
Change in quality of life of stage IA non-small cell lung cancer after surgery or radiation therapy
Highlight box
Key findings
• Surgery showed better physical health & lung cancer symptom scores than stereotactic body radiotherapy (SBRT) in early-stage non-small cell lung cancer (NSCLC) patients post-treatment. Both groups improved in overall mental health.
What is known and what is new?
• Previous studies have established the effects of NSCLC treatments on patient quality of life (QoL), but a direct longitudinal comparison between surgery and SBRT remained under-explored.
• This manuscript adds a detailed longitudinal comparison of QoL scores between NSCLC patients treated with surgery and SBRT. It offers new insights into how patients’ QoL changes post-treatment and how these changes differ over time.
What is the implication, and what should change now?
• The study highlights the importance of post-operative care within 2 months after surgery and persistent post-SBRT care throughout the 12 months after SBRT.
Introduction
The implementation of low-dose computed tomography (CT) screening has led to increased diagnoses of early-stage lung cancer and thus reduced lung cancer-related mortality (1). Surgical resection is considered the standard treatment for patients diagnosed with early-stage non-small cell lung cancer (NSCLC) (2). However, approximately 20% of early-stage NSCLC patients have underlying medical conditions which make them unsuitable for surgery (2) and others refuse to undergo surgery, making stereotactic body radiotherapy (SBRT) a viable alternative minimally invasive treatment option for patients ineligible for surgical resection (3).
Several studies and meta-analysis, including a propensity score-matching analysis in 2020, indicated no significant differences in overall and disease-free survival between SBRT and surgery, after adjustment (4-6). With improved mortality rates and the advancement in treatment options, patients’ quality of life (QoL) has received more attention in treatment decision making. A 2018 quantitative analysis of focus groups with surgeons and lung cancer patients revealed a divergence between the two in treatment decision-making priorities; surgeons favor clinical indicators while patients value physical and mental well-being more (7). A 2019 study corroborated this, indicating patients prioritize independence and QoL over survival and recurrence in early-stage lung cancer treatments (8). Therefore, as patients are more involved in the decision-making process, addressing their perspectives and considering their QoL is essential in evaluating treatment options.
A previous study (9) showed a short-term decrease in QoL outcomes like physical health and lung cancer symptoms within 2 months after surgery but rebound afterwards. Conversely, studies on SBRT are limited; one identified that early-stage lung cancer patients undergoing SBRT reported lower initial QoL, potentially due to older age and more comorbidities, compared to those opting for surgery (10). These results also suggested a greater decline in physical health QoL score for SBRT patients from baseline to post-treatment follow-up (10). Improved understanding of the impact of treatment on the change in QoL may be helpful in developing post-treatment guidelines and providing targeted resources for lung cancer patients. By utilizing additional QoL and social support instruments, our study aims to identify trends and critical time points at which QoL outcomes significantly change for each treatment group, using data from a large prospective cohort study. We hypothesize that there will be a differential impact of treatment options (surgery vs. SBRT) on QoL outcomes during the first year after treatment for lung cancer patients. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1201/rc).
Methods
We reviewed all patients diagnosed with NSCLC who participated in the prospective cohort study, the Initiative for Early Lung Cancer Research on Treatment (IELCART), since its start in 2016 who underwent surgery or SBRT for their NSCLC. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai (MSHS IRB Study-15-01021). Informed consent was taken from all the patients. We enrolled all patients who had a first primary clinical stage IA (T1a–1cN0M0) NSCLC [8th American Joint Committee on Cancer (AJCC)/International Union for Cancer Control (UICC) staging] who underwent either surgery or radiation therapy, who had more than one record of QoL forms and who did not have additional treatments within 1 year after their first treatment. Patients who had metachronous primaries, recurrences, or additional treatments within 1 year after surgery were excluded from our study.
We recorded demographic information, comorbidities, social support status, pre-surgical CT scan results, and post-surgical pathologic findings. During the pre-treatment clinic visit, interviews were conducted to collect patients’ pre-treatment QoL scores. If patients were unable to go to the in-person interviews, telephone interviews or mailed questionnaires were completed by patients. QoL scores during follow-up were acquired at clinic visits scheduled at 2, 6, and 12 months after either surgery or the completion of radiation therapy.
Sociodemographic and medical characteristics
During the enrollment process for IELCART, we gathered baseline demographic information for each patient, which included age, gender, and education level (categorized as less than a college degree or college degree or higher). Smoking status (current, former, never smoker), pack-years of cigarette smoking, and 12 different self-reported comorbidities—presence of asthma, emphysema or chronic obstructive pulmonary disease, high blood pressure, high cholesterol, angioplasty or stent, myocardial infarction, stroke, peripheral vascular disease, liver disease, diabetes, kidney disease and history of cancers other than lung—were also documented. A comorbidity ordinal score was calculated by summing the number of documented comorbidities for each patient, ranging from 0 to 12. Height and weight were documented and body mass index (BMI) was calculated in kg/m2. Race was divided into four categories (White, African American or Black, Asian, and other races). Patients’ ethnicities (Hispanic vs. non-Hispanic) were also documented. The nodule consistency on the CT scan was documented as solid, part-solid, or nonsolid according to published criteria (11).
