
Prognosis of immunotherapy for non-small cell lung cancer with CDKN2A loss of function
Highlight box
Key findings
• Immunotherapy functions less effectively in cyclin-dependent kinase inhibitor 2A (CDKN2A) loss of function (LOF) non-small cell lung cancer (NSCLC) patients than CDKN2A wild-type NSCLC patients. However, it also works quite effectively in selective CDKN2A LOF patients.
What is known and what is new?
• Immunotherapy only functions in about 20% of NSCLC patients. As one of most frequent mutation genes, CDKN2A LOF plays a controversial role in the effect of immunotherapy in NSCLC.
• Immunotherapy could work effectively in CDKN2A LOF patients.
What is the implication, and what should change now?
• Both CDKN2A LOF and wild-type NSCLC patients should also be prescribed immune checkpoint inhibitors.
Introduction
Non-small cell lung cancer (NSCLC), which mainly includes adenocarcinoma (ADC) and squamous cell carcinoma (SCC), accounts for about 75–80% of all lung cancers, and is the most deadly malignancy worldwide (1). The wide usage of tyrosine kinase inhibitors (TKIs) has greatly improved the prognosis of patients with driving gene mutations, such as epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) mutations; however, the rest of NSCLC patients are driving gene negative, which means that more than half of ADC and most SCC patients cannot benefit from targeted TKIs (2).
Fortunately, for patients without a driving gene mutation, immune checkpoint inhibitors (ICIs), such as the programmed death-1 (PD-1) or its ligand (PD-L1) inhibitor, offer hope. However, research has shown that immunotherapy is only effective in 20% of such patients, which narrows down the prescription of PD-1 (or PD-L1) greatly (3). It is often suggested that PD-L1 expression level, microsatellite instability status, and high tumor mutation burden (TMB-H) can be used to predict the effect of immunotherapy in NSCLC patients; however, research should be conducted to identify the other underlying mechanisms to determine why some patients are sensitive to immunotherapy, while others are not sensitive and are even resistant to immunotherapy.
Cyclin-dependent kinase inhibitor 2A (CDKN2A) is an important tumor suppressor gene. Its loss of function (LOF), which mainly includes a gene mutation and loss of copy number (LCN), is quite common in lung ADC (4) (occurring in about 8% of patients) and SCC (occurring in about 22% of patients) (5). CDKN2A can transcribe and then translate into two proteins (i.e., p16INK4A and p14ARF), of which the former is predominant. Both of these two proteins function in the cell cycle and can cause cell-cycle arrest or cell senescence (6). It appears likely that CDKN2A LOF is detrimental to immunotherapy, as many pre-clinical studies suggest that cell-cycle dysregulation in tumor cells promotes immune evasion (7,8). However, due to controversial results in NSCLC patients, debates continue, as research has shown that while sometimes CDKN2A LOF does not counteract the effect of immunotherapy, other times, it is harmful to immunotherapy (9,10).
In this study, we collected the data of CDKN2A LOF and CDKN2A wild-type NSCLC consecutive patients treated by any line of immunotherapy at our Tongji hospital. All the patients tested negative for the common driving gene mutations, such as the EGFR or ALK mutations. Using these data, we sought to identify the characteristics of CDKN2A LOF in NSCLC and determine the effect of CDKN2A LOF on immunotherapy. We present this article in accordance with the REMARK reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1017/rc).
Methods
Patients
All the patients were retrospectively recruited for this study from Tongji Hospital. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Tongji Hospital affiliated with Tongji Medical College of Huazhong University of Science and Technology (No. TJ-IRB20231236). Written informed consent for the use of all clinical data were provided by patients or their direct relatives. Specifically, we consecutively collected the data of CDKN2A LOF NSCLC patients and CDKN2A wild-type NSCLC patients from February 2018 to February 2022. These patients were all stage IV before the commencement of the initial treatment. All the patients received any line of immunotherapy, with or without chemotherapy, radiotherapy, or targeted therapy.
