
Clinical value of the red blood cell distribution width to albumin ratio in the assessment of prognosis in critically ill patients with sepsis: a retrospective analysis
Highlight box
Key findings
• The red blood cell distribution width (RDW) to albumin ratio was independently associated with mortality in patients with sepsis, even after a broad array of potential confounders were considered. The area under the curve of the RDW to albumin ratio in discriminating 30-day mortality was similar to that of the lactate to albumin ratio and higher than that of the neutrophil percentage to albumin ratio.
What is known and what is new?
• To date, some studies have shown that the RDW-albumin ratio is related to increased mortality from several disease.
• To our knowledge, this is one of the first studies to examine the association between the RDW to albumin ratio and the risk of mortality in critically ill patients with sepsis and to demonstrate that the RDW to albumin ratio may be a useful and easy-to-use marker for clinical prognosis.
What is the implication, and what should change now?
• The RDW to albumin ratio may be a convenient and reliable marker that can be used to predict mortality in critically ill patients with sepsis. This perspective opens new approaches for personalized sepsis management.
Introduction
Sepsis remains a major challenge in critically ill patients accounting for approximately 30–50% of short-term mortality (1,2). Early diagnosis and timely treatment of sepsis can greatly improve outcomes and reduce the rate of fatality (3). In recent years progress has been made in the identification of sepsis based on complex biological characteristics and auxiliary examinations, but these are still highly time-consuming and thus unsatisfactory for identifying high-risk patients (4-6). There is therefore, a demand for simpler and more cost-effective biomarkers for predicting the prognosis of patients with sepsis and identifying those at high risk of death (7).
Red blood cell (RBC) distribution width (RDW) is a hematological index that reflects the volume heterogeneity of RBCs and is one of the more commonly used hematologic indices in clinics. As part of complete blood count, the RDW may be biased by variables in the preanalytical and analytical phases of the total testing process. The heterogeneous algorithms for calculating the RDW applied by different instrumentation suggest that harmonization may still be regarded as an unmet need (8). RDW has been shown to increase during systemic inflammation and infection and is associated with poor prognosis (9,10). Accumulating evidence supports the prognostic role of RDW in several clinical conditions, such as pneumonia, cardiovascular diseases, and critically ill patients with sepsis and those with coronavirus disease 2019 (COVID-19) (9-12).
Serum albumin plays critical physiological roles, including maintaining oncotic pressure, transporting various substances such as hormones, fatty acids, and drugs, and contributing to antioxidant defense, making it a multifunctional protein with significant implications for overall health (13). Studies have demonstrated that the serum albumin level is inversely associated with severity of illness and the extent of hypoalbuminemia correlates with the degree of sepsis (14,15). Moreover, serum albumin has been used as a marker of poor prognosis in critically ill patients, and, along with other hematologic parameters, such as the lactic acid to albumin ratio and neutrophil percentage to albumin ratio, can also predict the severity of sepsis in patients and their associated prognosis (16-18).
The RDW to albumin ratio is a novel biomarker based on both RDW and albumin levels and is associated with short-term mortality in critically ill patients with acute respiratory distress syndrome and pneumonia (19,20). Additionally, the RDW to albumin ratio can also predict mortality in patients with diabetic ketoacidosis, stroke, cancer, or aortic aneurysm (21-24). However, only few studies have been conducted on the clinical value of the RDW to albumin ratio in evaluating the prognosis of patients with sepsis in the intensive care unit (ICU) suggesting a predictive role for acute kidney injury (AKI) development in sepsis (25,26). Moreover, it remains unclear whether the RDW to albumin ratio is more valuable than are other albumin-based indicators in predicting mortality.
Therefore, this study aimed to investigate the relationship between the RDW to albumin ratio and the prognosis of critically ill patients with sepsis and to compare its performance with that of the lactate to albumin ratio and neutrophil percentage to albumin ratio. We present this article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1696/rc).
Methods
Data source
Patient data were obtained from the publicly available Medical Information Mart for Intensive Care III (MIMIC-III) database version 1.4. The database holds the records of deidentified health data for more than 50,000 patients admitted to the ICU at the Beth Israel Deaconess Medical Center (Boston, MA, USA) from 2001 to 2012 (27). To ensure robustness of our findings, the sample size was guided by precedent in similar biomarker studies in sepsis, although a formal calculation was not performed due to the retrospective nature of our analysis and reliance on existing clinical data.
This database was approved by the institutional review boards of the Massachusetts Institute of Technology (Cambridge, MA, USA) and Beth Israel Deaconess Medical Center (Boston, MA, USA). All personal data were anonymized to protect the privacy of the enrolled patients. Author CY Ma obtained access to the MIMIC-III database (record ID: 34907227). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Data extraction
The selection criteria in our study included adult ICU patients with sepsis at their first hospital admission and a length of ICU stay longer than 24 hours. We identified a sepsis population according to the Martin criteria, a widely used approach for identifying sepsis in administrative health data (28,29). Sequential imputation using chained equations (SICE) method was used to address any missing data (<5%) (30). SICE is a robust multiple imputation technique that allows for the imputation of missing values in a dataset with different types of variables, such as continuous, binary, and ordinal. The process involves imputing missing values multiple times to create several complete datasets. Each variable with missing data is imputed sequentially, with the method taking into account the distribution of the observed data. By employing this technique, we aimed to minimize bias and improve the reliability of our analysis, ensuring that the missing data did not significantly skew the results. The imputed datasets were then analyzed separately, and the results were combined to produce estimates that reflect the uncertainty due to the missing data. Patients were excluded if they had no data on RDW or albumin within the first 24 hours of admission, or if more than 5% of the data were absent.
