
Prognostic value of lactic dehydrogenase-to-albumin ratio in critically ill patients with acute respiratory distress syndrome: a retrospective cohort study
Highlight box
Key findings
• In this study, we researched the association between lactic dehydrogenase-to-albumin ratio (LAR) and 30-day, 90-day, in-hospital mortality of acute respiratory distress syndrome (ARDS).
What is known and what is new?
• One previous study has shown that LAR was an independent risk factor for mortality for the patients with ARDS due to coronavirus disease 2019 (COVID-19).
• In this study, we analyzed the association between LAR and 30-day, 90-day, and in-hospital mortality of patients with ARDS in intensive care unit.
What is the implication, and what should change now?
• This study may reveal the relationship between LAR and overall dynamics of the condition in non-COVID-19 ARDS patients.
Introduction
Acute respiratory distress syndrome (ARDS) is noncardiogenic pulmonary edema, which manifests as rapidly progressive dyspnea, tachypnea, and hypoxemia and has considerable morbidity and mortality in both the short and long term (1,2). In ARDS, lung inflammation causes increased alveolar endothelial and epithelial permeabilities, which resulting in the accumulation of pulmonary edema fluid and decreased effective pulmonary ventilation area (3). Treatment strategy of ARDS is generally supportive and mechanical ventilation and supportive care is still the major treatment option (1). Despite the pathogenesis, pathophysiology and epidemiology of ARDS that we have known, which have led to several new treatment strategies that have improved survival rates significantly in patients with ARDS, the mortality of severe ARDS is still over 40.0% (4,5). Therefore, reliable prognostic biomarkers are of vital importance for classifying patients to assist doctors in better treatment management.
A lot of studies have shown that lactic dehydrogenase (LDH) appears to be a significant prognostic indicator (6-8). In the report of Jin et al., retrospective analysis was conducted on the correlation between pre-treatment and post-treatment LDH levels to treatment response and survival rate in metastatic nasopharyngeal carcinoma (NPC) treated with palliative chemotherapy and they thought that LDH seemed to be an important independent prognostic indicator for patients with disseminated NPC (6). In recent years, LAR as a new predictive indicator has been shown to be associated with the prognosis of many diseases, including stroke-associated pneumonia (SAP) (9), severe infection (10) and acute kidney injury (11).
One previous study showed that LAR was an independent risk factor for mortality for the patients with ARDS due to COVID-19 (12), but the study only revealed the relationship between LAR and the death outcome, which may not reflect the overall dynamics of the patient’s condition.
The goal of this study was to evaluate the association between LAR and 30-day, 90-day, and in-hospital mortality of patients with ARDS. We present this article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1238/rc).
Methods
Study design
This is a retrospective cohort study involving 1,211 patients with ARDS according to the Berlin definition. We obtained age, sex, vital signs, laboratory parameters, comorbidities and survival time from the Medical Information Mart for Intensive Care IV (MIMIC-IV). We have completed the online course and passed the online exams (No. 45104989) to gain access to the database. The establishment of the MIMIC IV database was approved by the Institutional Review Board of Beth Israel deacons Medical Center and Massachusetts Institute of Technology. Because of the anonymity of hospitalization information, informed consent was not required. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Participants
The selection of study participants is shown in Figure 1. We screened all the patients in the database. Our inclusion criteria were as follows: of age 16 years old or above, with more than 24-hour hospital stay, ARDS diagnosis meeting Berlin standard at the time of intensive care unit (ICU) admission, with LDH and albumin within 24 hours after ICU admission. We also excluded patients lacking the baseline of LDH and albumin records. If a patient was admitted multiple times during the study period, it was defaulted to use only the record of the patient’s first ICU admission.

The Berlin standard contains the following items: acute onset, arterial oxygen partial pressure (PaO2)/fraction of inspired oxygen (FiO2) <300 mmHg and positive end-expiratory pressure (PEEP) ≥5 cmH2O on the first day of ICU admission, bilateral infiltrates on chest radiograph, absence of heart failure.
