment and its priority should be individualised and depend on many factors, including the size of the lesion, its location, the presence of symptoms or functional impairment and patient preference (85). Beside surgery, other systemic and loco regional treatments have been reported, such as chemotherapy or administration of interferon-α, intralesional sclerotherapy or administration of propranolol, but there is still lack of follow-up data and the real effectiveness of these new therapies has not been proved (86). Surgical radical excision of the tumor is still considered the best treatment to be proposed to patients, even though relapse after surgery is not rare (87).
Lipoma
It is a benign tumor which exhibits mature adipocytes enveloped by a capsule (Figure 6). It is probably the most common soft tissue tumor and is mostly localized at a subcutaneous level and with a diameter of 5 cm or less. Imaging would show the same pattern seen in fat and grossly and microscopically have the same characteristics as normal fat (Figure 7). However, the large adipose neoplasms with a diameter of 10 cm or more and located deeply need to be carefully grossed and evaluated to rule out an atypical lipomatous tumor/well-differentiated liposarcoma and the exclusion of MDM2 amplification is substantially required for diagnosis. Epidemiologically, there is no sex difference, despite some reports disclosing a male predominance. It remains rare in children. The upper back location is the most frequent in the thorax (4,11). Pathophysiologically, there is a reactivation of the expression of HMGA2 protein, but despite this data, the etiology remains elusive (88). On the clinical side, the tumor is painless, but some discomfort can occur during some physical activities. In one of 20 cases, the tumor may occur at multiple sites (89). It has been associated with Cowden syndrome and Bannayan-Riley-Ruvalcaba syndrome (90). Histologically, a mature adipocyte proliferation is separated from the surrounding tissue by a capsule. There are paucicellular fibrous septa and fat necrosis can occur in large tumors. In intramuscular lipoma, skeletal muscle fibers are infiltrated. Subtypes include intramuscular lipoma, chondrolipoma, and endobronchial lipoma. Immunohistochemistry is not needed in most of cases. However, positive stains include Rb (G3-245), which is lost in spindle cell pleomorphic lipoma, HMGA2 is negative in normal fat, while negative stains include MDM2, CDK4, p16 (91-94). From the molecular and cytogenetic point of view, there are some features which have been described. They include structural rearrangement of chromosome bands12q13-q15 or HMGA2 gene, structural rearrangement of chromosome band 6p21 or HMGA1 gene, absence of MDM2 amplification, lack of CDK4 amplification, absence of giant marker/ring chromosome, and absence of 13p loss (95,96).

Treatment
Treatment of lipomas is related to their position and their growth pattern. Radiological or clinical follow-up is frequently proposed in case of small, superficial lesions, while a wide surgical resection can be proposed in case of aesthetical issues, rapidly growing or intrathoracic lesions (97). The surgical approach is strictly related to the location of the lesion: subcutaneous lipomas or those growing in the chest wall soft tissues can be approached by open surgery, while intrathoracic lipomas can be removed both by thoracotomy or, more frequently, by minimally invasive techniques (98). Given the low aggressiveness and slow progression, no systemic treatment or the use of radiation are described.
Recurrence is variably reported ranging from 3% to 62%, and it occurs up to 10 years after resection (99). Two cases of local liposarcoma recurrence at the site of a previously excised lipoma are described in the literature; these cases might be due to a malignant transformation of the lipoma. Both patients received a new radical surgery, and no recurrence was detected in the follow-up (100,101).
Hemangioma
Hemangiomas are benign vascular tumors made up of dilated, tortuous blood vessels that may primarily arise from the soft tissue of the chest wall or can per continuitatem into the chest wall from the mediastinum or the chest cavities. Capillary, cavernous, arteriovenous, and venous types have been described based on the predominant type of vascular channel identified in the lesion (102). Hemangiomas are usually congenital tumors, developing from abnormal embryonic sequestration, although trauma may represent a trigger factor in young patients.
