
Surgical lung biopsy for suspected interstitial lung disease with video-assisted thoracoscopic surgery is safe, providing exact histological and disease specific diagnosis for tailoring treatment
Highlight box
Key findings
• Long-term results from a single center in Iceland performing video-assisted thoracoscopic surgery (VATS) surgical lung biopsy (SLB) in patients with suspected interstitial lung disease (ILD), reflected in low complications rates and short hospital stay. A definitive histological and disease diagnosis was provided in 92.6% of cases.
What is known and what is new?
• Patients diagnosed with ILD often have poor long-term survival; it is therefore vital that they receive prompt diagnoses so that a tailored treatment can be initiated.
• VATS technique is safe and can be used safely to provide a SLB with low complication rates and high diagnostic yield.
What is the implication, and what should change now?
• VATS technique with the use of double lumen intubation can be used safely in patients with suspected ILD where less invasive techniques have been unable to provide a definitive diagnosis so that a tailored treatment can be initiated.
Introduction
Interstitial lung disease (ILD) is a collective term for diffuse and often progressive interstitial diseases that can cause irreversible damage to the lung parenchyma, and where in extreme cases a lung transplantation can be required (1). Over time the lung fibrosis affects oxygen delivery to the bloodstream which most commonly results in dyspnea (2,3). As modern developments in treatment can mitigate symptoms, slow down progression and decrease mortality, it is important that patients are diagnosed early; especially as a delay in diagnosis is attributed to a higher risk of death (4). An exact diagnosis is therefore of key importance for a tailored up-to-date treatment that involves both immunosuppressive agents such as corticosteroids and mycophenolate mofetil, but also antifibrotic agents such as pirfenidone and nintedanib (1,5-7).
Although significant advances have been made in reading patterns of interstitial changes on high-resolution computed tomography (HRCT) scans the structural changes seen can be unspecific, and blood biomarkers [autoimmune, angiotensin converting enzyme (ACE)] are useful but not diagnostic by themselves without evidence of connective tissue disease in the diagnosis of ILD (8). Therefore, in cases where noninvasive techniques have been inconclusive, a histological biopsy is required for a more exact diagnosis (1). Transbronchial biopsy (TBB), using a flexible bronchoscopy can provide adequate histological specimens in 77.6% [95% confidence interval (CI), 74.6–80.3%] of patients where a definitive diagnosis can be obtained and avoiding surgical lung biopsy (SLB) in 36.1% (95% CI, 33.4–38.9%) of patients (2). However, 64% (95% CI, 61–67%) are left without a diagnosis (2). Transbronchial lung cryobiopsy (TBLC) is an alternative to TBB with an estimated diagnostic yield of 80%, but the diagnostic yield can be further increased when three or more histological samples are collected (9,10). After the publication of the American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Asociación Latinoamericana de Tórax collaborative clinical guidelines in 2022, TBLC may be considered as an alternative to SLB since it costs less, is less invasive and provides a similar diagnostic confidence in a multidisciplinary discussion (MDD). It may also be considered more suitable to patients that are unable to tolerate surgery. However, it is only recommended in centers that are experienced with TBLC and have standardized their protocols as postoperative complication rate is higher when compared to SLB, warranting further studies on safety and standardization (2,9-11). Therefore, SLB in those cases were indicated, may still be the best suitable technique for histological sampling of lung parenchyma, providing a diagnostic yield of over 88% (95% CI, 86.9–89.4%) in patients (2,3,12). However, as patients with ILD often have impaired lung function perioperative risk can be increased, especially if conventional thoracotomy is used rather than video-assisted thoracoscopic surgery (VATS); with 30-day mortality being 4.3% and 2.1%, respectively (13). The aim of this study was to study the yield of VATS lung biopsies for ILD, clinical indications, and outcome, in a population-based cohort of patients. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1107/rc).
Methods
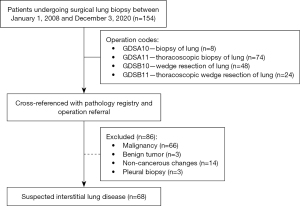
This retrospective nationwide study included all patients that underwent diagnostic SLB for suspected ILD in Iceland between January 1, 2008 and December 3, 2020. Patients were identified using a centralized Operation Registry at Landspitali University Hospital; the single institution performing cardiothoracic surgery in Iceland (370,000 inhabitants in 2020). Patient list was generated from the following operative codes and then evaluated: biopsy of lung (GDSA10, n=8), thoracoscopic biopsy of lung (GDSA11, n=74), wedge resection of lung (GDSB10, n=48) and thoracoscopic wedge resection of lung (GDSB11, n=24). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Icelandic National Bioethics Committee (VSN 14-111-V1-S2). As individuals were not identified in the study, individual consent was waived.