Data extraction
We collected baseline parameters such as age and gender of ICU patients at first ICU admission. The patients had complications such as myocardial infarction, cerebrovascular disease, chronic lung disease, liver disease, diabetes, renal failure and malignancy. Laboratory parameters included bicarbonate, bilirubin, creatinine, glucose, hemoglobin, blood urea nitrogen (BUN), white blood cell count, sodium, potassium, anion gap, albumin, alanine transaminase (ALT), aspartate aminotransferase (AST), LDH, LAR, prothrombin (PT) and international normalized ratio (INR) in the first 24 hours after admission. In addition, Charlson comorbidity index, Simplified Acute Physiology Score II (SASP II) score data and Sequential Organ Failure Assessment (SOFA) were also included. The LAR was calculated by initial LDH (U/L)/albumin (g/L).
Statistical analysis
We processed and analyzed all the data by SPSS software (version 25.0, IBM, USA) and R software (version 4.2.1). For clarity and effectiveness of the study, we divided participants in tertiles based on LAR. The data of continuous variables were represented as mean ± standard deviation (SD) or median (interquartile range), and the differences between groups were compared through one-way analysis of variance (ANOVA) or Kruskal-Wallis test. The data of categorical variables were represented by frequencies or percentages and analyzed using Chi-squared test. We used a restricted cubic spline analysis to assess the association between LAR and HR of different survival outcomes. We used the Kaplan-Meier curve method to compare the survival probabilities of LAR groups at different levels. We constructed multivariate Cox proportional hazards models to study the association between LAR and 30-day, 90-day, and in-hospital mortality and the hazard ratio (HR) of mortality was calculated. In the multiple regression analysis models, adjusted covariates were selected based on the difference of baseline characteristics among the three groups in which P<0.05 or P<0.001. Sex and age were adjusted in model I. In model II, the covariates were adjusted for model I plus mean blood pressure (BP), heart rate, pulse oxygen (SPO2), potassium, diabetes. In model III, systolic blood pressure (SBP), respiratory rate, bicarbonate, bilirubin, creatinine, anion gap, albumin, ALT, AST, LDH, PT, INR, SASP II score, SOFA score and liver disease were further adjusted. ROC curve analysis was used to evaluate whether SOFA score and LAR combined with SAPS II score could improve the predictive value of the 30-day mortality of ARDS patients. A two-tailed P<0.05 was considered statistically significant.
Results
Clinical and laboratory characteristics of ARDS patients