Treatment
Wide local excision with an adequate tumor-free margin is strongly recommended because hemangiomas may bleed spontaneously or after minor trauma. Embolization to the supplying artery followed by tumor resection is often the chosen surgical approach to prevent intraoperative bleeding and recruitment of a collateral blood supply.
Elastofibroma dorsi
It is a benign, poorly circumscribed tumor of the subscapular area of the thorax. It is composed of collagen and coarse enlarged elastic fibers. It some considered a pseudotumor as several authors suggest it represents a reactive hyperplasia involving abnormal elastogenesis. It was described in 1961 by Jarvi and Saxen first and most often seen in female adults older than 50 (103). The most common site is the apex of scapula, usually on the right. Infrequently, it has been described in deltoid muscle, infra-olecranon area, hip, thigh, and stomach. It may occur in a multiple fashions. Bilateral and familial cases have been described in the literature (Figure 8). Grossly, it is usually ill-defined. It is often rubbery, gray-white with mixed with yellow streaks. Microscopically, there are collagen bundles, which alternate with large, thick eosinophilic bands called “elastic cylinders with a dense central core”. Elastic fibers may occur fragmented into linear globules and labeled as “beads on a string”. There is often an irregular interdigitation into the surrounding adipose tissue. Verhoeff elastin stain is useful for identifying elastic fibers. The immunohistochemistry discloses positive vimentin and CD34 (spindle cells) (104). Negativity is found for S100, desmin, smooth muscle actin, p53 (105). The ultrastructural examination discloses central core contains mature fibers and cylinders composed of immature amorphous elastic tissue (106,107). Molecular biological analysis revealed Xq12-q22 or #19 gains in 30% of the cases (108). The differential diagnosis should include desmoid fibromatosis (high cellularity, skeletal muscle infiltration, and absence of elastic fibers) and fibrolipoma (absence of elastic fibers).
Treatment
The role of preoperative biopsy for elastofibroma dorsi is debated: Hayes et al. recommended core biopsy to obtain a pretreatment tissue diagnosis, while Massengill et al. suggested that clinical and radiological findings alone might allow an accurate diagnosis (109,110).
Surgery is the therapy of choice in patients with symptomatic disease, impairment of shoulder movement, or in patients with diagnostic doubts (111,112). In asymptomatic patients with typical radiological and clinical characteristics, conservative treatment with follow-up can be adopted.
Recurrence seems not to be an issue as it is not frequently reported in the literature. On the other hand, contralateral metachronous occurrence or bilateral synchronous presentation is reported: these cases are evaluated with the same treatment algorithm (113).
Conclusions
Benign tumors of the chest wall are rare entities that require a scrupulous imaging and pathological assessment in order to manage the affected patients with the proper treatment.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Erik de Loos, José Ribas de Campos and Jean Daemen) for the series “Chest Wall Resections and Reconstructions” published in Journal of Thoracic Disease. The article has undergone external peer review.
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-464/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-464/coif). The series “Chest Wall Resections and Reconstructions” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- David EA, Marshall MB. Review of chest wall tumors: a diagnostic, therapeutic, and reconstructive challenge. Semin Plast Surg 2011;25:16-24. [Crossref] [PubMed]
- Hsu PK, Hsu HS, Lee HC, et al. Management of primary chest wall tumors: 14 years’ clinical experience. J Chin Med Assoc 2006;69:377-82. [Crossref] [PubMed]
- Ito T, Suzuki H, Yoshino I. Mini review: surgical management of primary chest wall tumors. Gen Thorac Cardiovasc Surg 2016;64:707-14. [Crossref] [PubMed]
- Watt AJ. Chest wall lesions. Paediatr Respir Rev 2002;3:328-38.