All patient data were reviewed by at least three of the authors (L.T., G.G., T.G.). A total of 154 patients underwent SLB during the observation period. Furthermore, we cross-referenced all surgical patients (n=154) with the operation referral and also with the centralized Pathology Registry at Landspitali University Hospital which is the tertiary referral center for lung diseases in Iceland, in order to identify those patients with suspected ILD diagnosis. Patients that did not meet the operation indication for suspected ILD such as by having a primary lung cancer, metastasis and other non-cancerous findings were 86, they were subsequently excluded from the study and not evaluated further; leaving 68 patients for further analysis as shown in Figure 1.
Clinical data was collected in a retrospective manner by surveying both hospital- and private offices records by using a standardized data sheet. Clinical data containing percentages were recorded with one decimal while reported data from other studies was left unformatted. When available the following information was recorded; age, sex, year and month of diagnosis, clinical symptoms and comorbidities, duration of symptoms, smoking history, and if TBB or bronchoalveolar lavage (BAL) had been obtained. Newest results of spirometry and diffusing capacity for carbon monoxide (DLCO) was always recorded if available. Finally, the preoperative diagnosis or primary differential diagnosis was recorded, together with a subsequent postoperative primary diagnosis. MDD was conducted in an informal way in the beginning of the study period, therefore MDD registration was limited and could not be evaluated as we would have liked to.
Imaging studies were reviewed prior to the operation by the responsible surgeon and the best site suitable for biopsy selected if not indicated by MDD. Usually, the most inflamed part of the lung was chosen rather than the most fibrotic part, and if both sides were similar the right side was usually chosen as it had two fissures, making the biopsy technically easier. There was a consensus of avoiding the lingula if possible. Intraoperative assessment of the selected lung and lobe was then made which aided in the final biopsy site. All surgical biopsies were performed using a 3 port VATS-technique under general anesthesia using a double-lumen single lung ventilation. All patients were either placed in right or left lateral recumbent position depending on the selected lung for biopsy. A standard surgical stapler (Endo GIA, Medtronic, Minneapolis, MN, USA) with regular purple staples was used. Length- and the number of staples used were not registered in the study. Operative time (skin-to-skin) was registered in minutes.
Patient morbidity was registered as intra- and postoperative complications collected from patient charts and surgical reports. The time which the patient had a chest tube was registered in whole days, and if it was removed at the day of surgery it was registered as 0. Prolonged air leakage was defined as more than 4 days (>96 hours) and excessive bleeding intraoperatively more than 100 mL. Length of stay was recorded in days. Operative mortality was defined as death occurring within 30- and 90-days after surgery while hospital mortality was defined as death occurring within hospital stay. Overall survival was registered by comparing every patient at the end of the observation period of December 3rd, 2020 with the Icelandic Death Registry.
A radiologist (A.B.H.) reviewed all preoperative chest radiographs and CT imaging studies the patients had undergone. The findings of both the chest radiographs and CT were recorded on a standardized sheet (see Table S1). The date of study was recorded. Location of central or peripheral parenchymal and structural changes within the lung and different lobes were recorded. We then compared those changes and differences (localized, diffuse, interstitial changes in both lungs) between both lung sides and lobes. Specific findings of lung abnormalities are listed on Table S1 in the supplementary material, for instance, traction bronchiectasis, honeycombing, fibrosis and reticular changes. Signs of fibrosis independent of bronchiectasis and honeycombing was defined as reticular pattern with or without ground-glass opacities and architectural distortion. Reticular changes were defined as everything from small subtle reticulations to coarse reticular pattern. Variables for lung abnormalities on HRCT in this study were chosen based on known findings and patterns in ILDs (2).
All surgical specimens were analyzed by an attending pathologist at the Department of Pathology at Landspitali and later reviewed by one senior pulmonary pathologist (H.J.I.), using the Systematized Nomenclature of Medicine (SNOMED) coding system. The study recorded the surgical specimens on a standardized sheet (see Table S2) which included SNOMED coding, sample size and if the size was ≥2 cm × 2 cm, and if one or two or more different lobes had been biopsied. The final pathological evaluation was then recorded.
Finally, the two most common histology groups usual interstitial pneumonia (UIP) and nonspecific interstitial pneumonia (NSIP) were compared with each other by comparing demographics, lung function tests, bronchoscopy, operative time, intra-and postoperative complications, survival, and length of stay.
Statistical analysis
For data collection a databank was constructed and managed in REDCap, version 11.1.24 (Research Electronic Data Capture – 2021, Vanderbilt University, USA). All clinical data was then subsequently recorded and stored in the REDcap databank. Fisher’s exact test was used to compare categorical variables and Mann-Whitney U test to compare continuous variables. Mortality was compared between histological groups using Kaplan-Meier curves and Cox regression. All statistical tests were two-tailed and a P value less than 0.05 was considered significant. Statistical analysis was performed using R software version 2023.06.2+561 (R Foundation for Statistical Computing, Vienna, Austria).