Structure query language (SQL) was used to extract the following admission data in the first record within 24 hours after their ICU admission: demographic information, including sex, age, ethnicity, and service units; disease severity, as assessed by the Simplified Acute Physiology Score II (SAPS II) and Sequential Organ Failure Assessment (SOFA) score; vital signs, including heart rate, respiratory rate, temperature, mean blood pressure (MBP), systolic blood pressure (SBP), diastolic blood pressure (DBP), and percutaneous oxygen saturation (SpO2); laboratory parameters, including white blood cell (WBC) count, hemoglobin concentration, platelet count, RDW, and levels of hematocrit, bicarbonate, chloride, serum creatinine, blood urea nitrogen (BUN), serum potassium, serum sodium, albumin, and serum lactate; comorbidities, including congestive heart failure, chronic renal disease, liver disease, chronic obstructive pulmonary disease (COPD), stroke, and malignancy; and ICU intervention measures, including mechanical ventilation (MV) use, renal replacement therapy (RRT) use, and vasopressor use.
Study outcome and definitions
The study outcome was 30- and 90-day mortality. The start date was the date of the patient’s admission.
We used a previously proposed equation to calculate the RDW to albumin ratio: RDW (%) divided by albumin concentration (g/dL). The lactate to albumin ratio was calculated as serum lactate (mmol/L) divided by albumin concentration (g/dL); the neutrophil percentage to albumin ratio was calculated as the neutrophil percentage (%) divided by albumin concentration (g/dL).
We identified a sepsis population according to the Martin criteria, a widely used approach for identifying sepsis in administrative health data (28,29). Patients were excluded if: (I) they were not first ICU admission; (II) age less than 18 years old; (III) length of ICU stay less than 1 day; (IV) without sepsis diagnosis; and (V) they had no data on RDW or albumin within the first 24 hours of admission, or if more than 5% of the data were absent.
Statistical analysis
The study population was subdivided into three groups based on the RDW to albumin ratio. Data conforming to a normal distribution are presented as the mean ± standard deviation (SD); they are presented as the median and interquartile range (IQR) for continuous variables not conforming to a normal distribution, and as the number and frequency for categorical data. The logarithmic transformation Mann-Whitney test was used for comparisons between the survivor and non-survivor groups, the Kruskal-Wallis test was used for continuous variables, and the χ2 test or Fisher exact test (expected frequency <10) was used for categorical variables.
Cox proportional risk models were used to determine factors associated with 30- and 90-day mortality, expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). The RDW to albumin ratio values were divided into tertiles or quintiles, and the first tertile or quintile was selected as the reference group. The variables with P<0.1 in the univariate model were subjected to multivariate Cox regression analysis. The following models were used for covariance correction: model 1 was adjusted for the confounders of age, sex, ethnicity, and service units; model 2 was adjusted for model 1 and other confounders, including vital signs (heart rate, SBP, DBP, MBP, respiratory rate, SpO2, and temperature), laboratory parameters (WBC, hemoglobin, platelet count, bicarbonate, sodium, glucose, BUN, creatinine, and lactate level), severity scores (SOFA and SAPS II), comorbidities (congestive heart failure, chronic renal disease, liver disease, stroke, and malignancy), and ICU intervention measures (MV use, RRT use, and vasopressor use) as covariates. The associations between the crude and adjusted HR (95% CI) and RDW to albumin ratio as a continuous variable were evaluated using a restricted cubic spline with 5 knots (29,31), and the confounders were adjusted as shown in model 2.
Receiver operating characteristic (ROC) curves were generated to calculate the area under the curve (AUC), and the predictive values of the RDW to albumin ratio, lactate to albumin ratio, and neutrophil percentage to albumin ratio for all-cause mortality were compared. The Youden index was used to determine the optimal cutoff value. The population was divided into low (<5.246) and high (≥5.246) RDW to albumin groups, and the survival difference between the two groups was indicated with a Kaplan-Meier curve. The participants in this study were for analysis of the influence of patient characteristics including disease severity then subdivided into the following three groups based on the RDW to albumin ratio: <4.915, 4.915–6.405, and >6.405.
All analyses of data were performed using Stata 14.0 (StataCorp, College Station, TX, USA), SPSS software version 22.0 (IBM Corp., Armonk, NY, USA), and R software (https://www.r-project.org/). A two-sided P value <0.05 was considered statistically significant.
Results
Patient characteristics
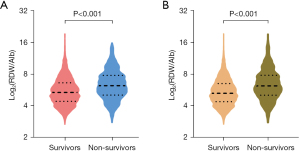
A total of 3,969 patients who met the screening criteria were ultimately included in this study (Figure 1). The comparison of baseline characteristics of survivors and non-survivors at 30 and 90 days is shown in Tables S1,S2, respectively. The overall median RDW to albumin ratio at 30 days in the non-survivor group was significantly higher than that in the survival group (6.24 vs. 5.39, P<0.001), and the same trend was observed at 90 days (6.19 vs. 5.27, P<0.001) (Figure 2).