At the time of baseline enrollment, we assessed the social support perceived by each patient using the Medical Outcomes Study (MOS) Social Support Survey, which comprises five subscales: emotional/informational support, tangible support, positive interaction, affection, and the presence of someone to help distract from worries or concerns (12). The questionnaire consists of 19 items and the overall MOS Social Support Survey score was obtained by calculating the average of the non-missing items. The score has a range from 1 to 5 with a higher score indicating better social support perceived by patients.
QoL instruments
Physical component summary (PCS) and mental component summary (MCS)
The PCS score and the MCS score are computed using the 12-item Short Form Health Survey version 2 (SF-12v2), which reflect eight health domains, including physical functioning, role limitations due to physical health, bodily pain, general health perceptions, vitality, social functioning, role limitations due to emotional problems, and mental health, all within the context of the past 4 weeks (13). The norm-based PCS and MCS scores for the general United States population have a mean of 50 and a standard deviation of 10, with higher scores indicating better physical and mental health. A minimum difference of 3 points is considered clinically significant for both scores (13).
Functional Assessment of Cancer Therapy-Lung Cancer Subscale (FACT-LCS)
The FACT-Lung (FACT-L) is a multi-dimensional validated self-report instrument measuring the QoL of patients diagnosed with lung cancer (14). We only used the LCS which asks about dyspnea, weight loss, mental clarity, coughing, appetite, tightness in the chest, and difficulty breathing. The scores range from 0 to 28 with higher score indicating better overall health and fewer symptoms. A change of 2 to 3 points is considered clinically significant (15).
The Patient Health Questionnaire-4 (PHQ-4)
The PHQ-4 is a short self-report questionnaire asking about patients’ anxiety and depression symptoms (16). It includes the Generalized Anxiety Disorder 2-item (GAD-2) and PHQ-2 subscales, each comprising two questions. The GAD-2 scores can range from 0 to 6 with lower scores indicating fewer anxiety symptoms. A score of 3 or higher is the recommended cut-off point for possible generalized anxiety disorder (17). The PHQ-2 scores can range from 0 to 6 with lower scores indicating fewer symptoms of depression (16). A score of 3 or higher is the suggested cut-off point for clinical depression (18).
Statistical analysis
We summarized the results of sociodemographic and clinical characteristics using means and standard deviations for continuous and ordinal data, and medians and interquartile ranges (IQRs) for continuous and ordinal data. For categorical data, we presented frequencies and percentages. To compare surgical and SBRT patients, two-sample t-tests were used for normally distributed continuous variables, and Mann-Whitney U tests were used for non-normally distributed continuous variables. For comparisons of categorical variables, Pearson’s χ2 tests (or Fisher’s exact tests where appropriate) were used.
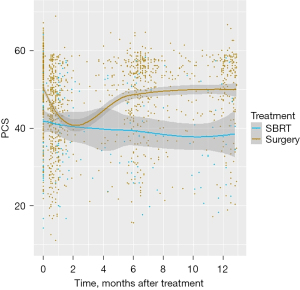
We used a similar approach to our previous study (9) to compare the QoL scores between surgical and SBRT patients within the first post-treatment year. We used non-parametric locally weighted scatterplot smoothing (LOWESS) curve to identify the crucial time points at which the trajectory of QoL scores exhibited changes, and then utilized a piecewise linear mixed effects model to estimate each of the QoL scores before treatment and over the course of the post-treatment period, which offer a robust method for handling missing data or data collected at irregular time intervals. The LOWESS curve was estimated using 80% of the data points that were nearest to each time point in the analysis (Figure 1), which allowed us to visualize the changes in QoL and identify when the trends shifted.
The model accounted for correlations between QoL scores for each patient by including a random intercept and two different rates of change for each QoL measure, one from time =0 (baseline) to 2 months after treatment, and another from 2.1 to 12 months after treatment. The effects of treatment option, time, and their interaction, as well as sociodemographic and clinical variables (age, sex, race, BMI, pack-years, smoking status, maximum nodule size on CT, and comorbidity) were adjusted for in the model. Missing independent variables were handled by creating five imputed datasets using Multiple Imputation by Chained Equation (MICE) approach, and the normality of the scaled residuals was assessed to evaluate the model fit.
The final model estimated the QoL scores using least square means for each measure at baseline (time =0), and at 2 and 12 months after treatment. The estimates were separately calculated for surgical and SBRT patients using sample averages of continuous variables and were averaged over categorical variables. R version 4.2.1 was used for all statistical analyses.
Results
Patient demographics
In total, 708 participants with a first primary NSCLC, clinical stage IA, were identified. After excluding 34 without any QoL forms, 40 with additional treatment within one year after their first treatment, and 66 with only one QoL form, 568 patients were included in the analysis. Among the 568 patients, surgery was performed more frequently than SBRT [503 (88.6%) vs. 65 (11.4%), P<0.001] (Table 1). Surgical patients were significantly younger (median age 69.0 vs. 74.0 years, P<0.001) and had a lower median of pack-years (median 15.0 vs. 24.5, P=0.014) compared to SBRT patients. Racial distribution was significantly different with more surgical patients identifying themselves as white (63.0% vs. 55.4%, P=0.032) and more SBRT patients identifying themselves as African American or Black (29.2% vs. 15.3%, P=0.032). Surgical patients had more frequently quit smoking or had never smoked (P<0.001), and had fewer comorbidities (median 2.00 vs. 3.00, P<0.001). Nodule diameter and consistency on CT were not significantly different between treatments. Surgical patients had more atypical and typical carcinoid tumors, while SBRT patients had more squamous cell carcinomas.