End of follow-up was December 3rd, 2021, with mean follow-up 61.3 months (range, 3–155 months). No patients were lost to follow-up.
Results
During the 13-year study period a total of 68 patients underwent SLB for diffuse lung disease, or on average 5.2 patients annually; ranging between 9 operations in 2014 to one in the years 2010 and 2012. The location of the SLBs is shown in Table 1; with all samples being larger than 2 cm × 2 cm in size and within the range of recommended sample size (14). Right superior lobe (29.6%) was the most common lobe selected for biopsy, followed by the right inferior lobe and middle lobe. A single biopsy was performed in 51 (75%) of cases and two or more biopsies were either obtained from the same lobe in 5 cases (7.4%) or different lobes in 12 cases (17.7%) as is shown in Table 2. Ninety-seven biopsies were taken in total.
Table 1
| Location | Values (n=81), n (%) |
|---|---|
| Right superior lobe | 24 (29.6) |
| Right medial lobe | 18 (22.2) |
| Right inferior lobe | 21 (25.9) |
| Left superior lobe‡ | 13 (16.0) |
| Left inferior lobe | 5 (6.2) |
†, the table shows the distribution of different locations selected for biopsy but does not take into account if multiple biopsies were taken from the same lobe. ‡, includes both biopsies from superior lobe and lingula.
Table 2
| Characteristics | Values (n=68), n (%) |
|---|---|
| Single biopsy, same lobe | 51 (75.0) |
| Two biopsies, different lobes | 8 (11.8) |
| Two biopsies, same lobe | 5 (7.4) |
| >2 biopsies, different lobes (range, 3 to 10) | 4 (5.9) |
The table shows if one or more biopsies were taken from single or multiple lobes during surgery.
Patient demographics are shown in Table 3. The average age of the patients was 55.6±15.1 years (range, 13–76 years) with 40 (58.8%) of them being males. Thirty-eight (55.9%) of patients had history of smoking with 34 (50.0%) having a smoking cessation of >12 months while 5 (7.4%) patients were still actively smoking at the time of surgery. Pack years were registered for 49 (72.1%) with a mean of 12.8±16.4 years. Most patients had been symptomatic for more than 1 year before the operation. Comorbidities were common with hypertension, rheumatic disease and ischemic heart disease being the most common.
Table 3
| Characteristics | Values (n=68) |
|---|---|
| Gender (male), n (%) | 40 (58.8) |
| Age (years), mean ± SD | 55.6±15.1 |
| Smoking | |
| Non-smoker, n (%) | 23 (33.8) |
| Ex-smoker >12 months, n (%) | 34 (50.0) |
| Ex-smoker but within 12 months†, n (%) | 4 (5.9) |
| Active smoker‡, n (%) | 5 (7.4) |
| Pack years, n (%), mean ± SD | 49 (72.1), 12.8±16.4 |
| Comorbidities, n (%) | |
| Ischemic heart disease | 13 (19.1) |
| Hypertension | 29 (42.6) |
| Diabetes | 7 (10.3) |
| COPD | 5 (7.4) |
| History of malignancy | 7 (10.3) |
| Rheumatic disease | 23 (33.8) |
| Duration of symptoms, n (%) | |
| Not known | 2 (2.9) |
| 0–3 months | 16 (23.5) |
| 6–12 months | 13 (19.1) |
| >12 months | 37 (54.4) |
| Spirometry | 58 (85.3) |
| FVC (L) with % (pre/pred), n (%), mean ± SD |
57 (83.8), 3.0±0.9/73.0±17.8 |
| FEV1 (L) with % (pre/pred), n (%), mean ± SD |
58 (85.3), 2.3±0.7/71.6±18.7 |
| DLCO, n (%) | 45 (66.2) |
| DLCO percentage of estimate, mean ± SD |
50.0±11.2 |
| Bronchoscopy, n (%) | 41 (60.3) |
| Biopsy and lavage | 39 (57.4) |
| Biopsy only | 1 (1.5) |
| Lavage only | 1 (1.5) |
| Histopathology, non-specific inflammation | 21 (30.9) |
Epidemiology and spirometry values of 68 patients undergoing surgical lung biopsies. †, greater than >28 days but <12 months. ‡, still smoking but not >28 days. SD, standard deviation; COPD, chronic obstructive pulmonary disease; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; DLCO, diffusing capacity for carbon monoxide.