The participants in this study were then subdivided into the following three groups based on the RDW to albumin ratio: <4.915 (n=1,325), 4.915–6.405 (n=1,322), and >6.405 (n=1,322). Table 1 shows the baseline and clinical characteristics of the different RDW to albumin ratio groups. Patients with sepsis and a higher RDW to albumin ratio tended to have a higher heart rate, respiratory rate, WBC count, RDW, and chloride, BUN, creatinine, and lactate levels; however, they had lower blood pressure, temperature, and hemoglobin, platelet count, bicarbonate, sodium, glucose, and albumin levels. They were also more likely to exhibit higher critical scores of SAPS II and SOFA, and to require a greater degree of ICU intervention, including MV, RRT, and vasopressor use. The prevalence of liver disease and malignancy was also significantly increased in patients with a high RDW to albumin ratio.
Table 1
| Characteristics | RDW to albumin ratio | P value | ||
|---|---|---|---|---|
| <4.915 | 4.915–6.405 | >6.405 | ||
| RDW to albumin ratio | 4.267±0.476 | 5.603±0.427 | 8.289±1.856 | <0.001 |
| Number | 1,325 | 1,322 | 1,322 | |
| Age (years) | 66.16 [52.62–78.72] | 66.92 [54.17–79.32] | 65.04 [53.64–77.13] | 0.044 |
| Male | 701 (52.9) | 708 (53.6) | 703 (53.2) | 0.945 |
| Ethnicity | 0.383 | |||
| White | 951 (71.8) | 912 (69.0) | 914 (69.1) | |
| Black | 97 (7.3) | 106 (8.0) | 117 (8.9) | |
| Others | 277 (20.9) | 304 (23.0) | 291 (22.0) | |
| Service unit | <0.001 | |||
| MICU | 622 (46.9) | 819 (62.0) | 816 (61.7) | |
| SICU/TSICU | 378 (28.5) | 283 (21.4) | 334 (25.3) | |
| CCU/CSRU | 325 (24.5) | 220 (16.6) | 172 (13.0) | |
| SOFA score | 5 [3–7] | 6 [4–9] | 7.5 [5–11] | <0.001 |
| SAPS II score | 38 [29–47] | 43 [34–53] | 48 [37–59] | <0.001 |
| Interventions | ||||
| MV use (first 24 h) | 780 (58.9) | 76 (5.7) | 872 (66.0) | <0.001 |
| RRT use (first 24 h) | 50 (3.8) | 93 (7.0) | 119 (9.0) | <0.001 |
| Vasopressor use (first 24 h) | 386 (29.1) | 540 (40.8) | 694 (52.5) | <0.001 |
| Vital signs | ||||
| Heart rate (bpm) | 86.8 [75.3–98.4] | 90.7 [78.2–104.0] | 93.1 [81.3–106.0] | <0.001 |
| SBP (mmHg) | 117.8 [106.9–133.3] | 112.2 [104.1–123.5] | 108.1 [100.8–118.2] | <0.001 |
| DBP (mmHg) | 60.9 [54.6–68.7] | 58.7 [52.6–65.5] | 56.4 [50.6–63.0] | <0.001 |
| MBP (mmHg) | 77.9 [71.5–86.4] | 74.5 [69–82.5] | 72.2 [67.0–79.1] | <0.001 |
| Respiratory rate (bpm) | 19.3 [16.8–22.2] | 20 [17.3–23.3] | 20.3 [17.4–23.8] | <0.001 |
| SpO2 (%) | 97.5 [96.0–98.8] | 97.2 [95.8–98.5] | 97.5 [96.0–98.7] | 0.008 |
| Temperature (℃) | 37.0 [36.6–37.4] | 36.9 [36.4–37.4] | 36.8 [36.3–37.3] | <0.001 |
| Laboratory tests | ||||
| WBC (×109/L) | 13.2 [10.1–18.1] | 14.1 [9.3–19.9] | 14.9 [9.5–22.1] | <0.001 |
| Hemoglobin (g/dL) | 11 [9.6–12.3] | 9.6 [8.5–10.8] | 8.8 [7.9–9.9] | <0.001 |
| Platelet count (×109/L) | 191 [140.5–251] | 164.5 [102–262] | 141.5 [71–240.3] | <0.001 |
| RDW (%) | 14.2 [13.4–14.9] | 15.4 [14.5–16.7] | 17.1 [15.6–19.1] | <0.001 |
| Bicarbonate (mmol/L) | 22 [19–25] | 20 [17–24] | 19 [15–23] | <0.001 |
| Potassium (mmol/L) | 3.6 [3.3–4.0] | 3.7 [3.3–4.1] | 3.7 [3.3–4.1] | 0.196 |
| Sodium (mmol/L) | 137 [134–140] | 137 [133–140] | 136 [132–140] | <0.001 |
| Glucose (mg/dL) | 138 [116.8–167.3] | 134.3 [112.0–167.0] | 131.1 [107.6–159] | <0.001 |
| Albumin (g/dL) | 3.4 [3.2–3.7] | 2.8 [2.6–3.0] | 2.2 [1.9–2.5] | <0.001 |
| BUN (mg/dL) | 24 [16–40] | 33 [20–54.3] | 34 [20–56] | <0.001 |
| Creatinine (mg/dL) | 1.2 [0.9–1.9] | 1.5 [1.0–2.6] | 1.5 [0.9–2.6] | <0.001 |
| Chloride (mmol/L) | 107 [103–110] | 108 [103–113] | 109 [104–114] | <0.001 |
| Lactate level (mmol/L) | 2.33 [1.57–3.51] | 2.56 [1.6–4.0] | 3.0 [1.9–5.1] | <0.001 |
| Comorbidities | ||||
| Congestive heart failure | 449 (33.9) | 472 (35.7) | 363 (27.5) | <0.001 |
| Chronic renal disease | 162 (12.2) | 215 (16.3) | 189 (14.3) | 0.012 |
| Liver disease | 98 (7.4) | 174 (13.2) | 266 (20.1) | <0.001 |
| COPD | 190 (14.3) | 206 (15.6) | 174 (13.2) | 0.207 |
| Stroke | 183 (13.8) | 82 (6.2) | 63 (4.8) | <0.001 |
| Malignancy | 132 (10.0) | 283 (21.4) | 389 (29.4) | <0.001 |