Table 1
| Characteristics | Total (n=568) | Surgery (n=503) | SBRT (n=65) | P value |
|---|---|---|---|---|
| Age (years) | 70.0 [64.0, 76.0] | 69.0 [63.0, 76.0] | 74.0 [69.0, 81.0] | <0.001* |
| Sex | 0.3 | |||
| Female | 329 (57.9) | 296 (58.8) | 33 (50.8) | |
| Male | 239 (42.1) | 207 (41.2) | 32 (49.2) | |
| Race | 0.032* | |||
| White | 353 (62.1) | 317 (63.0) | 36 (55.4) | |
| African American/Black | 96 (16.9) | 77 (15.3) | 19 (29.2) | |
| Asian | 63 (11.1) | 59 (11.7) | 4 (6.2) | |
| Other | 56 (9.9) | 50 (9.9) | 6 (9.2) | |
| Ethnicity | >0.9 | |||
| Non-Hispanic | 489 (86.1) | 433 (86.1) | 56 (86.2) | |
| Hispanic | 79 (13.9) | 70 (13.9) | 9 (13.8) | |
| Education | 0.2 | |||
| No college degree | 209 (36.8) | 180 (35.8) | 29 (44.6) | |
| College degree | 347 (61.1) | 313 (62.2) | 34 (52.3) | |
| NA | 12 (2.1) | 10 (2.0) | 2 (3.1) | |
| Pack-years | 17.0 [0.0, 40.0] | 15.0 [0.0, 40.0] | 24.5 [10.0, 50.0] | 0.014 |
| Smoking status | <0.001* | |||
| Current | 63 (11.1) | 46 (9.1) | 17 (26.2) | |
| Former | 357 (62.9) | 319 (63.4) | 38 (58.5) | |
| Never | 148 (26.1) | 138 (27.4) | 10 (15.4) | |
| BMI (kg/m2) | 26.2 [22.9, 30.2] | 26.2 [23.2, 30.2] | 26.1 [22.0, 30.2] | 0.5 |
| Comorbidity ordinal score | 2.00 [1.00, 3.00] | 2.00 [1.00, 3.00] | 3.00 [2.00, 5.00] | <0.001* |
| Nodule consistency | 0.2 | |||
| Solid | 425 (74.8) | 375 (74.6) | 50 (76.9) | |
| Non/part-solid | 141 (24.8) | 127 (25.2) | 14 (21.5) | |
| Other | 2 (0.4) | 1 (0.2) | 1 (1.5) | |
| Max nodule diameter (mm) | 15.0 [10.3, 20.0] | 15.0 [10.0, 20.0] | 15.9 [13.0, 20.0] | 0.2 |
| Histology | <0.001* | |||
| Adenocarcinoma | 427 (75.2) | 389 (77.3) | 38 (58.5) | |
| Squamous | 66 (11.6) | 47 (9.3) | 19 (29.2) | |
| Carcinoid typical and atypical | 58 (10.2) | 58 (11.5) | 0 (0.0) | |
| Other | 17 (3.0) | 9 (1.8) | 8 (12.3) | |
| Days between pre-treatment QoL form and treatment |
19 [8, 32] | 18 [7, 31] | 28 [19, 42] | <0.001* |
| MOS social support survey | 100 [89, 100] | 100 [92, 100] | 100 [78, 100] | 0.6 |
Data are presented as median [IQR] or n (%). *, P≤0.05. SBRT, stereotactic body radiotherapy; NA, not available; BMI, body mass index; QoL, quality of life; MOS, Medical Outcomes Study; IQR, interquartile range.
Among the 568 patients, 402 had baseline QoL forms, and 173 patients had all four QoL forms completed at baseline, 2, 6, and 12 months after treatment. There are 22 patients with missing BMI information (20 surgical patients vs. 2 SBRT patients) and 12 patients with missing education information (10 surgical patients vs. 2 SBRT patients), and they were imputed as mentioned in the “Methods” section.
Each of the five scores (PCS, MCS, FACT-LCS, GAD-2, and PHQ-2) measuring QoL outcomes were comprehensively assessed. The least square means of the QoL scores, estimated by the adjusted piecewise linear mixed-effects model, are presented in Table 2. Figure 2 offers a visual comparison of the estimated QoL scores for surgical and SBRT patient groups at baseline, 2, and 12 months post-treatment. The estimated rate of change in QoL scores for patients undergoing surgery and those receiving SBRT is detailed in Table 3. Additionally, Table 4 illustrates the comparisons of the estimated QoL scores from baseline to the 12-month follow-up within each treatment group.