The most common presenting symptoms was dyspnea 57 (83.8%), followed by coughing 47 (69.1%), malaise 25 (36.8%), mucus production was seen in 24 (35.3%) patients, with fever being seen in 12 (17.6%) and 11 (16.2%) had unintentional weight loss.
Flexible bronchoscopy was performed preoperatively as shown in Table 3. It included TBB in 41 (60.3%) patients, and BAL in 39 (57.4%) patients. In half of the TBB and BAL-samples that were sent for histological analysis a non-specific inflammation was seen in 21 (30.9%) with the other half of the samples being normal in 5 (7.4%) cases, 5 (7.4%) had plausible cryptogenic organizing pneumonia (COP) changes, while the rest of the samples either showed without any diagnosis, chronic inflammatory changes with or without interstitial changes. Whereas samples sent for bacterial culture were only positive in 1 (1.5%) out of 39 cases for pathological bacteria (Staphylococcus aureus), 27 (39.7%) patients had normal upper respiratory flora with the rest having either very few bacterial colonies or a negative bacterial culture.
Spirometry was obtained in 58 (85.3%) patients with mean forced vital capacity (FVC) being 3.0±0.9 L and 73.0%±17.8% of predicted value. Similar values were registered for forced expiratory volume in 1 second (FEV1) 2.3±0.7 L and 71.6%±18.7% of predicted, respectively. DLCO was within normal limits in 2 (2.9%) patients but lowered in 43 (63.2%) patients, with a mean 50.0%±11.2% of the predicted value, it was not performed in 23 (33.8%) of the patients.
All the patients had a conventional chest radiograph performed preoperatively and in all but one case a chest CT was done. Table 4 shows the most common radiographic changes seen on chest radiographs, scattered reticular changes were seen in 51 (75%) patients with 45 (66.2%) having interstitial lung changes. Reticular changes were seen in 58 (85.3%) patients on CT, they were also the most common changes seen, followed by ground class changes in 45 (66.2%) and fibrosis in 34 (50%).
Table 4
| Characteristics | Value, n (%) |
|---|---|
| Chest radiography | 68 (100.0) |
| Normal | 4 (5.9) |
| Scattered reticular changes | 51 (75.0) |
| Interstitial lung changes | 45 (66.2) |
| Patchy bilateral airspace opacities | 16 (23.5) |
| Focal opacity | 7 (10.3) |
| Pleural effusion | 7 (10.3) |
| Scattered nodular changes | 3 (4.4) |
| Unilateral diffuse airspace opacities | 2 (2.9) |
| Other | 3 (4.4) |
| Chest CT | 67 (98.5) |
| Reticular changes | 58 (85.3) |
| Ground glass changes | 45 (66.2) |
| Fibrosis | 34 (50.0) |
| Enlarged lymph nodes | 30 (44.1) |
| Nodules | 27 (39.7) |
| Multiple nodules | 25 (36.8) |
| Single nodule | 2 (2.9) |
| Bronchiectasis | 30 (44.1) |
| Traction bronchiectasis | 24 (35.3) |
| Honeycombing | 23 (33.8) |
| Peripheral consolidations | 15 (22.1) |
| Emphysema | 11 (16.2) |
| Pulmonary cysts | 9 (13.2) |
| Pleural effusion | 8 (11.8) |
| Other | 2 (2.9) |
The table shows the number of patients that underwent either chest radiography or CT prior to surgery. It also reports the different changes seen on both imaging studies. CT, computed tomography.
Table 5 shows the postoperative diagnosis obtained with SLB. In 63 out of 68 patients (92.6%) a definite diagnosis was obtained. However, in 2 (2.9%) cases tissue samples were non-diagnostic, while in 3 (4.4%) patients they were suspected of having ILD but a histopathological diagnosis could not be obtained. These 5 cases were therefore subsequently classified together as non-classifiable cases (5/68, 7.4%). NSIP (29.4%) and UIP (23.5%) were the most common postoperative diagnoses, followed by hypersensitivity pneumonitis (HP) (7.4%) and pulmonary sarcoidosis (7.4%).