- Colucci PG, Cohen SA, Baad M, et al. Pediatric chest wall masses: spectrum of benign findings on ultrasound. Pediatr Radiol 2022;52:429-44. [Crossref] [PubMed]
- Tateishi U, Gladish GW, Kusumoto M, et al. Chest wall tumors: radiologic findings and pathologic correlation: part 1. Benign tumors. Radiographics 2003;23:1477-90. [Crossref] [PubMed]
- Nam SJ, Kim S, Lim BJ, et al. Imaging of primary chest wall tumors with radiologic-pathologic correlation. Radiographics 2011;31:749-70. [Crossref] [PubMed]
- Kaplan T, Gunal N, Gulbahar G, et al. Painful Chest Wall Swellings: Tietze Syndrome or Chest Wall Tumor? Thorac Cardiovasc Surg 2016;64:239-44. [Crossref] [PubMed]
- Cerase A, Priolo F. Skeletal benign bone-forming lesions. Eur J Radiol 1998;27:S91-7. [Crossref] [PubMed]
- Berry M, Mankin H, Gebhardt M, et al. Osteoblastoma: a 30-year study of 99 cases. J Surg Oncol 2008;98:179-83. [Crossref] [PubMed]
- De Salvo S, Pavone V, Coco S, et al. Benign Bone Tumors: An Overview of What We Know Today. J Clin Med 2022;11:699. [Crossref] [PubMed]
- Golant A, Lou JE, Erol B, et al. Pediatric osteoblastoma of the sternum: a new surgical technique for reconstruction after removal: case report and review of the literature. J Pediatr Orthop 2004;24:319-22.
- Behjati S, Tarpey PS, Presneau N, et al. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nat Genet 2013;45:1479-82. [Crossref] [PubMed]
- Wu Y, Guan J, Zhang K, et al. Rare chondroblastoma of the 6th left rib, video-assisted thoracoscopy resected: one case report and literature review. J Cardiothorac Surg 2021;16:192. [Crossref] [PubMed]
- Liu J, Ahmadpour A, Bewley AF, et al. Chondroblastoma of the Clivus: Case Report and Review. J Neurol Surg Rep 2015;76:e258-64. [Crossref] [PubMed]
- Baumhoer D, Harder D, Ameline B, et al. Metastasizing chondroblastoma: a rare bone tumor no longer supported by the WHO classification. Skeletal Radiol 2021;50:255-60. [Crossref] [PubMed]
- Focaccia M, Gambarotti M, Hakim R, et al. Chondroblastoma’s Lung Metastases Treated with Denosumab in Pediatric Patient. Cancer Res Treat 2021;53:279-82. [Crossref] [PubMed]
- Singh VP, Mir R, Kaul S. Aneurysmal bone cyst of sternum. Ann Thorac Surg 2010;89:e43-5. [Crossref] [PubMed]
- Moraitis S, Moraitis D, Chounti M, et al. Aneurysmal rib cyst. Monaldi Arch Chest Dis 2017;87:860. [Crossref] [PubMed]
- Restrepo R, Zahrah D, Pelaez L, et al. Update on aneurysmal bone cyst: pathophysiology, histology, imaging and treatment. Pediatr Radiol 2022;52:1601-14. [Crossref] [PubMed]
- Varshney MK, Rastogi S, Khan SA, et al. Is sclerotherapy better than intralesional excision for treating aneurysmal bone cysts? Clin Orthop Relat Res 2010;468:1649-59. [Crossref] [PubMed]
- Deventer N, Schulze M, Gosheger G, et al. Primary Aneurysmal Bone Cyst and Its Recent Treatment Options: A Comparative Review of 74 Cases. Cancers (Basel) 2021;13:2362. [Crossref] [PubMed]
- Bavan L, Wijendra A, Kothari A. Efficacy of treatment interventions for primary aneurysmal bone cysts: a systematic review. Bone Jt Open 2021;2:125-33. [Crossref] [PubMed]
- Guo W, Xu W, Huvos AG, et al. Comparative frequency of bone sarcomas among different racial groups. Chin Med J (Engl) 1999;112:1101-4.