According to the inclusion and exclusion criteria, a total of 358 patients with ARDS were included in the current study. Baseline characteristics of the patients are shown in Table 1. Based on LAR values, participants in this study were divided into three tertiles (<8.7, 8.7–30.9 and >30.9). Patients with higher LAR had a higher respiratory rate, heart rate, bilirubin, creatinine, potassium, anion gap, ALT, AST, LDH, PT, INR, SASP II score and SOFA score (all P<0.05), while lower LAR had a higher SBP, bicarbonate and albumin (all P<0.05). For the study endpoints, the 30-day, 90-day and in-hospital were 72.1%, 84.9% and 66.8%, respectively. The median length of hospital stay and ICU stay were 6.4 and 4.4 days, respectively. Patients with elevated LAR had significantly higher 30-day, 90-day, and in-hospital mortality as well as the longer length of hospital stay (all P<0.05).
Table 1
| Characteristics | LAR | P value | |||
|---|---|---|---|---|---|
| Total (n=358) | Tertile 1 (<8.7) (n=89) |
Tertile 2 (8.7–30.9) (n=180) |
Tertile 3 (>30.9) (n=89) |
||
| Age (years) | 62.6±16.0 | 65.7±16.0 | 63.7±15.2 | 57.2±16.4 | 0.001 |
| Sex | 0.682 | ||||
| Male | 221 (61.7) | 57 (64.0) | 107 (59.4) | 57 (64.0) | |
| Female | 137 (38.3) | 32 (36.0) | 73 (40.6) | 32 (36.0) | |
| SBP (mmHg) | 109.5 (102.2, 119.6) | 116.8 (105.1, 126.0) | 108.8 (101.0, 117.3) | 107.7 (101.0.116.2) | <0.001 |
| DBP (mmHg) | 61.0±9.4 | 61.4±7.9 | 60.2±9.4 | 62.2±10.6 | 0.207 |
| Mean BP (mmHg) | 75.9±9.7 | 76.8±7.8 | 74.8±9.4 | 77.0±11.8 | 0.049 |
| Respiratory rate (beats/min) | 21.4±4.9 | 19.6±3.8 | 21.4±4.8 | 23.5±5.3 | <0.001 |
| Heart rate (beats/min) | 90.7±18.7 | 85.3±17.4 | 90.7±19.0 | 96.1±17.8 | 0.001 |
| SpO2 (%) | 97.2 (95.4, 98.8) | 97.7 (96.0, 98.9) | 97.2 (95.7, 98.8) | 96.3 (94.0, 98.5) | 0.009 |
| Laboratory parameters | |||||
| Bicarbonate (mmol/L) | 20.0 (17.0, 24.0) | 22.0 (19.0, 26.0) | 21.0 (18.0, 24.0) | 17.0 (11.0, 20.5) | <0.001 |
| Bilirubin (mg/dL) | 0.8 (0.4, 2.5) | 0.5 (0.3, 1.2) | 0.8 (0.4, 2.8) | 1.1 (0.6, 3.2) | <0.001 |
| Creatinine (mg/dL) | 1.4 (0.9, 2.2) | 1.1 (0.8, 1.8) | 1.4 (0.8, 2.2) | 1.8 (1.2, 2.5) | <0.001 |
| Glucose (mg/dL) | 145.0 (110.8, 207.0) | 132.0 (110.5, 188.5) | 143.0 (111.0, 197.0) | 160.0 (109.5, 228.0) | 0.217 |
| Hemoglobin (g/dL) | 11.2±2.6 | 11.1±2.7 | 11.0±2.5 | 11.6±2.7 | 0.236 |
| BUN (mg/dL) | 26.0 (17.0, 42.3) | 24.0 (15.0, 37.0) | 27.0 (18.0, 47.8) | 28.0 (16.0, 44.5) | 0.125 |
| WBC (109/L) | 13.4 (8.8, 18.7) | 12.3 (8.7, 16.3) | 13.0 (8.4, 18.0) | 14.3 (9.7, 22.2) | 0.054 |
| Sodium (mmol/L) | 139.0 (135.0, 142.0) | 139.0 (135.5, 142.0) | 139.0 (135.0, 142.0) | 138.0 (133.0, 143.0) | 0.830 |
| Potassium (mmol/L) | 4.3 (3.8, 5.1) | 4.2 (3.8, 4.9) | 4.2 (3.7, 4.8) | 4.8 (4.0, 5.5) | 0.002 |
| Anion gap (mmol/L) | 18.0 (14.0, 27.0) | 16.0 (14.0, 19.0) | 17.0 (14.0, 21.0) | 21.0 (19.0, 27.0) | <0.001 |
| Albumin (g/dL) | 2.9±0.7 | 3.4±0.6 | 2.8±0.6 | 2.7±0.7 | <0.001 |
| ALT (U/L) | 43.5 (21.0, 158.5) | 22.0 (15.0, 37.5) | 38.5 (23.3, 78.0) | 374.0 (120.5, 937.5) | <0.001 |
| AST (U/L) | 83.0 (36.0, 249.5) | 34.0 (22.0, 59.0) | 69.0 (41.0, 142.0) | 900.0 (298.0, 2,170.5) | <0.001 |
| LDH (U/L) | 394.0 (261.0, 766.8) | 212.0 (182.0, 254.0) | 397.0 (298.3, 548.3) | 1,724.0 (1,121.0, 3,287.5) | <0.001 |
| LAR | 14.3 (8.7, 30.9) | 6.6 (5.6, 7.6) | 14.3 (10.9, 21.2) | 68.7 (41.5, 127.2) | <0.001 |
| PT (seconds) | 15.0 (13.0, 20.5) | 13.7 (12.2, 16.0) | 15.0 (13.0, 20.5) | 18.0 (14.5, 27.4) | <0.001 |