Table 5
| Characteristics | Values (n=68), n (%) |
|---|---|
| Idiopathic interstitial pneumonia | |
| NSIP | 20 (29.4) |
| Idiopathic pulmonary fibrosis | |
| UIP | 16 (23.5) |
| COP | 4 (5.9) |
| PPFE | 1 (1.5) |
| Granulomatous | |
| HP | 5 (7.4) |
| Sarcoidosis | 5 (7.4) |
| Autoimmune related | |
| Collagen vascular disease | 1 (1.5) |
| Graft vs. host interstitial lung fibrosis | 1 (1.5) |
| IPAF | 1 (1.5) |
| SOP with associated RA | 1 (1.5) |
| Vasculitis | 1 (1.5) |
| Various diagnosis | |
| Asbestosis | 1 (1.5) |
| Bronchiolitis obliterans | 1 (1.5) |
| Drug-induced | |
| Nitrofurantoin induced interstitial lung disease | 1 (1.5) |
| Follicular bronchiolitis | 1 (1.5) |
| Giant cell interstitial pneumonitis | 1 (1.5) |
| Respiratory bronchiolitis | 1 (1.5) |
| Non-classifiable | 5 (7.4) |
| Non-interstitial diagnosis | |
| Emphysema | 1 (1.5) |
ILD, interstitial lung disease; NSIP, nonspecific interstitial pneumonia; UIP, usual interstitial pneumonia; COP, cryptogenic organizing pneumonia; PPFE, pleuroparenchymal fibroelastosis; HP, hypersensitivity pneumonitis; IPAF, interstitial pneumonitis with autoimmune features; SOP with associated RA, secondary organizing pneumonia with associated rheumatoid arthritis.
Operative data and peri- and postoperative complications are shown in Table 6. Mean operative time was 40±17 minutes (range, 11–109 minutes). Intraoperative complications were seen in 1 case (1.5%) and included perforation of the diaphragm with subsequent laceration of the liver after trocar placement, the liver laceration was observed and required no major surgical intervention or admission to the ICU. Major postoperative complications were only seen in 1 patient (1.5%) which had excessive postoperative bleeding from the trocar insertion site, requiring a reoperation with subsequent mechanical ventilation and ICU stay of 4 days. No patient developed acute exacerbation of interstitial lung disease (AE-ILD). One patient that had biopsies taken from right superior- and inferior lobe had prolonged air-leakage. No other complications were registered for those that had multiple biopsies taken. Minor postoperative complications were seen in 7 (10.3%) patients, with the most common being pneumonia seen in 4 (5.9%) patients followed by 3 (4.4%) having a prolonged air leakage of more than 4 days (>96 hours). Total registered postoperative complications were 10 (14.8%) for 8 (11.8%) patients. Median chest tube time was 1 [mean 1.8±1.6 days (range, 0–8 days)].
Table 6
| Characteristics | Values (n=68) |
|---|---|
| Type of operation, n (%) | |
| VATS | 66 (97.1) |
| Conversion to open mini-thoracotomy | 2 (2.9) |
| Operative time (minutes), mean ± SD | 40±17 |
| Intraoperative complications, n (%) | 1 (1.5) |
| Liver laceration from trocar | 1 (1.5) |
| Postoperative complications, n (%) | 8 (11.8) |
| Pneumonia | 4 (5.9) |
| Prolonged air leakage >4 days (>96 hours) | 3 (4.4) |
| Need for mechanical ventilation | 1 (1.5) |
| Excessive bleeding | 1 (1.5) |
| Pneumothorax requiring a new chest tube | 1 (1.5) |
| AE-ILD | 0 (0.0) |
| Operative mortality, n (%) | |
| Hospital mortality | 0 (0.0) |
| 30 days | 0 (0.0) |
| 90 days | 0 (0.0) |
| Length of stay (days) | |
| Median [range] | 2 [1–13] |
| Mean ± SD | 2.8±2.5 |
| Chest tube duration (days) | |
| Median [range] | 1 [0–8] |
| Mean ± SD | 1.8±1.6 |
| Follow-up time (months) | |
| Median [range] | 54.5 [3–155] |
| Mean ± SD | 61.3±41.3 |
VATS, video-assisted thoracoscopic surgery; SD, standard deviation; AE-ILD, acute exacerbation of interstitial lung disease.
The median length of postoperative stay was 2 days [mean 2.8±2.5 days (range, 1–13 days)]. No patient died during hospital stay, at 30- (operative mortality 0%) or 90-day.
A comparison of the two main histology groups; NSIP versus UIP, is shown in Table 7. Patients with NSIP were on average 10 years younger and more often females. Ischemic heart disease, hypertension and rheumatological disease were more commonly seen in the UIP group with ischemic heart disease being statistically significant. Bronchoscopy was only performed preoperatively in 31.3% of the UIP cases compared to 75.0% for NSIP.