- Viswanathan S, Jambhekar NA. Metastatic giant cell tumor of bone: are there associated factors and best treatment modalities? Clin Orthop Relat Res 2010;468:827-33. [Crossref] [PubMed]
- Özyüksel G, Ardıçlı B, Özcan HN, et al. Giant cell tumor arising from the anterior arc of the rib: an extremely rare site in an adolescent girl. Turk J Pediatr 2022;64:940-5. [Crossref] [PubMed]
- Mogi A, Kosaka T, Yamaki E, et al. Surgical resection and reconstruction for a giant cell tumor of the anterior rib. Gen Thorac Cardiovasc Surg 2012;60:233-6. [Crossref] [PubMed]
- Kundu ZS, Sen R, Dhiman A, et al. Effect of Intravenous Zoledronic Acid on Histopathology and Recurrence after Extended Curettage in Giant Cell Tumors of Bone: A Comparative Prospective Study. Indian J Orthop 2018;52:45-50. [Crossref] [PubMed]
- Gouin F, Rochwerger AR, Di Marco A, et al. Adjuvant treatment with zoledronic acid after extensive curettage for giant cell tumours of bone. Eur J Cancer 2014;50:2425-31. [Crossref] [PubMed]
- Traub F, Singh J, Dickson BC, et al. Efficacy of denosumab in joint preservation for patients with giant cell tumour of the bone. Eur J Cancer 2016;59:1-12. [Crossref] [PubMed]
- Müller DA, Beltrami G, Scoccianti G, et al. Risks and benefits of combining denosumab and surgery in giant cell tumor of bone-a case series. World J Surg Oncol 2016;14:281. [Crossref] [PubMed]
- Yeshvanth SK, Shivamurthy V, Patil C, et al. Mesenchymal hamartoma of the chest wall- mimicker of malignancy. J Cancer Res Ther 2011;7:496-8. [Crossref] [PubMed]
- Lee MYW, Wang MQW, Quan DLW, et al. A Case of Mesenchymal Hamartoma of the Chest Wall in a 4-Month-Old Infant. Am J Case Rep 2019;20:511-6. [Crossref] [PubMed]
- Yilmaz E, Erol OB, Pekcan M, et al. Bilateral Multifocal Hamartoma of the Chest Wall in an Infant. Pol J Radiol 2015;80:283-5. [Crossref] [PubMed]
- Tanaka T, Fumino S, Shirai T, et al. Mesenchymal hamartoma of the chest wall in a 10-year-old girl mimicking malignancy: a case report. Skeletal Radiol 2019;48:643-7. [Crossref] [PubMed]
- Tanteles GA, Nicolaou M, Neocleous V, et al. Genetic screening of EXT1 and EXT2 in Cypriot families with hereditary multiple osteochondromas. J Genet 2015;94:749-54. [Crossref] [PubMed]
- Wuyts W, Van Hul W, De Boulle K, et al. Mutations in the EXT1 and EXT2 genes in hereditary multiple exostoses. Am J Hum Genet 1998;62:346-54. [Crossref] [PubMed]
- Bakhshi H, Kushare I, Murphy MO, et al. Chest wall osteochondroma in children: a case series of surgical management. J Pediatr Orthop 2014;34:733-7. [Crossref] [PubMed]
- Shackcloth MJ, Page RD. Scapular osteochondroma with reactive bursitis presenting as a chest wall tumour. Eur J Cardiothorac Surg 2000;18:495-6. [Crossref] [PubMed]
- Chun DI, Cho JH, Choi IH, et al. Osteochondroma of ventral scapula associated with chest pain due to rib cage compression: A case report. Medicine (Baltimore) 2018;97:e0510. [Crossref] [PubMed]
- Thomas M, Shen KR. Primary Tumors of the Osseous Chest Wall and Their Management. Thorac Surg Clin 2017;27:181-93. [Crossref] [PubMed]
- Dolores-Velázquez R, Lever-Rosas CD, Barrera-Franco JL, et al. Primary benign chest wall tumors: results of surgical treatment. Cir Cir 2007;75:419-24.
- Somers J, Faber LP. Chondroma and chondrosarcoma. Semin Thorac Cardiovasc Surg 1999;11:270-7. [Crossref] [PubMed]
- Joyce KM. Fibrous Dysplasia of the Spine — A Case Involving Three Levels of Thoracic Spine. J Orthop Case Rep 2014;4:73-7. [Crossref] [PubMed]
- Czerniak B. Dorfman and Czerniak’s Bone Tumors. 2nd Edition. Elsevier; 2015.