| INR | 1.4 (1.2, 1.9) | 1.2 (1.1, 1.5) | 1.4 (1.2, 1.9) | 1.7 (1.3, 2.6) | <0.001 |
| SASP II score | 53.8±16.0 | 48.8±14.7 | 53.4±15.0 | 59.4±17.6 | <0.001 |
| Charlson comorbidity index | 6.0 (4.0, 8.0) | 6.0 (4.0, 8.0) | 6.0 (4.3, 8.0) | 5.0 (3.0, 7.0) | 0.010 |
| SOFA score | 2.0 (0, 5.0) | 2.0 (0, 4.0) | 2.0 (0, 4.0) | 4.0 (1.0, 7.0) | <0.001 |
| Comorbidities | |||||
| Myocardial infarction | 68 (19.0) | 11 (12.4) | 35 (19.4) | 22 (24.7) | 0.115 |
| Cerebrovascular disease | 72 (20.1) | 23 (25.8) | 32 (17.8) | 17 (19.1) | 0.278 |
| Chronic lung disease | 81 (22.6) | 18 (20.2) | 46 (25.6) | 17 (19.1) | 0.406 |
| Liver disease | 121 (33.8) | 16 (18.0) | 64 (35.6) | 41 (46.1) | <0.001 |
| Diabetes | 82 (22.9) | 27 (30.3) | 42 (23.3) | 13 (14.6) | 0.047 |
| Renal disease | 44 (12.3) | 15 (16.9) | 23 (12.8) | 6 (6.7) | 0.118 |
| Malignancy | 75 (20.9) | 16 (18.0) | 39 (21.7) | 20 (22.5) | 0.730 |
| 30-day mortality | 258 (72.1) | 44 (49.4) | 136 (75.6) | 78 (87.6) | <0.001 |
| 90-day mortality | 304 (84.9) | 66 (74.2) | 154 (85.6) | 84 (94.4) | 0.001 |
| In-hospital mortality | 239 (66.8) | 37 (41.6) | 126 (70.0) | 76 (85.4) | <0.001 |
| LOS (hospital) (days) | 6.4 (2.6, 15.0) | 7.5 (4.2, 13.9) | 7.0 (2.7, 16.1) | 3.1 (1.9, 12.7) | 0.004 |
| LOS (ICU) (days) | 4.4 (2.2, 10.1) | 4.3 (2.3, 8.7) | 4.8 (2.5, 10.1) | 3.5 (1.9, 10.2) | 0.352 |
Data are presented as mean ± standard deviation, median (interquartile range), or n (%). ARDS, acute respiratory distress syndrome; LAR, lactic dehydrogenase-to-albumin ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; BP, blood pressure; SpO2, pulse oxygen; BUN, blood urea nitrogen; WBC, white blood cell count; ALT, alanine transaminase; AST, aspartate aminotransferase; LDH, lactic dehydrogenase; PT, prothrombin; INR, international normalized ratio; SASP II, Simplified Acute Physiology Score II; SOFA, Sequential Organ Failure Assessment; LOS, length of stay; ICU, intensive care unit.
Association between LAR and mortality
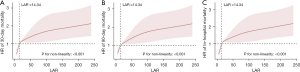
To evaluate the linear association between LAR and 30-day mortality in patients with ARDS, we applied restricted cubic spline analysis to model and visualize the relationship between HR of 30-day mortality and LAR in Figure 2A (P for non-linearity <0.001). In Figure 2A, when LAR is less than 14.34, HR is less than 1. Moreover, when LAR is greater than 14.34, HR is greater than 1 and LAR is positively correlated with HR. In addition, similar linear associations were also observed in the analysis of 90-day mortality and in-hospital mortality (Figure 2B,2C).
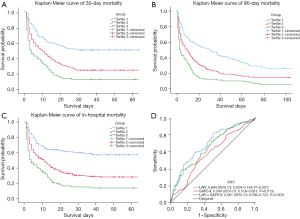
To evaluate cumulative survival at different levels of LAR, we generated 30-day survival curves for patients with ARDS by stratifying based on the LAR tertiles. Kaplan-Meier analysis showed that patients in the tertile1 group had a significantly higher 30-day survival probability (P<0.001) and as LAR increased, the survival probability also gradually decreased (Figure 3A). In addition, similar results were observed in the 90-day and in-hospital survival curves (Figure 3B,3C).