Pathologic confirmation
Pathologic specimens of the included patients were collected either by surgery or core-needle biopsy, and were then stored at the Pathologic Department of Tongji Hospital waiting further analysis. Hematoxylin-eosin staining was performed to verify the diagnosis of lung cancer, and if possible, subtypes of lung cancer (e.g., lung ADC or SCC) were confirmed. Additionally, immunohistochemical (IHC) and immunofluorescent (IF) staining of p16INK4A (Abcam, Shanghai, China) was also performed. Specifically, for the IHC staining, three sections per patient were stained with a mouse anti-p16INK4A monoclonal antibody (dilution: 1:400) using a standardized procedure detailed in the instructions of Abcam website. For the IF staining, three sections per patient were stained with same mouse anti-p16INK4A monoclonal antibody (dilution: 1:100) following the instructions provided as the same above.
DNA extraction and sequencing
Genomic DNA samples were subjected to whole exon sequencing according to standard protocols, and this sequencing was performed by the Novogene Corporation (Beijing, China) using the Agilent SureSelectV6 Human All Exon Kit; the sequencing was performed using Illumina HiSeq-nova instruments.
Statistical analysis
STATA (version 12.0) was used to analyze all the data in this study. An analysis of variance and chi-squared (χ2) test were carried out to compare different clinical features among different groups based on a two-way statistical analysis. Progression-free survival (PFS) and overall survival (OS) were assessed using Kaplan-Meier curves. A P value ≤0.05 was considered statistically significant.
Results
Basic information of NSCLC patients treated by immunotherapy
From February 2018 to February 2022, we collected the data of 49 CDKN2A LOF NSCLC patients undergoing any line of ICI, of whom 18 had mutations and 31 had LCNs. The main mutation types were pR80STOP (8/18), pW15STOP (4/18), and pG136D (2/18) (Figure 1). Most mutations occurred in exon 2 of the CDKN2A gene. The LCN coefficients ranged from 0.10–0.70 (mean: 0.49). We also collected the data of 173 CDKN2A wild-type NSCLC patients treated with ICIs. The basic information of all the patients is set out in Table 1. In general, all the patients including those with CDKN2A LOF NSCLC and CDKN2A wild-type NSCLC, were stage IV at the time of the initial treatment. In terms of their pathologic histology, most patients had ADC, and less than 20% had SCC. Most of these patients were prescribed ICIs as their first-line treatment concurrently, with or without chemotherapy, or targeted therapy, such as anti-angiogenic therapy. The ICI regimens included pembrolizumab, nivolumab, sintilimab, toripalimab, tislelizumab, and camrelizumab. Notably, no patient had more than 50% PD-L1 expression in the CDKN2A LOF group, and only 4 of the 173 patients (2%) had more than 50% PD-L1 expression in the CDKN2A wild-type group. However, 10% (5/49) of the patients in the CDKN2A LOF group had a high tumor mutation burden (TMB-H), and the ratio was the same in the wild-type group.

Table 1
| Characteristics | CDKN2A LOF (n=49) | CDKN2A wild type (n=173) | P value |
|---|---|---|---|
| Age (years) | 62 [41–69] | 64 [39–73] | 0.71 |
| Gender | 0.41 | ||
| Male | 31 | 98 | |
| Female | 18 | 75 | |
| Histology | 0.29 | ||
| ADC | 42 | 139 | |
| SCC | 6 | 31 | |
| Other | 1 | 3 | |
| ECOG before ICI | 0.65 | ||
| >2 | 4 | 11 | |
| ≤2 | 45 | 162 | |
| Smoking history | 29 | 93 | 0.50 |
| Combined therapy | 47 | 169 | 0.50 |
| TMB-H | 5 | 19 | 0.88 |
| PD-L1 | 0.35 | ||
| ≥50% | 0 | 4 | |
| ≥1%, <50% | 15 | 39 | |
| <1% | 34 | 130 | |
| First-line ICI | 38 | 142 | 0.47 |
CDKN2A, cyclin-dependent kinase inhibitor 2A; LOF, loss of function; ADC, adenocarcinoma; SCC, squamous cell carcinoma; ECOG, Eastern Cooperative Oncology Group; ICI, immune check inhibitor; TMB-H, high tumor mutation burden; PD-L1, programmed death ligand 1.
Effects of ICI in NSCLC patients with CDKN2A LOF
In our 49 CDKN2A LOF NSCLC patients, there are 18 mutations and 31 LCNs. First, we tested p16INK4A expression, which is the predominant transcription and translation protein of the CDKN2A gene, in all the patients (Figure 2A). As expected, the IHC and IF staining results showed that most CDKN2A LOF patients (36/49) were p16INK4A negative. Conversely, most CDKN2A wild-type patients (129/173) were p16INK4A positive (P<0.001) (Figure 2B). Additionally, p16INK4A was expressed in both the cytoplasm and nucleus in the CDKN2A wild-type group.