Table 2
| QoL instruments | Baseline estimated score | 2-month estimated score | 12-month estimated score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | P value† | Estimate | SE | P value† | Estimate | SE | P value† | |||
| PCS (SF-12) (physical) | |||||||||||
| Surgery | 46.62 | 0.99 | – | 42.40 | 1.10 | – | 48.66 | 1.02 | – | ||
| SBRT | 41.15 | 1.55 | – | 40.94 | 1.80 | – | 37.90 | 1.73 | – | ||
| Surgery-SBRT | 5.47 | 1.46 | <0.001* | 1.46 | 1.81 | 0.42 | 10.76 | 1.66 | <0.001* | ||
| MCS (SF-12) (mental) | |||||||||||
| Surgery | 53.84 | 0.92 | – | 54.38 | 1.01 | – | 55.74 | 0.94 | – | ||
| SBRT | 53.98 | 1.41 | – | 54.91 | 1.63 | – | 56.13 | 1.57 | – | ||
| Surgery-SBRT | −0.14 | 1.33 | 0.91 | −0.53 | 1.63 | 0.75 | −0.39 | 1.50 | 0.80 | ||
| FACT-LCS (lung cancer symptom) | |||||||||||
| Surgery | 23.30 | 0.38 | – | 22.55 | 0.42 | – | 24.44 | 0.39 | – | ||
| SBRT | 22.53 | 0.58 | – | 22.36 | 0.67 | – | 21.27 | 0.66 | – | ||
| Surgery-SBRT | 0.77 | 0.55 | 0.16 | 0.19 | 0.67 | 0.77 | 3.17 | 0.63 | <0.001* | ||
| Anxiety (GAD-2) | |||||||||||
| Surgery | 1.31 | 0.15 | – | 0.49 | 0.17 | – | 0.69 | 0.15 | – | ||
| SBRT | 1.24 | 0.23 | – | 0.73 | 0.27 | – | 0.71 | 0.26 | – | ||
| Surgery-SBRT | 0.07 | 0.22 | 0.75 | −0.24 | 0.27 | 0.37 | −0.02 | 0.25 | 0.96 | ||
| Depression (PHQ-2) | |||||||||||
| Surgery | 0.79 | 0.13 | – | 0.74 | 0.15 | – | 0.58 | 0.14 | – | ||
| SBRT | 0.91 | 0.20 | – | 0.73 | 0.23 | – | 0.72 | 0.23 | – | ||
| Surgery-SBRT | −0.12 | 0.19 | 0.54 | 0.01 | 0.23 | 0.96 | −0.14 | 0.22 | 0.52 | ||
†, P values test differences in estimated scores between surgery and SBRT groups. *, P≤0.05. QoL, quality of life; SE, standard error; PCS, physical component summary; SF-12, 12-item Short Form Health Survey; SBRT, stereotactic body radiotherapy; MCS, mental component summary; FACT-LCS, Functional Assessment of Cancer Therapy-Lung Cancer Subscale; GAD-2, Generalized Anxiety Disorder 2-item; PHQ-2, Patient Health Questionnaire-2.

Table 3
| QoL instruments | Per month rate (within 2 months after intervention) |
Per month rate (after 2 months after intervention) |
|||||
|---|---|---|---|---|---|---|---|
| Estimate | SE | P value† | Estimate | SE | P value† | ||
| PCS (SF-12) (physical) | |||||||
| Surgery | −2.11 | 0.41 | <0.001* | 0.63 | 0.08 | <0.001* | |
| SBRT | −0.10 | 0.97 | 0.92 | −0.30 | 0.21 | 0.15 | |
| Surgery-SBRT | −2.01 | 1.05 | 0.06 | 0.93 | 0.23 | <0.001* | |
| MCS (SF-12) (mental) | |||||||
| Surgery | 0.27 | 0.36 | 0.46 | 0.14 | 0.07 | 0.06 | |
| SBRT | 0.46 | 0.85 | 0.59 | 0.12 | 0.19 | 0.51 | |
| Surgery-SBRT | −0.19 | 0.93 | 0.84 | 0.02 | 0.20 | 0.94 | |
| FACT-LCS (lung cancer symptom) | |||||||
| Surgery | −0.37 | 0.15 | 0.01 | 0.19 | 0.03 | <0.001* | |
| SBRT | −0.09 | 0.35 | 0.81 | −0.11 | 0.08 | 0.17 | |
| Surgery-SBRT | −0.28 | 0.38 | 0.45 | 0.30 | 0.08 | <0.001 | |
| Anxiety (GAD-2) | |||||||
| Surgery | −0.41 | 0.07 | <0.001* | 0.02 | 0.01 | 0.10 | |
| SBRT | −0.25 | 0.16 | 0.07 | −0.002 | 0.03 | 0.94 | |
| Surgery-SBRT | −0.16 | 0.17 | 0.32 | 0.02 | 0.03 | 0.51 | |
| Depression (PHQ-2) | |||||||
| Surgery | −0.03 | 0.05 | 0.61 | −0.02 | 0.01 | 0.12 | |
| SBRT | −0.1 | 0.12 | 0.44 | −0.001 | 0.03 | 0.98 | |
| Surgery-SBRT | 0.07 | 0.13 | 0.61 | −0.02 | 0.03 | 0.59 | |
†, P values test per month rates of change vs. 0 (no change) within each group, and differences in rates of change between surgery and SBRT groups. *, P≤0.05. QoL, quality of life; SBRT, stereotactic body radiotherapy; SE, standard error; PCS, physical component summary; SF-12, 12-item Short Form Health Survey; MCS, mental component summary; FACT-LCS, Functional Assessment of Cancer Therapy-Lung Cancer Subscale; GAD-2, Generalized Anxiety Disorder 2-item; PHQ-2, Patient Health Questionnaire-2.