Table 7
| Characteristics | NSIP (n=20) | UIP (n=16) | P value |
|---|---|---|---|
| Gender (male), n (%) | 10 (50.0) | 12 (75.0) | 0.18 |
| Age (years), mean ± SD | 56.3±11.5 | 65.9±5.8 | 0.005 |
| Smoking | |||
| None-smoker, n (%) | 6 (30.0) | 4 (25.0) | 1 |
| Ex-smoker >12 months, n (%) | 14 (70.0) | 8 (50.0) | 0.31 |
| Ex-smoker but within 12 months†, n (%) | 0 (0.0) | 3 (18.8) | 0.08 |
| Active smoker‡, n (%) | 0 (0.0) | 0 (0.0) | N/A |
| Pack years, n (%), mean ± SD | 14 (70.0), 10.1±13.0 | 12 (75.0), 23.0±21.0 | 0.16 |
| Duration of symptoms, n (%) | |||
| Not known | 0 (0.0) | 1 (6.3) | 0.44 |
| 0–3 months | 5 (25.0) | 5 (31.3) | 0.72 |
| 6–12 months | 5 (25.0) | 3 (18.8) | 0.71 |
| >12 months | 10 (50.0) | 7 (43.8) | 0.75 |
| Comorbidities, n (%) | |||
| Ischemic heart disease | 2 (10.0) | 7 (43.8) | 0.049 |
| Hypertension | 6 (30.0) | 8 (50.0) | 0.31 |
| Diabetes | 1 (5.0) | 3 (18.8) | 0.30 |
| COPD | 0 (0.0) | 1 (6.3) | 0.44 |
| History of malignancy | 3 (15.0) | 1 (6.3) | 0.61 |
| Rheumatic disease | 6 (30.0) | 7 (43.8) | 0.49 |
| Spirometry | 16 (80.0) | 15 (93.8) | 0.35 |
| FVC (L) with % (pre/pred), mean ± SD | 2.9±0.6/74.0±7.5 | 2.9±0.7/72.5±18.9 | 0.74/0.68 |
| FEV1 (L) with % (pre/pred), mean ± SD | 2.3±0.5/75.2±6.0 | 2.4±0.6/77.4±18.0 | 0.71/0.72 |
| DLCO, n (%) | 16 (80.0) | 10 (62.5) | 0.29 |
| DLCO percentage of estimate, mean ± SD | 50.7±11.2 | 49.5±12.4 | 0.81 |
| Bronchoscopy, n (%) | 15 (75.0) | 5 (31.3) | 0.02 |
| Not done | 5 (25.0) | 11 (68.8) | 0.02 |
| Biopsy and lavage | 15 (75.0) | 4 (25.0) | 0.006 |
| Biopsy only | 0 (0.0) | 1 (6.3) | 0.44 |
| Lavage only | 0 (0.0) | 0 (0.0) | N/A |
| Histopathology, non-specific inflammation | 8 (40.0) | 2 (12.5) | 0.13 |
| BAL, culture—normal respiratory flora | 9 (45.0) | 3 (18.8) | 0.16 |
| Operative time (minutes), mean ± SD | 34±12 | 36±11 | 0.77 |
| Intraoperative complications, n (%) | 0 (0.0) | 0 (0.0) | N/A |
| Postoperative complications, n (%) | 2 (10.0) | 1 (6.3) | 1 |
| Pneumonia | 2 (10.0) | 0 (0.0) | 0.49 |
| Excessive bleeding | 1 (5.0) | 0 (0.0) | 1 |
| Need for mechanical ventilation | 1 (5.0) | 0 (0.0) | 1 |
| Reoperation | 1 (5.0) | 0 (0.0) | 1 |
| Pneumothorax requiring a chest tube drainage | 0 (0.0) | 0 (0.0) | N/A |
| Prolonged air leakage >4 days (>96 hours) | 0 (0.0) | 1 (6.3) | 0.44 |
| AE-ILD | 0 (0.0) | 0 (0.0) | N/A |
| Chest tube duration (days) | 0.87 | ||
| Median [range] | 1 [1–8] | 1 [0–6] | |
| Mean ± SD | 1.8±1.6 | 1.8±1.4 | |
| Length of stay (days) | 0.48 | ||
| Median [range] | 2 [1–13] | 2 [1–5] | |
| Mean ± SD | 3.0±2.6 | 2.3±1.3 |
†, greater than >28 days but <12 months. ‡, still smoking but not >28 days. NSIP, nonspecific interstitial pneumonia; UIP, usual interstitial pneumonia; SD, standard deviation; N/A, not applicable; COPD, chronic obstructive pulmonary disease; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; DLCO, diffusing capacity for carbon monoxide; BAL, bronchoalveolar lavage; AE-ILD, acute exacerbation of interstitial lung disease.
As shown in Table 7 postoperative complications were found in two patients with NSIP (10.0%) and in one patient with UIP (6.3%). The median chest tube time was 1 day for both NSIP [mean 1.8±1.6 days (range, 1–8 days)] and UIP [mean 1.8±1.4 days (range, 0–6 days)], as was the length of stay, or 2 days median in both groups.
Figure 2 shows long-term survival for all patients, comparing UIP, and NSIP to other diagnoses. Patients with UIP had higher long-term mortality compared to patients with NSIP (HR: 4.27, 95% CI: 1.53–11.92, P=0.006) and patients with other diagnosis than UIP or NSIP (HR: 4.99, 95% CI: 1.82–13.72, P=0.002).