- Shidham VB, Chavan A, Rao RN, et al. Fatty metamorphosis and other patterns in fibrous dysplasia. BMC Musculoskelet Disord 2003;4:20. [Crossref] [PubMed]
- Jour G, Oultache A, Sadowska J, et al. GNAS Mutations in Fibrous Dysplasia: A Comparative Study of Standard Sequencing and Locked Nucleic Acid PCR Sequencing on Decalcified and Nondecalcified Formalin-fixed Paraffin-embedded Tissues. Appl Immunohistochem Mol Morphol 2016;24:660-7. [Crossref] [PubMed]
- Fu CJ, Hsu CY, Shih TT, et al. Monostotic fibrous dysplasia of the thoracic spine with malignant transformation. J Formos Med Assoc 2004;103:711-4.
- Conner JR, Hornick JL. SATB2 is a novel marker of osteoblastic differentiation in bone and soft tissue tumours. Histopathology 2013;63:36-49. [Crossref] [PubMed]
- Shin SJ, Lee SJ, Kim SK. Frequency of GNAS R201H substitution mutation in polyostotic fibrous dysplasia: Pyrosequencing analysis in tissue samples with or without decalcification. Sci Rep 2017;7:2836. [Crossref] [PubMed]
- Lee SE, Lee EH, Park H, et al. The diagnostic utility of the GNAS mutation in patients with fibrous dysplasia: meta-analysis of 168 sporadic cases. Hum Pathol 2012;43:1234-42. [Crossref] [PubMed]
- Ahmad Z, Zubair I. Fibrous dysplasia of rib presenting as a cystic mass in the lung. Oxf Med Case Reports 2015;2015:196-9. [Crossref] [PubMed]
- Mahadevappa A, Patel S, Ravishankar S, et al. Monostotic fibrous dysplasia of the rib: a case report. Case Rep Orthop 2012;2012:690914. [Crossref] [PubMed]
- Tang J, Wang JJ, Zhai W, et al. Chest wall reconstruction in a patient with sternal fibrous dysplasia. Thorac Cardiovasc Surg 2011;59:58-60. [Crossref] [PubMed]
- Zorzin L, Palmieri G, Marrese C, et al. Polyostotic fibrous dysplasia involving the sternum. Clin Rheumatol 1988;7:107-9. [Crossref] [PubMed]
- Traibi A, El Oueriachi F, El Hammoumi M, et al. Monostotic fibrous dysplasia of the ribs. Interact Cardiovasc Thorac Surg 2012;14:41-3. [Crossref] [PubMed]
- Liu B, Gao S, Wu Q, et al. A case report of robotic-assisted resection of large fibrous benign tumor of second rib. J Cardiothorac Surg 2022;17:329. [Crossref] [PubMed]
- Nakahama H, Vigneswaran W. Robotic-Assisted Resection of Fibrous Dysplasia of the Ribs. Thoracic Surgery: 50 Challenging cases. CRC Press; 2019.
- Angelini A, Mavrogenis AF, Rimondi E, et al. Current concepts for the diagnosis and management of eosinophilic granuloma of bone. J Orthop Traumatol 2017;18:83-90. [Crossref] [PubMed]
- Jha SK, De Jesus O. Eosinophilic Granuloma. Treasure Island, FL, USA: StatPearls Publishing; 2022.