Multivariate Cox regression analysis revealed significant associations between LAR and different clinical outcomes (Table 2). For 30-day mortality, in non-adjusted model for variables, compared with the reference group tertile 1, the HR (95% CI) for tertile 2 was 1.96 (1.39, 2.76) (P<0.001) and for tertile 3 was 3.10 (2.14, 4.51) (P<0.001). This association still had statistical significance even after adjusting for age, gender, mean BP, heart rate, SPO2, potassium, diabetes, SBP, respiratory rate, bicarbonate, bilirubin, creatinine, anion gap, albumin, ALT, AST, LDH, PT, INR, SASP II score, SOFA score and liver disease. In model I, with the comparison of tertile 1, the adjusted HR (95% CI) of tertile 2 and tertile 3 were 1.94 (1.38, 2.73) (P<0.001) and 2.97 (2.04, 4.33) (P<0.001). In model II, the adjusted HR (95% CI) of tertile 2 and tertile 3 were 1.96 (1.39, 2.77) (P<0.001) and 2.99 (2.03, 4.40) (P<0.001). In model III, compared with the reference group tertile 1, the adjusted HR (95% CI) was 2.00 (1.37, 2.92) (P<0.001) and 2.50 (1.50, 4.15) (P<0.001) for tertile 2 and tertile 3. The significant associations between LAR and 90-day mortality and in-hospital mortality could also be seen in non-adjusted model, model I, model II and model III.
Table 2
| LAR | Non-adjusted | Model I | Model II | Model III | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||||
| 30-day mortality | |||||||||||
| Tertile 1 (<8.7) | Ref. | Ref. | Ref. | Ref. | |||||||
| Tertile 2 (8.7–30.9) | 1.96 (1.39, 2.76) | <0.001 | 1.94 (1.38, 2.73) | <0.001 | 1.96 (1.39, 2.77) | <0.001 | 2.00 (1.37, 2.92) | <0.001 | |||
| Tertile 3 (>30.9) | 3.10 (2.14, 4.51) | <0.001 | 2.97 (2.04, 4.33) | <0.001 | 2.99 (2.03, 4.40) | <0.001 | 2.50 (1.50, 4.15) | <0.001 | |||
| 90-day mortality | |||||||||||
| Tertile 1 (<8.7) | Ref. | Ref. | Ref. | Ref. | |||||||
| Tertile 2 (8.7–30.9) | 1.61 (1.20, 2.15) | 0.001 | 1.60 (1.19, 2.13) | 0.002 | 1.60 (1.19, 2.15) | 0.002 | 1.54 (1.11, 2.14) | 0.009 | |||
| Tertile 3 (>30.9) | 2.51 (1.81, 3.49) | <0.001 | 2.45 (1.76, 3.41) | <0.001 | 2.41 (1.72, 3.39) | <0.001 | 1.88 (1.19, 2.96) | 0.006 | |||
| In-hospital mortality | |||||||||||
| Tertile 1 (<8.7) | Ref. | Ref. | Ref. | Ref. | |||||||
| Tertile 2 (8.7–30.9) | 2.14 (1.48, 3.09) | <0.001 | 2.12 (1.47, 3.06) | <0.001 | 2.15 (1.48, 3.12) | <0.001 | 2.11 (1.41, 3.17) | <0.001 | |||
| Tertile 3 (>30.9) | 3.51 (2.36, 5.21) | <0.001 | 3.37 (2.26, 5.02) | <0.001 | 3.36 (2.23, 5.07) | <0.001 | 2.67 (1.57, 4.54) | <0.001 | |||
Model I covariates were adjusted for age and gender. Model II covariates were adjusted for model I plus mean BP, heart rate, SpO2, potassium, and diabetes. P<0.05. Model III covariates were adjusted for model II plus SBP, respiratory rate, bicarbonate, bilirubin, creatinine, anion gap, albumin, ALT, AST, LDH, PT, INR, SASP II score, SOFA score, and liver disease. P<0.001. HR, hazard ratio; CI, confidence interval; LAR, lactic dehydrogenase-to-albumin ratio; BP, blood pressure; SpO2, pulse oxygen; SBP, systolic blood pressure; ALT, alanine transaminase; AST, aspartate aminotransferase; LDH, lactic dehydrogenase; PT, prothrombin; INR, international normalized ratio; SASP II, Simplified Acute Physiology Score II; SOFA, Sequential Organ Failure Assessment.
Receiver operating characteristic (ROC) analysis
To further evaluate the predictive value of LAR, SAPS II score and LAR combined with SAPS II score in patients with ARDS for 30-day mortality, we generated ROC curves (Figure 3D). The area under the curve (AUC) was 0.694 for the LAR (95% CI: 0.634–0.754, P<0.001), which was higher than the AUC of LAR + SAPS II (0.661, 95% CI: 0.599–0.722, P<0.001) and was significantly higher than the AUC of SAPS II (0.584, 95% CI: 0.518–0.651, P=0.013). This indicates that the predictive performance of LAR alone is even better than that of LAR combined with SAPS II score in patients with ARDS for 30-day mortality.