Table 4
| QoL instruments | Surgery | SBRT | |||||
|---|---|---|---|---|---|---|---|
| Estimate | SE | P value† | Estimate | SE | P value† | ||
| PCS (SF-12) (physical) | |||||||
| Baseline | 46.62 | 0.99 | – | 41.15 | 1.55 | – | |
| 12-month | 48.66 | 1.02 | – | 37.90 | 1.73 | – | |
| 12-month-baseline | 2.04 | 0.57 | <0.001* | −3.25 | 1.64 | 0.05* | |
| MCS (SF-12) (mental) | |||||||
| Baseline | 53.84 | 0.92 | – | 53.98 | 1.41 | – | |
| 12-month | 55.74 | 0.94 | – | 56.13 | 1.57 | – | |
| 12-month-baseline | 1.90 | 0.5 | <0.001* | 2.15 | 1.45 | 0.14 | |
| FACT-LCS (lung cancer symptom) | |||||||
| Baseline | 23.30 | 0.38 | – | 22.53 | 0.58 | – | |
| 12-month | 24.44 | 0.39 | – | 21.27 | 0.66 | – | |
| 12-month-baseline | 1.14 | 0.21 | <0.001* | −1.26 | 0.60 | 0.04* | |
| Anxiety (GAD-2) | |||||||
| Baseline | 1.31 | 0.15 | – | 1.24 | 0.23 | – | |
| 12-month | 0.69 | 0.15 | – | 0.71 | 0.26 | – | |
| 12-month-baseline | −0.62 | 0.09 | <0.001* | −0.53 | 0.25 | 0.03* | |
| Depression (PHQ-2) | |||||||
| Baseline | 0.79 | 0.13 | – | 0.91 | 0.20 | – | |
| 12-month | 0.58 | 0.14 | – | 0.72 | 0.23 | – | |
| 12-month-baseline | −0.21 | 0.07 | <0.001* | −0.19 | 0.2 | 0.34 | |
†, P values test differences in estimated score between baseline and 12 months within each group. *, P≤0.05. QoL, quality of life; SE, standard error; SBRT, stereotactic body radiotherapy; PCS, physical component summary; SF-12, 12-item Short Form Health Survey; MCS, mental component summary; FACT-LCS, Functional Assessment of Cancer Therapy-Lung Cancer Subscale; GAD-2, Generalized Anxiety Disorder 2-item; PHQ-2, Patient Health Questionnaire-2.
SF-12 PCS
The average PCS score estimated by the adjusted piecewise linear mixed effect model at baseline was 46.62 and 41.15 for surgical and SBRT patients, respectively (Table 2). Post-treatment, a significant decrease was observed in the PCS scores for surgical patients (−2.11/month, P<0.001) in the first 2 months. Conversely, for SBRT patients, the change was minimal and indicated essentially no significant change (−0.10/month, P=0.92) (Table 3, Figure 2A). The difference in rates of change between the two groups was only borderline significant (P=0.06). PCS scores increased over the subsequent 10 months for surgical patients but continued to decrease for SBRT patients (0.63/month vs. −0.30/month, P<0.001). At 12 months, the estimated average PCS scores were 48.66 and 37.90 for surgical and SBRT patients, showing a significant difference between the two groups (P<0.001). Compared to baseline, the estimated average PCS score at 12 months was significantly higher for surgical patients (difference =2.04, P<0.001) (Table 4), but the increase was lower than the three-point difference considered clinically significant. For SBRT patients, the estimated PCS score at 12 months was significantly lower compared to baseline (difference =−3.25, P=0.05), and the decrease was both statistically and clinically significant.
SF-12 MCS
The estimated average MCS scores for surgical and SBRT patients at baseline were similar and slightly above the general population average (53.84 vs. 53.98, P=0.91) (Table 2). Neither surgical nor SBRT patients had statistically significant changes in their MCS scores within the first 2 months after treatment, and the monthly rates of change were not statistically significant throughout the first post-treatment year for either treatment (Table 3, Figure 2B). The estimated average MCS score for surgical patients at 12 months after treatment was significantly higher compared to baseline (55.74 vs. 53.84, difference =1.90, P<0.001) (Table 4) but the difference was not clinically significant. For SBRT patients, the improvement of estimated average MCS score from baseline to 12 months after treatment was neither statistically nor clinically significant (56.13 vs. 53.98, difference =2.15, P=0.14).
FACT-LCS
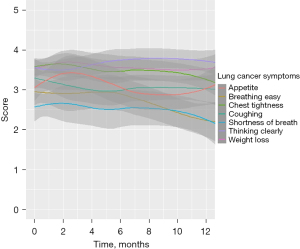
The estimated average FACT-LCS scores at baseline were 23.30 and 22.53, respectively for surgical and SBRT patients (Table 2). Within the first 2 months after treatment, the average FACT-LCS score decreased significantly for surgical patients (−0.37/month, P=0.01) but the decrease for SBRT was minimal and not statistically significant (−0.09/month, P=0.81). The difference in rate of change was not statistically significant between the two treatments (P=0.45) (Table 3, Figure 2C). However, FACT-LCS scores increased in the subsequent 10 months for surgical patients but decreased for SBRT patients (0.19/month, P<0.001 vs. −0.11/month, P=0.17), with significantly different rates of change between the two groups (P<0.001). By 12 months, the estimated average FACT-LCS score had significantly improved over the estimated baseline score for surgical patients (24.44 vs. 23.30, difference =1.14, P<0.001) (Table 4), but significantly decreased for SBRT patients (21.27 vs. 22.53, difference =−1.26, P=0.04). A sub-analysis was conducted to identify which categories of FACT-LCS decreased for SBRT patients; it revealed declines in scores for questions related to “breathing easy”, “chest tightness”, and “shortness of breath” (Figure 3).