| Mortality | ||||
| 30-day mortality | 224 (16.9) | 337 (25.5) | 500 (37.8) | <0.001 |
| 90-day mortality | 288 (21.7) | 448 (33.9) | 633 (47.9) | <0.001 |
Data are presented as mean ± SD, n, median [IQR], or n (%). RDW, red blood cell distribution width; MICU, medical intensive care unit; SICU, surgical intensive care unit; TSICU, trauma/surgical intensive care unit; CCU, coronary care unit; CSRU, cardiac surgery recovery unit; SOFA, Sequential Organ Failure Assessment; SAPS II, Simplified Acute Physiology Score II; MV, mechanical ventilation; RRT, renal replacement therapy; SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; SpO2, percutaneous oxygen saturation; WBC, white blood cell; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; SD, standard deviation; IQR, interquartile range.
Association between RDW to albumin ratio and 30- and 90-day mortality
To investigate the relationship between the RDW to albumin ratio and the outcomes of patients with sepsis, we performed the Cox regression analysis, the results of which are shown in Table 2. The RDW to albumin ratio was found to be an independent predictor for 30- and 90-day mortality in patients with sepsis. For 30-day mortality, the HR for the second (4.915–6.405) and the third (>6.405) tertiles was 1.597 (95% CI: 1.348–1.890) and 2.598 (95% CI: 2.219–3.042), respectively, compared to the first tertiles (<4.915). For model 1, after adjustments were made for age, sex, ethnicity, and service units, the HR (of 30-day mortality for the second and third tertiles) was 1.505 (95% CI: 1.270–1.784) and 2.558 (95% CI: 2.181–2.999), respectively, compared to the first tertile. After further adjustment of potential covariates in model 2 (model 1 plus other multiple confounders), the trend was similar to that of previous models. The HR for the second and third tertiles was 1.169 (95% CI: 0.976–1.331) and 1.521 (95% CI: 1.263–1.833), respectively, compared to the reference. In quintile analyses, a similar trend was observed, and the fifth quintile (>7.40) had the highest HR compared with the reference (<4.35) in the three models. The tendency of the all-cause mortality rate in all models showed significant increases in HR (95% CI) values in tertile and quintile analyses. For the 90-day all-cause mortality rate, the same association was detected when the RDW to albumin ratio was divided into three and five categories, and a similar association with mortality was also observed.
Table 2
| Exposure | Non-adjusted | Model 1 | Model 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | P trend | HR (95% CI) | P value | P trend | HR (95% CI) | P value | P trend | |||
| 30-day mortality | |||||||||||
| Tertile | <0.001 | <0.001 | <0.001 | ||||||||
| <4.915 | 1 | 1 | 1 | ||||||||
| 4.915–6.405 | 1.597 (1.348–1.890) | <0.001 | 1.505 (1.270–1.784) | <0.001 | 1.169 (0.976–1.331) | 0.089 | |||||
| >6.405 | 2.598 (2.219–3.042) | <0.001 | 2.558 (2.181–2.999) | <0.001 | 1.521 (1.263–1.833) | <0.001 | |||||
| Quintile | |||||||||||
| <4.35 | 1 | <0.001 | 1 | <0.001 | <0.001 | ||||||
| 4.35–<5.15 | 1.132 (0.894–1.432) | 0.303 | 1.093 (0.863–1.385) | 0.459 | 0.973 (0.765–1.238) | 0.825 | |||||
| 5.15–<6.04 | 1.718 (1.382–2.134) | <0.001 | 1.602 (1.287–1.994) | <0.001 | 1.232 (0.977–1.552) | 0.077 | |||||
| 6.04–7.40 | 1.988 (1.607–2.459) | <0.001 | 1.919 (1.549–2.378) | <0.001 | 1.232 (1.045–1.672) | 0.020 | |||||
| >7.40 | 3.071 (2.510–3.758) | <0.001 | 3.044 (2.483–3.733) | <0.001 | 1.649 (1.300–2.091) | <0.001 | |||||
| 90-day mortality | |||||||||||
| Tertile | <0.001 | <0.001 | <0.001 | ||||||||
| <4.915 | 1 | 1 | 1 | ||||||||
| 4.915–6.405 | 1.685 (1.453–1.953) | <0.001 | 1.607 (1.384–1.865) | <0.001 | 1.212 (1.039–1.413) | 0.014 | |||||
| >6.405 | 2.679 (2.330–3.080) | <0.001 | 2.691 (2.338–3.098) | <0.001 | 1.522 (1.301–1.779) | <0.001 | |||||
| Quintile | <0.001 | <0.001 | <0.001 | ||||||||
| <4.35 | 1 | 1 | 1 | ||||||||
| 4.35–<5.15 | 1.255 (1.021–1.543) | 0.031 | 1.231 (1.001–1.515) | 0.049 | 1.080 (0.874–1.334) | 0.477 | |||||
| 5.15–<6.04 | 1.848 (1.523–2.242) | <0.001 | 1.753 (1.443–2.130) | <0.001 | 1.346 (1.095–1.653) | 0.005 | |||||