- Eroglu A, Kürkçüoglu IC, Karaoglanoglu N. Solitary eosinophilic granuloma of sternum. Ann Thorac Surg 2004;77:329-31. [Crossref] [PubMed]
- Bayram AS, Köprücüoglu M, Filiz G, et al. Case of solitary eosinophilic granuloma of the sternum. Thorac Cardiovasc Surg 2008;56:117-8. [Crossref] [PubMed]
- Zuo T, Jiang P, Yu J, et al. Langerhans cell histiocytosis of the rib presenting with pathological fracture: a case report. J Cardiothorac Surg 2020;15:332. [Crossref] [PubMed]
- Sai S, Fujii K, Masui F, et al. Solitary eosinophilic granuloma of the sternum. J Orthop Sci 2005;10:108-11. [Crossref] [PubMed]
- Meyerson SL, D’Amico TA. Intrathoracic desmoid tumor: brief report and review of literature. J Thorac Oncol 2008;3:656-9. [Crossref] [PubMed]
- Noda D, Abe M, Takumi Y, et al. Resection and postoperative radiation therapy for desmoid fibromatosis of the chest wall in a young woman. Surg Case Rep 2021;7:28. [Crossref] [PubMed]
- The management of desmoid tumours: A joint global consensus-based guideline approach for adult and paediatric patients. Eur J Cancer 2020;127:96-107. [Crossref] [PubMed]
- Salas S, Dufresne A, Bui B, et al. Prognostic Factors Influencing Progression-Free Survival Determined From a Series of Sporadic Desmoid Tumors: A Wait-and-See Policy According to Tumor Presentation. J Clin Oncol 2011;29:3553-8. [Crossref] [PubMed]
- Libertini M, Mitra I, van der Graaf WTA, et al. Aggressive fibromatosis response to tamoxifen: lack of correlation between MRI and symptomatic response. Clin Sarcoma Res 2018;8:13. [Crossref] [PubMed]
- Gounder MM, Mahoney MR, Van Tine BA, et al. Sorafenib for Advanced and Refractory Desmoid Tumors. N Engl J Med 2018;379:2417-28. [Crossref] [PubMed]
- Toulmonde M, Pulido M, Ray-Coquard I, et al. Pazopanib or methotrexate-vinblastine combination chemotherapy in adult patients with progressive desmoid tumours (DESMOPAZ): a non-comparative, randomised, open-label, multicentre, phase 2 study. Lancet Oncol 2019;20:1263-72. [Crossref] [PubMed]
- Azzarelli A, Gronchi A, Bertulli R, et al. Low-dose chemotherapy with methotrexate and vinblastine for patients with advanced aggressive fibromatosis. Cancer 2001;92:1259-64. [Crossref] [PubMed]
- Skapek SX, Ferguson WS, Granowetter L, et al. Vinblastine and methotrexate for desmoid fibromatosis in children: results of a Pediatric Oncology Group Phase II Trial. J Clin Oncol 2007;25:501-6. [Crossref] [PubMed]
- Mir O, Rahal C, Rimareix F, et al. Efficacy of oral vinorelbine in advanced/progressive desmoid tumours: An updated retrospective study in 50 patients. J Clin Oncol 2016;34:abstr 11050.
- de Camargo VP, Keohan ML, D’Adamo DR, et al. Clinical outcomes of systemic therapy for patients with deep fibromatosis (desmoid tumor). Cancer 2010;116:2258-65. [Crossref] [PubMed]
- Garbay D, Le Cesne A, Penel N, et al. Chemotherapy in patients with desmoid tumors: a study from the French Sarcoma Group (FSG). Ann Oncol 2012;23:182-6. [Crossref] [PubMed]
- Ullah A, Patterson GT, Ghleilib I, et al. Primary Extra-Pleura Leiomyoma: A Case Report and Literature Review. Curr Oncol 2022;29:2935-40. [Crossref] [PubMed]
- Batihan G, Usluer O, Kaya SO, et al. Atypical deep somatic soft-tissue leiomyoma of extrathoracic chest wall: first case of the literature. BMJ Case Rep 2018;11:e226668. [Crossref] [PubMed]