The PHQ-4
The estimated average GAD-2 anxiety scores at baseline for surgical and SBRT patients were both below the cut-off point for generalized anxiety disorder, and there was no significant difference between the two groups (P=0.75) (Table 2). Within the first 2 post-treatment months, GAD-2 scores decreased for both groups, and the rates of change were similar (P=0.32) (Table 3, Figure 2D). No significant changes were observed for the next 10 post-treatment months, and the rates of change were again similar for both treatments (P=0.51). At 12 months, the estimated average GAD-2 score was significantly lower than baseline for both surgical (0.69 vs. 1.31, P<0.001) (Table 4) and SBRT (0.71 vs. 1.24, P=0.03) patients. These findings suggest that anxiety had decreased over the year for the patients in both groups, although they started below the cut-off point for possible generalized anxiety disorder.
The estimated average PHQ-2 depression scores at baseline for surgical and SBRT patients were 0.79 and 0.91, P=0.54 (Table 2), which were both below the preferred cut-off point of 3 for clinical depression. The scores decreased slightly for the first 2 post-treatment months for both surgical (−0.03/month, P=0.61) and SBRT patients (−0.1/month, P=0.44) (Table 3, Figure 2E). In the subsequent 10 months, no significant changes in depression score were observed in either surgical or SBRT patients (−0.02/month, P=0.12) vs. (−0.001/month, P=0.98). The estimated average PHQ-2 score at 12 months was lower than baseline for both surgical (0.58 vs. 0.79, P<0.001) and SBRT patients (0.72 vs. 0.91, P=0.34) (Table 4).
Other significant predictors
Older age was associated with better mental health (MCS, P=0.02), fewer lung cancer symptoms (FACT-LCS, P<0.001), and lower levels of anxiety (GAD-2, P<0.001) and depression (PHQ-2, P=0.002). More comorbidities were significantly related to worse lung cancer symptoms (FACT-LCS, P<0.001), physical QoL (PCS, P=0.003), mental health QoL (MCS, P=0.03), higher levels of anxiety symptoms (GAD-2, P=0.03), and higher levels of depression symptoms (PHQ-2, P=0.04). African Americans or Blacks (P=0.04), and those of other races (P=0.04) had significantly lower average PCS scores compared to Whites. No significant associations were observed between QoL outcomes and the gender, smoking status, or nodule size of patients.
Sensitivity analysis
Propensity score-matched analyses were also conducted to enhance the robustness of our comparison between the SBRT and surgery groups. Results from propensity score approach were consistent with results from the adjusted piece-wise linear mixed-effect model for all QoL outcomes. As a result, we chose to detail the adjusted results in this report as they included a broader patient population, so that they can reflect the diverse demographics and clinical characteristics represented in our study, and enhance the external validity of our findings.
Discussion
The choice of treatment and its subsequent effect on QoL is an important consideration for the management of early-stage NSCLC patients. Our study found a differential impact of surgery and SBRT on QoL outcomes within the first year post-treatment. Physical health (PCS) decreased within the first 2 months after treatment for both SBRT and surgical patients, with a greater decrease observed for surgical patients but without significant differences in the rates of change between the two groups. Over the following 10 months, surgical patients’ QoL rebounded significantly while SBRT patients continued to show a decline in physical health QoL. This corroborates with our previous study (10). The lung cancer symptom (FACT-LCS) scores followed a similar trend to the PCS score but did not differ significantly at baseline between the two treatments. Mental health summary (MCS) scores remained above the general population average throughout the 12-month span for both treatments. GAD-2 anxiety scores and PHQ-2 depression scores also remained below the diagnosis point throughout the 12-month period and improved at 12-month compared to baseline, without significant differences from the general population rates.
Our study examined the changes in multiple measures of QoL among early-stage NSCLC patients using a longitudinal approach and it allowed us to identify the QoL trends after treatment and the critical time points when QoL outcomes significantly change. It is a crucial finding that surgical patients’ physical health worsened significantly within 2 months post-treatment, emphasizing the need for focused interventions and close monitoring in that period to improve patients’ QoL outcomes. This is consistent with our previous study (9). In addition, the current study revealed that for post-SBRT care, a more sustained support system is needed as these patients showed a consistent decline in physical health throughout the year following treatment. Interventions could include rehabilitative therapies, pain management, and personalized exercise and diet plans.