| 6.04–7.40 | 2.100 (1.737–2.540) | <0.001 | 2.065 (1.705–2.500) | <0.001 | 1.402 (1.137–1.728) | 0.002 | |||||
| >7.40 | 3.346 (2.794–4.008) | <0.001 | 3.438 (2.865–4.126) | <0.001 | 1.829 (1.478–2.264) | <0.001 | |||||
Model 1 was adjusted for the confounder of: age, sex, ethnicity, and service units; model 2 was adjusted for model 1 plus other factors (heart rate, SBP, DBP, MBP, respiratory rate, SpO2, temperature, WBC, hemoglobin, platelet count, bicarbonate, sodium, glucose, BUN, creatinine, chloride, lactate level, SOFA score, SAPS II, congestive heart failure, chronic renal disease, liver disease, stroke, malignancy, MV use, RRT use, and vasopressor use). RDW, red blood cell distribution width; HR, hazard ratio; CI, confidence interval; SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; SpO2, percutaneous oxygen saturation; WBC, white blood cell; BUN, blood urea nitrogen; SOFA, Sequential Organ Failure Assessment; SAPS II, Simplified Acute Physiology Score II; MV, mechanical ventilation; RRT, renal replacement therapy.
The crude and adjusted HR for 30-day mortality was further investigated when the RDW to albumin ratio was used as a continuous variable based on restricted cubic spline analysis of the Cox model, as shown in Figure 3. From the figure, it is evident that an increased RDW to albumin ratio is associated with the risk of death in patients with sepsis.

Subgroup analysis
Subgroup analysis revealed the relationship between the RDW to albumin ratio and 30-day mortality in critically ill patients with sepsis with different comorbidities (Figure 4). After adjustments were made for confounding factors in model 1, there was no significant difference in other subgroup factors except chronic heart failure (P=0.015) and chronic renal disease (P=0.014).

Predictive value of RDW to albumin ratio for 30-day mortality in patients with sepsis
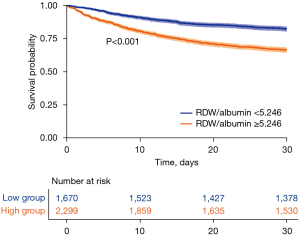
The value of the RDW to albumin ratio for predicting 30-day mortality in critically ill patients with sepsis was analyzed through the ROC curve and compared to that of the other two albumin-based indicators: the lactate to albumin ratio and neutrophil percentage to albumin ratio (Figure 5, Table 3). The AUC value of the RDW to albumin ratio in all patients was 0.633 (95% CI: 0.614–0.653), this was similar to the lactate to albumin ratio (AUC =0.617; 95% CI: 0.597–0.636; P=0.133) but higher than that of the neutrophil percentage to albumin ratio (AUC =0.559; 95% CI: 0.539–0.580; P<0.001). According to the Youden index, the optimal cutoff point of the RDW to albumin ratio was 5.246, with a sensitivity of 72.48% and a specificity of 47.39%. Accordingly, the study population was divided into a high RDW to albumin ratio group (≥5.246) and a low RDW to albumin ratio group (<5.246), with the Kaplan-Meier curve showing that the 30-day all-cause mortality in the high RDW to albumin ratio group was significantly higher than that in the low group (log-rank P<0.001; Figure 6).

Table 3
| Variables | AUC | 95% CI | Cutoff value | P1 | P2 |
|---|---|---|---|---|---|
| RDW to albumin ratio | 0.633 | 0.614–0.653 | 5.246 | <0.001 | |
| Lactate to albumin ratio | 0.617 | 0.597–0.636 | 1.301 | <0.001 | 0.133 |
| Neutrophil percentage to albumin ratio | 0.559 | 0.539–0.580 | 28.41 | <0.001 | <0.001 |
P1: P value for the AUC of each variable; P2: P value compared to RDW to albumin ratio. According to the AUC, the predictive ability of the RDW to albumin ratio for 30-day mortality was similar to that of the lactate to albumin ratio (P=0.133) and was better than that of the neutrophil percentage to albumin ratio (P<0.001). AUC, area under the curve; CI, confidence interval; RDW, red blood cell distribution width.
Discussion
To the best of our knowledge, this is one of the first studies to examine the association between the RDW to albumin ratio and the risk of mortality in critically ill patients with sepsis and to demonstrate that the RDW to albumin ratio may be a useful and easy-to-use marker for clinical prognostic prediction.