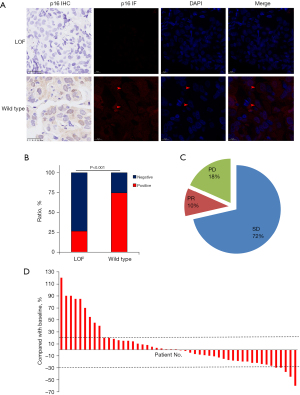
We then examined the effects of immunotherapy in the CDKN2A LOF group (Figure 2C). In total, five patients achieved partial response (PR), 35 achieved stable disease (SD), and nine had progressive disease (PD) after the first evaluation after ICI therapy (Figure 2D). Specifically, in one SCC patient with both CDKN2A pR80STOP and TMB-H, ICI worked effectively for 3 years as a second-line therapy after first-line concurrent radio-chemotherapy. Additionally, one patient was prescribed ICI therapy combined with a cyclin-dependent kinase 4/6 (CDK4/6) inhibitor as a second-line therapy, which worked effectively for about 10 months. However, there were also nine PD patients after 2 to 3 months of first-line ICI therapy, one of whom had super PD and died shortly into the 3rd month after receiving the first dose of ICI. Taken together, though these results indicated that CDKN2A might promote PD when the patients are treated with immunotherapy, CDKN2A might also work effectively in selective NSCLC patients treated with ICI.
Clinical outcomes of CDKN2A LOF compared to CDKN2A wild type in NSCLC patients treated with ICI
We also sought to examine the effects of ICI therapy in the CDKN2A LOF group compared to the CDKN2A wild-type group. Using the Kaplan-Meier method, we determined that the median PFS of the LOF group was 4.67 months, while that of the wild-type group was 8.63 months [hazard ratio (HR): 0.54; 95% confidence interval (CI): 0.38–0.77; P<0.001] (Figure 3A). Meanwhile, CDKN2A LOF also had a negative effect on OS (Figure 3B). The median OS of the CDKN2A LOF group was 9.07 months and that of the CDKN2A wild-type group LOF was 21.37 months (HR: 0.42; 95% CI: 0.29–0.61; P<0.001). We also conducted a subgroup analysis to compare the CDKN2A mutation to LCN in the CDKN2A LOF group (Figure 3C,3D). As expected, there was no statistically significant difference in terms of PFS and OS between these two subgroups, which indicates that both the CDKN2A LCN and mutation may exert the same function in immunotherapy in NSCLC.

Discussion
In our study, CDKN2A LOF was common in the NSCLC patients, and the CDKN2A LOF patients had a worse prognosis than the CDKN2A wild-type patients treated by ICI mono-therapy or combined therapy. However, it is still difficult to determine whether or not patients with CDKN2A LOF are suitable for ICI, as the results also suggested that some selective patients in the LOF group could have PR or achieve SD following ICI treatment.
CDKN2A is a well-known tumor suppressor gene, and it is a very common alteration, second only to that of tumor suppressor protein 53 (P53) in all cancers (6). CDKN2A LOF is associated with most common tumors, including breast cancer, colorectal cancer, and melanoma (11-13). It has been reported that the CDKN2A germline mutation could work as a driving gene in melanoma (such as pM53I and pS56I) (14), and breast cancer (such as pA148T) (15). CDKN2A LOF is also one of the most frequent alterations in NSCLC. CDKN2A LOF has been reported in up to 10% of ADC patients and more than 20% of SCC patients (4,5). In patients with the EGFR mutation, CDKN2A LOF could also work as a secondary resistance mechanism to TKIs (16). However, until now, there was no evidence to suggest that CDKN2A could work as a driving gene in NSCLC, especially in ADC. Interestingly, CDKN2A LOF was shown to drive small cell lung cancer tumorigenesis in a mouse model (17).