The trends of the lung cancer symptom (FACT-LCS) scores have similar implications. Although the FACT-LCS score stayed stable within the first two months after SBRT, it dropped in the following 10 months and was lower compared to baseline. Sub-questions of the FACT-LCS measure were examined and the scores for questions related to “breathing easy”, “chest tightness”, and “shortness of breath” were found to be worsening, which point to specific needs among SBRT patients regarding the management of these symptoms which could also result from chronic respiratory or cardiac conditions that caused them to be limited to non-surgical treatment. Previous literature also indicated the association between dyspnea and toxicity related to SBRT (19). These findings highlight the necessity to limit the toxicity that might affect patients’ QoL during SBRT treatment and the importance of considering the potential impact of SBRT on QoL and toxicity in treatment decision making. However, it is also important to point out that SBRT patients started with lower physical health at baseline that might be attributed to more comorbidities, older age, and fear of surgery. Therefore, the sustained poorer physical health outcomes of SBRT patients were likely not due to the treatment, but due to overall poorer baseline health and other factors.
Unlike physical health outcomes, throughout the 12-month period, mental component of QoL, anxiety, and depression levels remained stable and even improved, with no significant difference observed between surgical and SBRT patients regarding these outcomes. This aligns with a prior study on SBRT patients, where they displayed significant improvement in emotional functioning, insomnia, anxiety, and depression levels (20). This consistency in mental health outcomes across both groups suggests a resilience and adaptability in patients’ psychological responses, regardless of the treatment method. It also highlights the importance of early intervention for lung cancer, as it can help relieve mental distress in patients, regardless of the treatment method chosen. However, despite the overall stability in mental health, our findings suggested a relatively slower improvement in anxiety scores for SBRT patients within the first two months post-treatment. While this was not statistically significant, understanding the nuances in patient anxiety post-treatment is essential. Consequently, we believe exploring additional mental health support avenues, including counseling or support groups, could be beneficial for post-SBRT patients.
We also found African Americans and other races had significantly lower physical health QoL outcomes compared to Whites. A growing body of research has shown that heterogeneity in social determinants of health, such as socioeconomic conditions and access to quality health care have disproportionally affect some racial/ethnic groups (21,22), resulting in lower physical function among racial minorities compared with Whites (23). Similarly, in a previous study to examine the differences in supportive care needs over time among patients with advanced stages of lung cancer, it was found that minority patients had higher initial and sustained levels of supportive care needs when compared to non-minority patients (24). These disparities should be addressed in the future for post-treatment care of lung cancer patients by providing racial and ethnic minority patients with comprehensive social support as well as psychosocial and behavioral supportive interventions (25).
We acknowledge the potential for selection bias in our study. The choice between surgical resection and SBRT is not random but is driven by patient and disease characteristics. In our cohort, factors such as patients’ overall health status, presence of comorbidities, patient preference, perceived surgical risk, and feasibility of surgery all impacted the decision making between SBRT and surgery. Additionally, patients who were older, with more comorbidities or had a fear of surgery were often more inclined towards SBRT. Despite our efforts to utilize propensity score matching and to adjust for known confounders such as age, sex, race, BMI, pack-years, smoking status, and comorbidities in the models, the difference in physical health (PCS) scores between surgical and SBRT patients at baseline was still significant, suggesting there may be unmeasured factors that influenced treatment choice and could potentially impact the estimated QoL outcomes observed in our study.
Another challenge encountered in this study was the presence of incomplete QoL measures for several patients. This is not uncommon in longitudinal studies, particularly those tracking QoL in clinical settings, where data may be missed or collected at irregular intervals. Despite this limitation, our study employed linear mixed models in our statistical analysis. These models offer a robust method for handling missing data or data collected at irregular time intervals. Therefore, instead of excluding these patients with incomplete QoL measures, which could introduce bias, linear mixed models allowed us to utilize all available data and appropriately handle these missing or mistimed QoL outcome measurements. This approach significantly enhanced the robustness of our results and prevented the potential loss of valuable information.
Conclusions
Our study highlights the needs for focused interventions within the early post-treatment period, particularly within the first two months after surgical procedures. Consistent support is crucial for patients undergoing SBRT, suggesting a need for long-term monitoring and care. With physical health outcomes showing varied trajectories between the two treatments, and with mental health outcomes demonstrating resilience and potential areas for additional support, our findings emphasize the complexity of post-treatment care. The stable mental health outcomes also suggest that timely and appropriate treatment can foster mental resilience in NSCLC patients. In addition, post-SBRT mental health management, effective communication, and thorough explanation of the SBRT’s side effects and slow-acting nature can be beneficial for patients’ mental health. It is crucial to consider these findings in clinical practice to improve the overall QoL for patients undergoing treatment for early-stage NSCLC.