- Corrivetti F, Stati G, Carpineta E, et al. Minimally Invasive Microsurgical Removal of Giant Dumbbell Thoracic Neurofibroma: Usefulness of Current Technology for Minimizing the Approach-Illustrative Case and Technical Video. World Neurosurg 2021;147:157. [Crossref] [PubMed]
- Kumar S, Gupta R, Handa A, et al. Totally cystic intradural schwannoma in thoracic region. Asian J Neurosurg 2017;12:131-3. [Crossref] [PubMed]
- Muneeb A, Khan MS, Iqbal H, et al. Chest Wall Schwannoma: Case Report and a Review of Imaging Findings. Cureus 2018;10:e3694. [Crossref] [PubMed]
- Galetta D, Spaggiari L. Primary Intrathoracic Neurogenic Tumors: Clinical, Pathological, and Long-Term Outcomes. Thorac Cardiovasc Surg 2021;69:749-55. [Crossref] [PubMed]
- Feng WH, Liu T, Huang TW, et al. Schwannoma of the Intercostal Nerve Manifesting as Chest Pain. Ann Thorac Surg 2020;110:e281-3. [Crossref] [PubMed]
- Castro EC, Galambos C. Prox-1 and VEGFR3 antibodies are superior to D2-40 in identifying endothelial cells of lymphatic malformations—a proposal of a new immunohistochemical panel to differentiate lymphatic from other vascular malformations. Pediatr Dev Pathol 2009;12:187-94. [Crossref] [PubMed]
- Alkwai H, Alkwai H, Al Namshan M. Sudden Appearance of a Palpable Chest Wall Mass Secondary to Macrocystic Lymphatic Malformation: A Case Report. Children (Basel) 2023;10:235. [Crossref] [PubMed]
- Song S, Chang D, Li H, et al. Rare cystic lymphangioma in the chest wall of an adult patient: A case report and comprehensive review of the literature. Thorac Cancer 2020;11:3388-90. [Crossref] [PubMed]
- Lee WS, Kim YH, Chee HK, et al. Cavernous lymphangioma arising in the chest wall 19 years after excision of a cystic hygroma. Korean J Thorac Cardiovasc Surg 2011;44:380-2. [Crossref] [PubMed]
- Ligon AH, Moore SD, Parisi MA, et al. Constitutional rearrangement of the architectural factor HMGA2: a novel human phenotype including overgrowth and lipomas. Am J Hum Genet 2005;76:340-8. [Crossref] [PubMed]
- Rydholm A, Berg NO. Size, site and clinical incidence of lipoma. Factors in the differential diagnosis of lipoma and sarcoma. Acta Orthop Scand 1983;54:929-34. [Crossref] [PubMed]
- Celebi JT, Tsou HC, Chen FF, et al. Phenotypic findings of Cowden syndrome and Bannayan-Zonana syndrome in a family associated with a single germline mutation in PTEN. J Med Genet 1999;36:360-4.
- Chen BJ, Mariño-Enríquez A, Fletcher CD, et al. Loss of retinoblastoma protein expression in spindle cell/pleomorphic lipomas and cytogenetically related tumors: an immunohistochemical study with diagnostic implications. Am J Surg Pathol 2012;36:1119-28. [Crossref] [PubMed]
- Dreux N, Marty M, Chibon F, et al. Value and limitation of immunohistochemical expression of HMGA2 in mesenchymal tumors: about a series of 1052 cases. Mod Pathol 2010;23:1657-66. [Crossref] [PubMed]
- Thway K, Flora R, Shah C, et al. Diagnostic utility of p16, CDK4, and MDM2 as an immunohistochemical panel in distinguishing well-differentiated and dedifferentiated liposarcomas from other adipocytic tumors. Am J Surg Pathol 2012;36:462-9. [Crossref] [PubMed]
- Kammerer-Jacquet SF, Thierry S, Cabillic F, et al. Differential diagnosis of atypical lipomatous tumor/well-differentiated liposarcoma and dedifferentiated liposarcoma: utility of p16 in combination with MDM2 and CDK4 immunohistochemistry. Hum Pathol 2017;59:34-40. [Crossref] [PubMed]