Subgroup analysis
To explore the differences in the predictive value of LAR among different subgroups, we performed subgroup analysis as shown in Table 3. There was an interaction among sex, liver disease and LAR on 30-day mortality (P for interaction =0.009 and 0.002). For male patients, 30-day mortality was higher with increasing LAR. The HRs (95% CIs) for tertile 2 and tertile 3 were 1.56 (1.03, 2.36) and 2.74 (1.75, 4.30), respectively, compared with tertile 1 (P<0.001). For the patients with liver disease, 30-day mortality was also higher with increasing LAR. The HRs (95% CIs) for tertile 2 and tertile 3 were 2.20 (0.99, 4.87) and 4.22 (1.87, 9.51), respectively, compared with tertile 1 (P<0.001). No significant interactions were observed in other subgroups (P for interaction >0.05).
Table 3
| Subgroup | Total | LAR | P for interaction | ||
|---|---|---|---|---|---|
| <8.7 | 8.7–30.9 | >30.9 | |||
| Age (years) | 0.223 | ||||
| <60 | 152 | 1 | 1.76 (1.03, 3.00) | 3.33 (1.93, 5.77) | |
| ≥60 | 206 | 1 | 2.08 (1.34, 3.24) | 2.55 (1.50, 4.32) | |
| Sex | 0.009 | ||||
| Male | 221 | 1 | 1.56 (1.03, 2.36) | 2.74 (1.75, 4.30) | |
| Female | 137 | 1 | 3.01 (1.64, 5.51) | 3.93 (2.02, 7.67) | |
| Myocardial infarction | 0.268 | ||||
| No | 290 | 1 | 1.78 (1.24, 2.55) | 3.13 (2.09, 4.67) | |
| Yes | 68 | 1 | 4.43 (1.34, 14.65) | 5.16 (1.51, 17.61) | |
| Cerebrovascular disease | 0.237 | ||||
| No | 286 | 1 | 2.17 (1.45, 3.26) | 3.89 (2.51, 6.03) | |
| Yes | 72 | 1 | 1.60 (0.84, 3.04) | 1.47 (0.69, 3.13) | |
| Chronic lung disease | 0.728 | ||||
| No | 277 | 1 | 1.89 (1.29, 2.78) | 3.57 (2.36, 5.42) | |
| Yes | 81 | 1 | 2.34 (1.12, 4.90) | 1.80 (0.76, 4.29) | |
| Liver disease | 0.002 | ||||
| No | 237 | 1 | 1.96 (1.34, 2.88) | 2.74 (1.75, 4.30) | |
| Yes | 121 | 1 | 2.20 (0.99, 4.87) | 4.22 (1.87, 9.51) | |
| Diabetes | 0.139 | ||||
| No | 276 | 1 | 2.09 (1.38, 3.15) | 3.26 (2.10, 5.07) | |
| Yes | 82 | 1 | 1.74 (0.94, 3.22) | 2.79 (1.29, 6.00) | |
| Renal disease | 0.719 | ||||
| No | 314 | 1 | 2.11 (1.45, 3.08) | 3.27 (2.18, 4.91) | |
| Yes | 44 | 1 | 1.28 (0.56, 2.89) | 2.31 (0.81, 6.60) | |
| Malignancy | 0.520 | ||||
| No | 283 | 1 | 1.80 (1.25, 2.60) | 2.97 (1.98, 4.44) | |
| Yes | 75 | 1 | 3.23 (1.24, 8.40) | 4.46 (1.61, 12.32) | |
LAR, lactic dehydrogenase-to-albumin ratio.
Discussion
In this study, we investigated the correlation between LAR and adverse clinical outcomes in ARDS patients. We found that the association between elevated LAR and 30-day, 90-day, and in-hospital mortality was significant for the patients with ARDS. Moreover, ROC curves showed that LAR may have a good predictive power for 30-day mortality in patients with ARDS.
High LDH is a result of tissue breakdown, which is the prognostic marker in patients with metastatic NPC (6), metastatic colorectal cancer (7), sepsis (8) and several respiratory illnesses (13). Albumin levels is an indicator of the severity of inflammation (14). Therefore, albumin has a competent prognostic value in many diseases, including cancer (15,16), infections (17) and pneumonia (18).