Acknowledgments
Funding: This study was supported by generous grants from
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1201/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1201/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1201/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1201/coif). All authors report that this study was supported by generous grants from the Simons Foundation (International, Ltd.). D.F.Y. is a named inventor on a number of patents and patent applications related to the evaluation of chest diseases including measurements of chest nodules. D.F.Y. has received financial compensation for the licensing of these patents. In addition, he is a consultant and co-owner of Accumetra, a private company developing tools to improve the quality of CT imaging. He is on the advisory board and owns equity in HeartLung, a company that develops software related to CT scans of the chest. He is on the medical advisory board of Median Technology that is developing technology related to analyzing pulmonary nodules and is on the medical advisory board of Carestream, a company that develops radiography equipment. C.I.H. is also an inventor of the patents and pending patents owned by Cornell Research Foundation. As of April 2009, she has divested herself of all royalties and other interests arising from these. She is on the medical advisory board for LungLife Al. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai (MSHS IRB Study-15-01021). Informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vansteenkiste J, Crinò L, Dooms C, et al. 2nd ESMO Consensus Conference on Lung Cancer: early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann Oncol 2014;25:1462-74. [Crossref] [PubMed]
- Wisnivesky JP, Bonomi M, Henschke C, et al. Radiation therapy for the treatment of unresected stage I-II non-small cell lung cancer. Chest 2005;128:1461-7. [Crossref] [PubMed]
- Timmerman RD, Kavanagh BD, Cho LC, et al. Stereotactic body radiation therapy in multiple organ sites. J Clin Oncol 2007;25:947-52. [Crossref] [PubMed]
- Zheng X, Schipper M, Kidwell K, et al. Survival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: a meta-analysis. Int J Radiat Oncol Biol Phys 2014;90:603-11. [Crossref] [PubMed]
- Tomita N, Okuda K, Osaga S, et al. Surgery versus stereotactic body radiotherapy for clinical stage I non-small-cell lung cancer: propensity score-matching analysis including the ratio of ground glass nodules. Clin Transl Oncol 2021;23:638-47. [Crossref] [PubMed]
- Henschke CI, Yip R, Sun Q, et al. Prospective Cohort Study to Compare Long-Term Lung Cancer-Specific and All-Cause Survival of Clinical Early Stage (T1a-b; ≤20 mm) NSCLC Treated by Stereotactic Body Radiation Therapy and Surgery. J Thorac Oncol 2023; Epub ahead of print. [Crossref]
- Schwartz RM, Gorbenko K, Kerath SM, et al. Thoracic surgeon and patient focus groups on decision-making in early-stage lung cancer surgery. Future Oncol 2018;14:151-63. [Crossref] [PubMed]
- Sullivan DR, Eden KB, Dieckmann NF, et al. Understanding patients' values and preferences regarding early stage lung cancer treatment decision making. Lung Cancer 2019;131:47-57. [Crossref] [PubMed]
- Février E, Yip R, Becker BJ, et al. Change in quality of life of stage IA lung cancer patients after sublobar resection and lobectomy. J Thorac Dis 2020;12:3488-99. [Crossref] [PubMed]
- Schwartz RM, Alpert N, Rosenzweig K, et al. Changes in quality of life after surgery or radiotherapy in early-stage lung cancer. J Thorac Dis 2019;11:154-61. [Crossref] [PubMed]
- International Early Lung Cancer Action Program Investigators Group. I-ELCAP: Screening Protocol. 2022. [Cited 2022 Mar 1]. Available online: https://www.ielcap.org/home/ielcap/clinical-resources/ielcap-protocols/
- Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med 1991;32:705-14. [Crossref] [PubMed]
- Ware JE Jr. SF-36 health survey update. Spine (Phila Pa 1976) 2000;25:3130-9. [Crossref] [PubMed]
- Cella DF, Bonomi AE, Lloyd SR, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer 1995;12:199-220. [Crossref] [PubMed]
- Cella D, Eton DT, Fairclough DL, et al. What is a clinically meaningful change on the Functional Assessment of Cancer Therapy-Lung (FACT-L) Questionnaire? Results from Eastern Cooperative Oncology Group (ECOG) Study 5592. J Clin Epidemiol 2002;55:285-95. [Crossref] [PubMed]
- Kroenke K, Spitzer RL, Williams JB, et al. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics 2009;50:613-21. [Crossref] [PubMed]
- Kroenke K, Spitzer RL, Williams JB, et al. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med 2007;146:317-25. [Crossref] [PubMed]
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 2003;41:1284-92. [Crossref] [PubMed]
- van der Weijst L, Azria D, Berkovic P, et al. The correlation between pre-treatment symptoms, acute and late toxicity and patient-reported health-related quality of life in non-small cell lung cancer patients: Results of the REQUITE study. Radiother Oncol 2022;176:127-37. [Crossref] [PubMed]
- Rutkowski J, Szymanik M, Blok M, et al. Prospective evaluation of anxiety, depression and quality of life in medically inoperable early stage non-small cell lung cancer patients treated with stereotactic ablative radiotherapy. Rep Pract Oncol Radiother 2017;22:217-22. [Crossref] [PubMed]
- Cockerham WC, Hamby BW, Oates GR. The Social Determinants of Chronic Disease. Am J Prev Med 2017;52:S5-S12. [Crossref] [PubMed]
- Dean LT, Gehlert S, Neuhouser ML, et al. Social factors matter in cancer risk and survivorship. Cancer Causes Control 2018;29:611-8. [Crossref] [PubMed]
- Rincon MA, Smith AW, Yu M, et al. Trends in Racial/Ethnic Disparity of Health-Related Quality of Life in Older Adults with and without Cancer (1998-2012). Cancer Epidemiol Biomarkers Prev 2020;29:1188-95. [Crossref] [PubMed]
- Mazor MB, Li L, Morillo J, et al. Disparities in Supportive Care Needs Over Time Between Racial and Ethnic Minority and Non-Minority Patients With Advanced Lung Cancer. J Pain Symptom Manage 2022;63:563-71. [Crossref] [PubMed]
- Raz DJ, Sun V, Kim JY, et al. Long-Term Effect of an Interdisciplinary Supportive Care Intervention for Lung Cancer Survivors After Surgical Procedures. Ann Thorac Surg 2016;101:495-502; discussion 502-3. [Crossref] [PubMed]