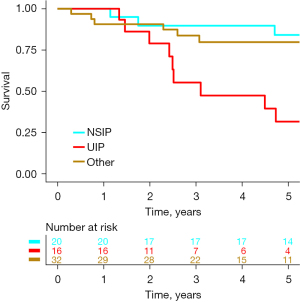
Overall, 1- and 5-year survival for all patients was 95.6% (95% CI: 87.6–99.1%) and 73.5% (95% CI: 61.4–83.5%), respectively. No mortality occurred within the first year of follow up for the NSIP- and UIP-patients, and at 5 years the overall survival rate was 85.0% (95% CI: 62.1–96.8%) and 43.7% (95% CI: 19.8–70.1%), respectively. At 1-year follow-up COP and secondary organizing pneumonia (SOP) survival was 80% (95% CI: 28.4–95.0%) with one reported mortality; no additional mortality was reported at 5 years. No mortality was reported for HP and sarcoidosis at neither 1- or 5-year follow-up.
Discussion
In this population-based retrospective study we reviewed all patients that underwent SLB in Iceland for suspected ILD over a 13-year period using a centralized database that covered the whole nation. The study addresses several issues related to ILD, such as diagnostic yield of SLB, the different histological and final diagnosis, intra-/postoperative complications, and length of stay together with both short- and long-term survival. We found that SLB resulted in definite diagnosis in almost all patients with a low rate of postoperative complications and no operative mortality.
The diagnostic yield for SLB in the current study was high, giving a definite diagnosis in roughly 93% of cases. The final diagnosis was reached after MDD using clinical, radiologic, histopathologic, and other data to reach the final diagnosis. For the first years of the study period MDD was informal but became more formalized for the latter part of the study period. In a prospective multicenter study from France including 103 patients that underwent SLB reported a definite diagnosis after MDD in 84.4% of cases (15). A meta-analysis of 2,148 patients from 23 studies which included both VATS and open thoracotomies reported a median diagnostic yield of 95% (range, 42–100%) (15).
UIP (23.5%) and NSIP (29.4%) were the most common histological groups while 7.4% remained non-classifiable. Our interpretation on why NSIP was more common in our study than UIP is that radiologic diagnosis has recently become more common for UIP, but is still more difficult for NSIP. Therefore, patients with unspecific radiologic changes on CT were more likely be referred to SLB than radiologic patterns suggesting UIP.
There are alternatives to surgical biopsy for ILD, such as conventional transbronchial forceps biopsy (TBFB) and more recently transbronchial cryobiopsy (10,11). Importantly, neither of these techniques have the same diagnostic yield for the diagnosis of ILD, ranging from 20–30% for TBFB and 73–81% for cryobiopsy, compared to SLBs 90–95% (16-19). The newly published CAN-ICE study from 2023 aimed to assess the diagnostic agreement between TBLC and SLB at both histologic and MDD level (20). In 56.7% [k value 0.38 (95% CI: 0.22–0.53)] of cases a histologic diagnostic agreement was reached, after MDD the diagnostic agreement between TBLC and SLB increased to 61.7% [k value 0.46 (95% CI: 0.29–0.63)] demonstrating only a moderate agreement for ILD (20). While the COLDICE study reported a histological agreement of 62.9% [k value 0.47 (95% CI: 0.30–0.64)] and an MDD agreement of 76.9% [k value 0.62 (95% CI: 0.47–0.78)] for TBLC and SLB (10). While MDD is important and recommended in the diagnosis of ILD and can increase the diagnostic yield of both TBLC and SLB, it is interesting that the diagnostic agreement can vary so much between the previous mentioned techniques (9,10,20). Furthermore, cryobiopsy has been reported to have higher rate of complications such as pneumothorax (8–12%) and moderate/severe bleeding (14–39%) with an overall complication rate of 23% (11,16,18). TBLC may be considered as an alternative of SLB especially for patients that are unable to tolerate surgery, however, it is only recommended in centers that are experienced with TBLC and have standardized their protocols to reduce complications rate and increase diagnostic yield (9). With the relatively small size of our center and TBLC being first described in 2009 it was decided by our center to prioritize SLBs over TBLC as the main diagnostic method for ILD (21). TBLC was therefore not performed in Iceland during the study period.