LAR as a new predictive indicator has been shown to be related to the prognosis of a variety of diseases. Yan et al. reported that high LAR was related to an increased risk of SAP in patients with acute ischemic stroke and LAR may be a potential predictor for the incidence of SAP (9). In addition, LAR had good predictive value for the prognosis of severe infection (10) and acute kidney injury (11). Therefore, we had ample reasons to believe that LAR is also associated with the prognosis of ARDS of the ICU. This study included 358 patients with ARDS in the ICU. The results indicated that LAR was related to the increase in patient mortality at 30-day, 90-day and in-hospital mortality, which suggested that the high LAR leads to the poor clinical prognosis of patients with ARDS.
SAPS II, as an established severity score, has a prognostic role in critically ill patients (19,20). But it seems that no research has shown a direct relationship between SAPS II scores and ARDS. In our study, we found significant differences in SAPS II scores among the LAR subgroup population (P<0.001). Therefore, we speculated that the SAPS II score also has predictive value in patients with ARDS which grouped by LAR. In the present study, we observed that the AUC of LAR was superior to that of LAR + SAPS II score, suggesting that LAR has a higher predictive value for 30-day morbidity and mortality in ARDS patients than LAR + SAPS II score. Therefore, more research is needed to prove whether the SAPS II scores can predict the prognosis of ARDS patients which grouped by LAR. In addition, we found that in the article of Yoo et al. (21), the area under the ROC curve (AUROC) was 0.681 (95% CI: 0.617–0.741, P<0.001) for red cell distribution width/albumin ratio (RDW/albumin). In the report of Zhang et al. (22), AUROC curve was 0.6613 (95% CI: 0.6238–0.6988, P<0.0001) for glucose-to-lymphocyte ratio (GLR). Both AUROCs were lower than the AUROC 0.694 (95% CI: 0.634–0.754, P<0.001) of LAR which may suggest that LAR may have a better predictive value than RDW/albumin and GLR. Therefore, LAR may have a good predictive power for 30-day mortality in patients with ARDS.
In the subgroup analysis, sex and liver disease showed an interaction with LAR in 30-day mortality. Due to the impact of liver disease on LDH and albumin, we hold reservations about the outcome of this interaction. Which suggested that more attention should be taken to female patients. Because of the heterogeneity among different population groups, we are cautious about the results of subgroup analysis and further research is needed to confirm these results.
Our study had some limitations. First, this study is a retrospective study, and although we controlled for confounding factors and conducted subgroup analysis, confounding bias is still inevitable, which would make it difficult to apply the results to all patients with ARDS. Second, one study has shown that direct and indirect ARDS differ in their pathophysiologic process (23); however, we did not further refine patients with ARDS into direct and indirect ARDS patients. Therefore, the predictive role of LAR in patients with direct and indirect ARDS is uncertain. Third, due to database research, some data sources may have data quality issues, such as incomplete or inaccurate data. Fourth, the study was single center research. Therefore, it is necessary to conduct our findings in multicenter studies.
Conclusions
LAR is a prognostic indicator for patients with ARDS. Elevated LAR levels are associated with increased 30-day mortality, 90-day mortality and in-hospital mortality in patients with ARDS.
Acknowledgments
Funding: The present study was supported by the ECCM Program of Clinical Research Center of Shandong University (No. 2021SDUCRCB001).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1238/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1238/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1238/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Saguil A, Fargo MV. Acute Respiratory Distress Syndrome: Diagnosis and Management. Am Fam Physician 2020;101:730-8.