CDKN2A can transcribe and then translate to both p16INK4A and p14ARF; the former is predominant, mainly works in the G1/S phase, and inhibits CDK4/6 and then causes cell cycle arrest (6). CDK4/6 inhibitors, such as palbociclib and abemaciclib, have been widely and successfully used to treat hormone positive late-stage breast cancer patients. Many clinical trials have used CDK4/6 inhibitors in NSCLC patients with CDKN2A LOF. In a phase II clinical trial, 29 patients with CDKN2A LOF received palbociclib; these patients had a medium PFS of 8.1 weeks, and one achieved a PR and six achieved SD (18). However, the CDK4/6 inhibitor single regimen has only been shown to have limited effects in treating NSCLC. It has been reported that CDK4/6 inhibitors up-regulate PD-L1 expression and promote CD8+ T cell memory formation (19). A clinical trial is being conducted that combines ICI therapy with the CDK4/6 inhibitor (20) (NCT02079636); however, the results of that study have yet to be published. In one of our patients, the single CDK4/6 inhibitor did not work initially, but later worked effectively when combined with ICI therapy for about 10 months.
Due to the results to date, controversy remains as to whether ICI should be prescribed to patients with CDKN2A LOF. In one study, ICI therapy improved the prognosis of melanoma patients with the germline or somatic CDKN2A mutation, compared to those with CDKN2A wild-type melanoma, with 6 out of the 19 patients achieving a complete response (21). However, a study by the American Society of Clinical Oncology in 2019, which included only 20 patients, reported that CDKN2A LOF could act as a potential molecular signature for hyper-PD in advanced NSCLC, meaning that CDKN2A could directly cause super PD (22). However, no additional data have been collected to confirm this conclusion.
In our CDKN2A LOF patients, 40 of 49 achieved disease control by immunotherapy (five achieved a PR and 35 achieved SD); however, 9 of the 49 patients had PD, and one patient had super PD. However, this evidence does not directly prove that CDKN2A directly causes PD or even super PD. Another study reported that CDKN2A LOF is one of the reasons NSCLC patients become resistant to ICI (10). However, in that study, the authors only compared CDKN2A LOF to CDKN2A wild-type patients undergoing ICI treatment, and the authors did not directly compare the usage of ICI with no ICI inside CDKN2A LOF patients. A cohort study of six kinds of solid tumors reported that CDKN2A LOF had no relationship with the effectiveness of ICI therapy in NSCLC (9). In our study, ICI therapy achieved a better effect in the CDKN2A wild-type group than the LOF group; however, ICI therapy also worked effectively in the LOF group, with most of the patients achieving disease control at the time of their first evaluation of ICI. Based on our data, it is difficult to determine whether CDKN2A is a resistance mechanism or even promotes super PD in patients receiving ICI treatments.
This study had some limitations. First, it lacked a head-to-head comparison of treatment with ICI or no ICI inside CDKN2A LOF patients. This is the direct evidence to show the effect of ICI on CDKN2A LOF patients. Second, because some patients achieved a PR or SD following ICI therapy in the CDKN2A LOF group, it is necessary to identify which patients are suitable for ICI among the CDKN2A LOF patients. Third, while our data demonstrates that the CDKN2A mutation functions the same as the CDKN2A LCN, our study only collected the data of 18 CDKN2A mutations, which limits the interpretation of our entire results.
Conclusions
This study demonstrated that ICIs function less effectively in CDKN2A LOF than CDKN2A wild-type NSCLC patients. However, it is still difficult to determine whether or not patients with CDKN2A LOF are suitable for ICI, as our findings also suggest that ICI could work quite effectively in selective CDKN2A LOF patients.
Acknowledgments
We thank all the patients mentioned in this manuscript. We also thank every person who helped us accomplish this paper.