Sepsis is a heterogeneous clinical disorder defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection (32). The inflammatory response plays an important role in the occurrence and development of sepsis, and early identification of new inflammatory biomarkers or combined indicators is crucial for the timely initiation of treatment and prediction of progression and outcome. Previous studies have suggested that identifying new markers of inflammation or combining already existing indicators, such as the lactate to albumin ratio and neutrophil percentage to albumin ratio, may be valuable in determining the severity or predicting the clinical prognosis of patients with sepsis (18,33-35).
RDW expresses changes in the size of RBCs and has been identified as a novel prognostic factor in many pathophysiological conditions, including severe inflammation and sepsis (9,36,37). Hu et al. (9) conducted a narrative review to summarize the relationship between RDW and sepsis and found that increased RDW on admission, both in adults and neonates, may be associated with unfavorable outcomes in the short- and long-term. The mechanisms underlying the association between increased RDW and poor outcomes remain largely unknown. Previous studies have shown that RDW is positively associated with some inflammatory biomarkers, suggesting that RDW may reflect the existence of an inflammatory response (37). In sepsis, erythrocyte heterogeneity and morphological changes are more prominent due to high oxidative stress and alterations in erythrocyte membrane glycoproteins and ion channels, leading directly to an increase in RDW (36). In addition, RDW is involved in the function of different organs in the body. Therefore, in patients with sepsis, an increase in RDW suggests possible organ dysfunction and poor outcomes (36).
Serum albumin, a biochemical marker of nutritional status, plays a role in the acute response to inflammation and in sepsis (38). In sepsis, acute and chronic states of low serum albumin are independently associated with an increased risk of mortality (39). Previous studies also showed that serum albumin level over time, serum albumin level at admission, and the lowest serum albumin level were significant unique predictors of mortality in ICU patients with sepsis (40). The increased vascular permeability in sepsis may increase albumin loss, accelerate the onset of hypoalbuminemia and compound the negative effects of hypoproteinemia in sepsis. Ultimately, a vicious cycle exists between the adverse outcomes of hypoproteinemia and sepsis (41).
The RDW to albumin ratio is a novel combined inflammatory-related indicator, and several studies have shown that the RDW to albumin ratio has predictive value in disease prognosis. Yoo et al. (20) evaluated an adult medical ICU and found that the RDW to albumin ratio was associated with 60-day mortality in patients with acute respiratory distress syndrome. Jeong et al. (19) recently conducted a prospective study in critically ill patients with pneumonia receiving invasive MV, and they found that a high RDW to albumin ratio has similar predictability in determining 28-day mortality to that of the lactate to albumin ratio. In addition, the RDW to albumin ratio was found to be a potential prognostic biomarker for predict mortality in patients afflicted with diabetic ketoacidosis, stroke, cancer, or aortic aneurysm (21-25). To the best of our knowledge, our study is one of the first studies to focus on the relationship between the RDW to albumin ratio and the prognosis of patients with sepsis. We found that the median RDW to albumin ratio was significantly higher in non-survivor patients with sepsis than that in survivors at 30 and 90 days. As the RDW to albumin ratio increased, the risk of both 30- and 90-day mortality increased, and the RDW to albumin ratio was independently associated with mortality. In addition, the AUC of the RDW to albumin ratio showed that it was comparable to the lactate to albumin ratio in predicting 30-day mortality while being superior to that of the neutrophil percentage to albumin ratio. Finally, patients with a high RDW to albumin ratio (≥5.246) had a significantly higher risk of 30-day mortality than patients with a low RDW to albumin ratio (<5.246). We found that a high RDW to albumin ratio was independently associated with increased 30- and 90-day mortality of patients with sepsis, even after a broad array of potential confounders were considered. This result suggests that the RDW to albumin ratio is a promising biomarker that can readily predict mortality in patients with sepsis. To contextualize our findings within the broader scope of existing research, it is noteworthy that elevated RDW-albumin ratios have been associated with increased mortality in heart failure and acute myocardial infarction, suggesting potential parallels in the systemic inflammatory and stress responses observed in our septic patient cohort.
Previously, albumin-based ratios, including the lactate to albumin ratio and neutrophil percentage to albumin ratio, were identified as prognostic factors for evaluating the outcome of patients with sepsis (18,33-35). Thus, we further compared the diagnostic value of these indicators to predict mortality. Notably, the RDW to albumin ratio has the same utility as the lactate to albumin ratio in assessing the 30-day mortality of patients with sepsis and a superior utility to that of neutrophil percentage to albumin ratio. In addition, given that regular monitoring of arterial lactic acid in critically ill patients is difficult to achieve, RDW and albumin are feasible measurements that can be obtained from venous blood and routine laboratory tests. Therefore, the RDW to albumin ratio is less restrictive in clinical practice and more easily used for risk stratification of patients with sepsis, serving as a reference for early recognition and intervention. Our findings suggest that the RDW/albumin ratio, beyond reflecting disease severity, might also have potential in guiding therapeutic strategies. Specifically, it could aid in the future identification of sepsis patients who are likely to benefit from albumin supplementation, akin to the targeted use of albumin in cirrhosis. This perspective opens new approaches for personalized sepsis management, where biomarkers are not only prognostic indicators but also guide therapeutic interventions.