- Gorman EA, O'Kane CM, McAuley DF. Acute respiratory distress syndrome in adults: diagnosis, outcomes, long-term sequelae, and management. Lancet 2022;400:1157-70. [Crossref] [PubMed]
- Huppert LA, Matthay MA, Ware LB. Pathogenesis of Acute Respiratory Distress Syndrome. Semin Respir Crit Care Med 2019;40:31-9. [Crossref] [PubMed]
- Pan C, Liu L, Xie JF, et al. Acute Respiratory Distress Syndrome: Challenge for Diagnosis and Therapy. Chin Med J (Engl) 2018;131:1220-4. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. Erratum in: JAMA 2016;316:350. JAMA 2016;316:350. [Crossref] [PubMed]
- Jin Y, Ye X, Shao L, et al. Serum lactic dehydrogenase strongly predicts survival in metastatic nasopharyngeal carcinoma treated with palliative chemotherapy. Eur J Cancer 2013;49:1619-26. [Crossref] [PubMed]
- Wei Y, Xu H, Dai J, et al. Prognostic Significance of Serum Lactic Acid, Lactate Dehydrogenase, and Albumin Levels in Patients with Metastatic Colorectal Cancer. Biomed Res Int 2018;2018:1804086. [Crossref] [PubMed]
- Duman A, Akoz A, Kapci M, et al. Prognostic value of neglected biomarker in sepsis patients with the old and new criteria: predictive role of lactate dehydrogenase. Am J Emerg Med 2016;34:2167-71. [Crossref] [PubMed]
- Yan D, Huang Q, Dai C, et al. Lactic Dehydrogenase to Albumin Ratio Is Associated With the Risk of Stroke-Associated Pneumonia in Patients With Acute Ischemic Stroke. Front Nutr 2021;8:743216. [Crossref] [PubMed]
- Jeon SY, Ryu S, Oh SK, et al. Lactate dehydrogenase to albumin ratio as a prognostic factor for patients with severe infection requiring intensive care. Medicine (Baltimore) 2021;100:e27538. [Crossref] [PubMed]
- Deng Y, Li X, Lai Q, et al. Prognostic implication of lactic dehydrogenase-to-albumin ratio in critically ill patients with acute kidney injury. Clin Exp Nephrol 2023;27:349-57. [Crossref] [PubMed]
- Sipahioglu H, Onuk S. Lactate dehydrogenase/albumin ratio as a prognostic factor in severe acute respiratory distress syndrome cases associated with COVID-19. Medicine (Baltimore) 2022;101:e30759. [Crossref] [PubMed]
- Gupta GS. The Lactate and the Lactate Dehydrogenase in Inflammatory Diseases and Major Risk Factors in COVID-19 Patients. Inflammation 2022;45:2091-123. [Crossref] [PubMed]
- Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: Pathogenesis and Clinical Significance. JPEN J Parenter Enteral Nutr 2019;43:181-93. [Crossref] [PubMed]
- Danan D, Shonka DC Jr, Selman Y, et al. Prognostic value of albumin in patients with head and neck cancer. Laryngoscope 2016;126:1567-71. [Crossref] [PubMed]
- Zhang X, Xing P, Hao X, et al. Clinical value of serum albumin level in patients with non-small cell lung cancer and anaplastic lymphoma kinase (ALK) rearrangement. Ann Palliat Med 2021;10:12403-11. [Crossref] [PubMed]
- Wiedermann CJ. Hypoalbuminemia as Surrogate and Culprit of Infections. Int J Mol Sci 2021;22:4496. [Crossref] [PubMed]
- Zhao L, Bao J, Shang Y, et al. The prognostic value of serum albumin levels and respiratory rate for community-acquired pneumonia: A prospective, multi-center study. PLoS One 2021;16:e0248002. [Crossref] [PubMed]
- Rocchetti NS, Egea-Guerrero JJ, Ruiz de Azua-Lopez Z, et al. APACHE II and SAPS II as predictors of brain death development in neurocritical care patients. Rev Neurol 2018;67:121-8.
- Takekawa D, Endo H, Hashiba E, et al. Predict models for prolonged ICU stay using APACHE II, APACHE III and SAPS II scores: A Japanese multicenter retrospective cohort study. PLoS One 2022;17:e0269737. [Crossref] [PubMed]
- Yoo JW, Ju S, Lee SJ, et al. Red cell distribution width/albumin ratio is associated with 60-day mortality in patients with acute respiratory distress syndrome. Infect Dis (Lond) 2020;52:266-70. [Crossref] [PubMed]
- Zhang Y, Zhang S. Prognostic value of glucose-to-lymphocyte ratio in critically ill patients with acute respiratory distress syndrome: A retrospective cohort study. J Clin Lab Anal 2022;36:e24397. [Crossref] [PubMed]
- Shaver CM, Bastarache JA. Clinical and biological heterogeneity in acute respiratory distress syndrome: direct versus indirect lung injury. Clin Chest Med 2014;35:639-53. [Crossref] [PubMed]