Funding: The study was supported by
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1017/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1017/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1017/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1017/coif). Y.J. reports funding by the Natural Science Foundation of Hubei Province, China (No. 2021CFB407). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Tongji Hospital affiliated with Tongji Medical College of Huazhong University of Science and Technology (No. TJ-IRB20231236). Written informed consent for the use of all clinical data was provided by patients or their direct relatives.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Cooper AJ, Sequist LV, Lin JJ. Third-generation EGFR and ALK inhibitors: mechanisms of resistance and management. Nat Rev Clin Oncol 2022;19:499-514. [Crossref] [PubMed]
- Reck M, Remon J, Hellmann MD. First-Line Immunotherapy for Non-Small-Cell Lung Cancer. J Clin Oncol 2022;40:586-97. [Crossref] [PubMed]
- Zhang B, Zhang L, Yue D, et al. Genomic characteristics in Chinese non-small cell lung cancer patients and its value in prediction of postoperative prognosis. Transl Lung Cancer Res 2020;9:1187-201. [Crossref] [PubMed]
- Satpathy S, Krug K, Jean Beltran PM, et al. A proteogenomic portrait of lung squamous cell carcinoma. Cell 2021;184:4348-4371.e40. [Crossref] [PubMed]
- Farooq U, Notani D. Transcriptional regulation of INK4/ARF locus by cis and trans mechanisms. Front Cell Dev Biol 2022;10:948351. [Crossref] [PubMed]
- Galvani E, Mundra PA, Valpione S, et al. Stroma remodeling and reduced cell division define durable response to PD-1 blockade in melanoma. Nat Commun 2020;11:853. [Crossref] [PubMed]
- Peng J, Sun BF, Chen CY, et al. Single-cell RNA-seq highlights intra-tumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma. Cell Res 2019;29:725-38. [Crossref] [PubMed]
- Adib E, Nassar AH, Akl EW, et al. CDKN2A Alterations and Response to Immunotherapy in Solid Tumors. Clin Cancer Res 2021;27:4025-35. [Crossref] [PubMed]
- Gutiontov SI, Turchan WT, Spurr LF, et al. CDKN2A loss-of-function predicts immunotherapy resistance in non-small cell lung cancer. Sci Rep 2021;11:20059. [Crossref] [PubMed]
- Hill VK, Gartner JJ, Samuels Y, et al. The genetics of melanoma: recent advances. Annu Rev Genomics Hum Genet 2013;14:257-79. [Crossref] [PubMed]
- Saponara M, Urbini M, Astolfi A, et al. Molecular characterization of metastatic exon 11 mutant gastrointestinal stromal tumors (GIST) beyond KIT/PDGFRα genotype evaluated by next generation sequencing (NGS). Oncotarget 2015;6:42243-57. [Crossref] [PubMed]
- Wang L, Tang L, Xie R, et al. p16 promoter hypermethylation is associated with increased breast cancer risk. Mol Med Rep 2012;6:904-8. [Crossref] [PubMed]
- Goldstein AM, Chan M, Harland M, et al. Features associated with germline CDKN2A mutations: a GenoMEL study of melanoma-prone families from three continents. J Med Genet 2007;44:99-106. [Crossref] [PubMed]
- Debniak T, Górski B, Huzarski T, et al. A common variant of CDKN2A (p16) predisposes to breast cancer. J Med Genet 2005;42:763-5. [Crossref] [PubMed]
- Zhang YC, Chen ZH, Zhang XC, et al. Analysis of resistance mechanisms to abivertinib, a third-generation EGFR tyrosine kinase inhibitor, in patients with EGFR T790M-positive non-small cell lung cancer from a phase I trial. EBioMedicine 2019;43:180-7. [Crossref] [PubMed]
- Hamad SH, Montgomery SA, Simon JM, et al. TP53, CDKN2A/P16, and NFE2L2/NRF2 regulate the incidence of pure- and combined-small cell lung cancer in mice. Oncogene 2022;41:3423-32. [Crossref] [PubMed]
- Ahn ER, Mangat PK, Garrett-Mayer E, et al. Palbociclib in Patients With Non-Small-Cell Lung Cancer With CDKN2A Alterations: Results From the Targeted Agent and Profiling Utilization Registry Study. JCO Precis Oncol 2020;4:757-66. [Crossref] [PubMed]
- Heckler M, Ali LR, Clancy-Thompson E, et al. Inhibition of CDK4/6 Promotes CD8 T-cell Memory Formation. Cancer Discov 2021;11:2564-81. [Crossref] [PubMed]
- Kim ES, Kelly K, Paz-Ares LG, et al. Abemaciclib in Combination with Single-Agent Options in Patients with Stage IV Non-Small Cell Lung Cancer: A Phase Ib Study. Clin Cancer Res 2018;24:5543-51. [Crossref] [PubMed]
- Helgadottir H, Ghiorzo P, van Doorn R, et al. Efficacy of novel immunotherapy regimens in patients with metastatic melanoma with germline CDKN2A mutations. J Med Genet 2020;57:316-21. [Crossref] [PubMed]
- Giusti R, Mazzotta M, Filetti M, et al. CDKN2A/B gene loss and MDM2 alteration as a potential molecular signature for hyperprogressive disease in advanced NSCLC: A next-generation-sequencing approach. J Clin Oncol 2019;37:e20628.
(English Language Editor: L. Huleatt)