- Sirvent N, Coindre JM, Maire G, et al. Detection of MDM2-CDK4 amplification by fluorescence in situ hybridization in 200 paraffin-embedded tumor samples: utility in diagnosing adipocytic lesions and comparison with immunohistochemistry and real-time PCR. Am J Surg Pathol 2007;31:1476-89. [Crossref] [PubMed]
- Sandberg AA. Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors: lipoma. Cancer Genet Cytogenet 2004;150:93-115. [Crossref] [PubMed]
- Bancroft LW, Kransdorf MJ, Peterson JJ, et al. Benign fatty tumors: classification, clinical course, imaging appearance, and treatment. Skeletal Radiol 2006;35:719-33. [Crossref] [PubMed]
- Hirai Y, Mikasa Y, Iguchi H, et al. Intrathoracic lipoma of the chest wall that appeared relatively rapidly and could be resected and diagnosed by minimally invasive thoracoscopic surgery: A case report. Respirol Case Rep 2022;10:e0946. [Crossref] [PubMed]
- Schicchi N, Tiberi M, Fogante M, et al. Chest wall lipoma mimicking intrathoracic mass: Imaging with surgical correlation. Radiol Case Rep 2019;14:956-61. [Crossref] [PubMed]
- Jeong JY, Park HJ, Lee JH, et al. Liposarcoma of the chest wall: a case potentially transformed from a recurrent lipoma. Gen Thorac Cardiovasc Surg 2011;59:310-1. [Crossref] [PubMed]
- Lee YJ, Cha WJ, Kim Y, et al. The recurrence of well-differentiated liposarcoma from benign giant intramuscular lipoma: A case (CARE-compliant) report. Medicine (Baltimore) 2021;100:e24711. [Crossref] [PubMed]
- Kransdorf MJ, Jelinek JS, Moser RP Jr. Imaging of soft tissue tumors. Radiol Clin North Am 1993;31:359-72.
- Jarvi O, Saxen E. Elastofibroma dorse. Acta Pathol Microbiol Scand Suppl 1961;51:83-4.
- Hisaoka M, Hashimoto H. Elastofibroma: clonal fibrous proliferation with predominant CD34-positive cells. Virchows Arch 2006;448:195-9. [Crossref] [PubMed]
- Kayaselçuk F, Demirhan B, Kayaselçuk U, et al. Vimentin, smooth muscle actin, desmin, S-100 protein, p53, and estrogen receptor expression in elastofibroma and nodular fasciitis. Ann Diagn Pathol 2002;6:94-9. [Crossref] [PubMed]
- Hemmi A, Tabata M, Homma T, et al. Application of a quick-freezing and deep-etching method to pathological diagnosis: a case of elastofibroma. J Electron Microsc (Tokyo) 2006;55:89-95. [Crossref] [PubMed]
- Kuroda N, Hamaguchi N, Ohara M, et al. Elastofibroma: a histochemical, immunohistochemical, and ultrastructural study of two patients. Med Mol Morphol 2008;41:179-82. [Crossref] [PubMed]
- Nishio JN, Iwasaki H, Ohjimi Y, et al. Gain of Xq detected by comparative genomic hybridization in elastofibroma. Int J Mol Med 2002;10:277-80.
- Hayes AJ, Alexander N, Clark MA, et al. Elastofibroma: a rare soft tissue tumour with a pathognomonic anatomical location and clinical symptom. Eur J Surg Oncol 2004;30:450-3. [Crossref] [PubMed]
- Massengill AD, Sundaram M, Kathol MH, et al. Elastofibroma dorsi: a radiological diagnosis. Skeletal Radiol 1993;22:121-3. [Crossref] [PubMed]
- Tsikkinis C, Balamoti S, Grigoropoulos P, et al. Elastofibroma dorsi. J BUON 2014;19:573-6.
- Fabien J, Patel V, Timpone M. Management of Symptomatic Elastofibroma Dorsi: A Case Report and Literature Review. Cureus 2022;14:e29163. [Crossref] [PubMed]
- Karakurt O, Kaplan T, Gunal N, et al. Elastofibroma dorsi management and outcomes: review of 16 cases. Interact Cardiovasc Thorac Surg 2014;18:197-201. [Crossref] [PubMed]