However, several limitations of this study should be noted. First, we employed a single-center, retrospective study design, using observational data from the MIMIC-III database. As this was a retrospective analysis of the database, the database is not up to date and includes patients from the first decade of the year 2000. Besides, some data we were unable to extract, such as the infection origin of sepsis and types of etiological agent, etc. Nonetheless, the conclusions are still reliable because of the large sample data analysis. Further multi-center prospective studies are required to validate our findings. Second, the RDW to albumin ratio was obtained from RDW and albumin within the first 24 hours of ICU admission, and the possible effects of different processes and dynamic changes on the RDW to albumin ratio were not addressed. Third, this was a retrospective cohort study, these results could be reinforced by prospective observational studies. Despite the deficiencies in this study, we have demonstrated that the RDW to albumin ratio is a statistically significant prognostic marker in critically ill patients with sepsis.
Conclusions
In summary, the RDW to albumin ratio was found to be an independent predictor for 30- and 90-day mortality in patients with sepsis, and its prognostic value was similar to that of the lactic acid to albumin ratio and superior to that of the neutrophil percentage to albumin ratio. According to our findings, the RDW to albumin ratio may be a convenient and reliable marker that can be used to predict mortality in critically ill patients with sepsis; it would thus be worthwhile to further verify these findings using a prospective, multicenter study.
Acknowledgments
The authors appreciate the great support from Dr. Robert Arnce (Kansas City University, Kansas City, MO, USA) in improving the quality of this paper.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1696/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1696/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1696/coif). C.J.W. reports fees for speaking and/or consulting from Biotest, CSL Behring, and Grifols. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Machado FR, Cavalcanti AB, Bozza FA, et al. The epidemiology of sepsis in Brazilian intensive care units (the Sepsis PREvalence Assessment Database, SPREAD): an observational study. Lancet Infect Dis 2017;17:1180-9. [Crossref] [PubMed]
- Xie J, Wang H, Kang Y, et al. The Epidemiology of Sepsis in Chinese ICUs: A National Cross-Sectional Survey. Crit Care Med 2020;48:e209-18. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Duncan CF, Youngstein T, Kirrane MD, et al. Diagnostic Challenges in Sepsis. Curr Infect Dis Rep 2021;23:22. [Crossref] [PubMed]
- Zheng YJ, Zhu XJ, Chen YW, et al. Establishment of a novel risk score for in-hospital mortality in adult sepsis patients. Ann Transl Med 2022;10:781. [Crossref] [PubMed]
- Komorowski M, Green A, Tatham KC, et al. Sepsis biomarkers and diagnostic tools with a focus on machine learning. EBioMedicine 2022;86:104394. [Crossref] [PubMed]
- Pierrakos C, Velissaris D, Bisdorff M, et al. Biomarkers of sepsis: time for a reappraisal. Crit Care 2020;24:287. [Crossref] [PubMed]
- Lippi G, Pavesi F, Bardi M, et al. Lack of harmonization of red blood cell distribution width (RDW). Evaluation of four hematological analyzers. Clin Biochem 2014;47:1100-3. [Crossref] [PubMed]
- Hu ZD, Lippi G, Montagnana M. Diagnostic and prognostic value of red blood cell distribution width in sepsis: A narrative review. Clin Biochem 2020;77:1-6. [Crossref] [PubMed]
- Parizadeh SM, Jafarzadeh-Esfehani R, Bahreyni A, et al. The diagnostic and prognostic value of red cell distribution width in cardiovascular disease; current status and prospective. Biofactors 2019;45:507-16. [Crossref] [PubMed]
- Bello S, Fandos S, Lasierra AB, et al. Red blood cell distribution width [RDW] and long-term mortality after community-acquired pneumonia. A comparison with proadrenomedullin. Respir Med 2015;109:1193-206.
- Lorente L, Martín MM, Argueso M, et al. Association between red blood cell distribution width and mortality of COVID-19 patients. Anaesth Crit Care Pain Med 2021;40:100777. [Crossref] [PubMed]
- Quinlan GJ, Martin GS, Evans TW. Albumin: biochemical properties and therapeutic potential. Hepatology 2005;41:1211-9. [Crossref] [PubMed]
- Rungsakulkij N, Vassanasiri W, Tangtawee P, et al. Preoperative serum albumin is associated with intra-abdominal infection following major hepatectomy. J Hepatobiliary Pancreat Sci 2019;26:479-89. [Crossref] [PubMed]
- Huang S, Zhou Z, Luo L, et al. Preoperative serum albumin: a promising indicator of early mortality after surgery for infective endocarditis. Ann Transl Med 2021;9:1445. [Crossref] [PubMed]
- Shin J, Hwang SY, Jo IJ, et al. Prognostic Value of The Lactate/Albumin Ratio for Predicting 28-Day Mortality in Critically ILL Sepsis Patients. Shock 2018;50:545-50. [Crossref] [PubMed]
- Gharipour A, Razavi R, Gharipour M, et al. Lactate/albumin ratio: An early prognostic marker in critically ill patients. Am J Emerg Med 2020;38:2088-95. [Crossref] [PubMed]
- Gong Y, Li D, Cheng B, et al. Increased neutrophil percentage-to-albumin ratio is associated with all-cause mortality in patients with severe sepsis or septic shock. Epidemiol Infect 2020;148:e87. [Crossref] [PubMed]
- Jeong JH, Heo M, Lee SJ, et al. Clinical Usefulness of Red Cell Distribution Width/Albumin Ratio to Discriminate 28-Day Mortality in Critically Ill Patients with Pneumonia Receiving Invasive Mechanical Ventilation, Compared with Lacate/Albumin Ratio: A Retrospective Cohort Study. Diagnostics (Basel) 2021;11:2344. [Crossref] [PubMed]
- Yoo JW, Ju S, Lee SJ, et al. Red cell distribution width/albumin ratio is associated with 60-day mortality in patients with acute respiratory distress syndrome. Infect Dis (Lond) 2020;52:266-70. [Crossref] [PubMed]
- Zhou D, Wang J, Li X. The Red Blood Cell Distribution Width-Albumin Ratio Was a Potential Prognostic Biomarker for Diabetic Ketoacidosis. Int J Gen Med 2021;14:5375-80. [Crossref] [PubMed]
- Zhao N, Hu W, Wu Z, et al. The Red Blood Cell Distribution Width-Albumin Ratio: A Promising Predictor of Mortality in Stroke Patients. Int J Gen Med 2021;14:3737-47. [Crossref] [PubMed]
- Long J, Xie X, Xu D, et al. Association Between Red Blood Cell Distribution Width-to-Albumin Ratio and Prognosis of Patients with Aortic Aneurysms. Int J Gen Med 2021;14:6287-94. [Crossref] [PubMed]
- Lu C, Long J, Liu H, et al. Red blood cell distribution width-to-albumin ratio is associated with all-cause mortality in cancer patients. J Clin Lab Anal 2022;36:e24423. [Crossref] [PubMed]
- Xu Y, Qi W. Association between red cell distribution width to albumin ratio and acute kidney injury in patients with sepsis: a MIMIC population-based study. Int Urol Nephrol 2023;55:2943-50. [Crossref] [PubMed]
- Xu W, Huo J, Chen G, et al. Association between red blood cell distribution width to albumin ratio and prognosis of patients with sepsis: A retrospective cohort study. Front Nutr 2022;9:1019502. [Crossref] [PubMed]
- Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data 2016;3:160035. [Crossref] [PubMed]
- Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003;348:1546-54. [Crossref] [PubMed]
- Feng M, McSparron JI, Kien DT, et al. Transthoracic echocardiography and mortality in sepsis: analysis of the MIMIC-III database. Intensive Care Med 2018;44:884-92. [Crossref] [PubMed]
- Gravesteijn BY, Sewalt CA, Venema E, et al. Missing Data in Prediction Research: A Five-Step Approach for Multiple Imputation, Illustrated in the CENTER-TBI Study. J Neurotrauma 2021;38:1842-57. [Crossref] [PubMed]
- Ng R, Kornas K, Sutradhar R, et al. The current application of the Royston-Parmar model for prognostic modeling in health research: a scoping review. Diagn Progn Res 2018;2:4. [Crossref] [PubMed]
- Yousof T, Sharma H, Austin RC, et al. Stressing the endoplasmic reticulum response as a diagnostic tool for sepsis. Ann Transl Med 2022;10:812. [Crossref] [PubMed]
- Bou Chebl R, Geha M, Assaf M, et al. The prognostic value of the lactate/albumin ratio for predicting mortality in septic patients presenting to the emergency department: a prospective study. Ann Med 2021;53:2268-77. [Crossref] [PubMed]
- Bou Chebl R, Jamali S, Sabra M, et al. Lactate/Albumin Ratio as a Predictor of In-Hospital Mortality in Septic Patients Presenting to the Emergency Department. Front Med (Lausanne) 2020;7:550182. [Crossref] [PubMed]
- Cakir E, Turan IO. Lactate/albumin ratio is more effective than lactate or albumin alone in predicting clinical outcomes in intensive care patients with sepsis. Scand J Clin Lab Invest 2021;81:225-9. [Crossref] [PubMed]
- Zhang L, Yu CH, Guo KP, et al. Prognostic role of red blood cell distribution width in patients with sepsis: a systematic review and meta-analysis. BMC Immunol 2020;21:40. [Crossref] [PubMed]
- Han YQ, Zhang L, Yan L, et al. Red blood cell distribution width predicts long-term outcomes in sepsis patients admitted to the intensive care unit. Clin Chim Acta 2018;487:112-6. [Crossref] [PubMed]
- Padkins M, Breen T, Anavekar N, et al. Association Between Albumin Level and Mortality Among Cardiac Intensive Care Unit Patients. J Intensive Care Med 2021;36:1475-82. [Crossref] [PubMed]
- Wiedermann CJ. Hypoalbuminemia as Surrogate and Culprit of Infections. Int J Mol Sci 2021;22:4496. [Crossref] [PubMed]
- Kendall H, Abreu E, Cheng AL. Serum Albumin Trend Is a Predictor of Mortality in ICU Patients With Sepsis. Biol Res Nurs 2019;21:237-44. [Crossref] [PubMed]
- Wiedermann CJ. Moderator Effect of Hypoalbuminemia in Volume Resuscitation and Plasma Expansion with Intravenous Albumin Solution. Int J Mol Sci 2022;23:14175. [Crossref] [PubMed]