Patient characteristics were similar to other studies; males being predominant, with cardiovascular disease, hypertension and diabetes being the most common comorbidities (22,23). History of smoking and pre-operative spirometry results were also in line with other studies (22,23). We, however, reported a lower age at diagnosis for both UIP (65.9 years) and the whole patient cohort (55.6 years), which is slightly lower in comparison with a previous study from Iceland where it was 58 years in patients operated between 1986 and 2007 (24). In other similar studies the mean age ranges between 68 and 73 years; which is considerably higher than in the current study (22,23,25). Furthermore, our NSIP patients were on average 10 years younger than those with UIP. This could in part be explained by pulmonologists referring younger patients for SLBs; in order for them to receive a tailored treatment regimen to reduce disease progression. In our study long-term mortality rate for UIP was four times greater when compared with NSIP and other diagnosis. Therefore, it is important that patients with suspected UIP changes receive a prompt diagnosis so that a targeted treatment can be initiated during the early disease phase; as a delay in treatment has been associated with higher risk of death (6). Disease-modifying therapy with nintedanib and pirfenidone has also been shown to reduce the decline of FVC by almost half when compared to the placebo group (5-7).
Radiologic findings were similar to what others have described (9,12). Chest radiographs were most commonly suggestive of diffuse ILDs and CT scans showed both reticular and ground glass changes, as was expected as NSIP and UIP were the most common diagnoses.
Intra- and postoperative severe complications were rare (n=2), with only one patient sustaining intraoperative liver laceration from a trocar, and another patient required a reoperation for excessive postoperative bleeding with subsequent mechanical ventilation and ICU stay. Other complications were minor, most often pulmonary infections, and occurred in 10.3% of cases. Similar complications rates have been reported in other studies, or ranging from 9.4% to 30% for minor complications, and 0% to 7.7% for major complications (25-28).
Only 2 out of 68 patients had to be converted to mini-thoracotomy, which is as comparable rate to other studies as was the length of chest tube drainage (1 day median) and postoperative stay (2 days median) (25,26,28). In patients with severe hypoxemia, a mini-thoracotomy has been suggested over double-lumen intubation with single lung ventilation, as it eases the application of continuous positive airway pressure (CPAP), inspiratory positive airway pressure (IPAP) and partial lung ventilation techniques (29). Our results, however, show that our approach with a double-lumen intubation and single lung ventilation is safe, and we find it technically easier to perform the biopsy with deflated lung. Another important advance is that postoperative pain is less following VATS compared to open thoracotomy (30,31). VATS offers a wide range of surgical techniques such as uni-, bi- and tri-portal but also awake and non-intubated. In our center we felt most comfortable with tri portal VATS approach. Awake non-intubated VATS approach with regional anesthesia (epidural, local) has been described in the literature as an alternative to standard SLB by removing the need for neuromuscular blocking agents and mechanical ventilation which may predispose the patient to barotrauma, upper airway muscle weakness, airway obstruction and diaphragmatic dysfunctions (32-34). Postoperative complication rates were few with short operative time and length of stay and a diagnostic yield of 97% (33,34). However, those surgeries were performed on few and highly selective patient’s groups that had been evaluated preoperatively to tolerate the surgical procedure (33,34).
In our center a single biopsy was performed in 75% of cases with right lung being the most commonly biopsied as it had two fissures, making it technically easier. Although it has been recommended to ideally take biopsies from multiple lobes to increase the likelihood that an area with UIP is not missed, it was our believe, although not scientific, that taking multiple samples from different lobes would both increase the rate for intra- and postoperative complications, most importantly air leakage (14,35). Therefore, instead of taking multiple biopsies we tried to select the most inflamed part of the lung rather than the most fibrotic part, which in most cases was located in the upper lobes. Our hospital-, 30- and 90-day mortality was zero. Hutchinson et al. reported 6.4% overall 30-day mortality in 30,000 SLB procedures where 1.7% in-hospital mortality rate was reported after elective surgery (36). There was significantly higher mortality rate of 16% for non-elective surgery (urgent and emergency), with possible complications to occur in 30% of elective procedures. However, their patient cohort included both open- and VATS cases. Median length of stay was reported as 5 days, which is more than twice compared to two days in the current study. Furthermore, Hutchinson et al. did not report 30- and 90-day mortality rate since most patients were discharged within 30 days (36). Park et al. reported similar mortality rates at 30- and 90 days when comparing VATS with thoracotomy. VATS being 4.0% and 8.0% compared to 4.0 and 10.0% respectively (37). Nguyen et al. reported similar findings with VATS 30-day mortality being 2.1% compared to 4.3% for open technique (13).
Overall survival for all groups was 95.6% and 73.5% at 1 and 5 years, respectively, which is in line with other studies. Kreider et al. reported a 95.6% survival rate at 60 days after SLB while Kim et al. reported a 90.6% rate at 2 years (38,39). Furthermore, Nagano et al reported a survival rate at 5 years of 50% for idiopathic pulmonary fibrosis (IPF) and 91% for non-IPF after SLB, respectively (25).
The main strength of the study lies in the two centralized databases used to identify the ILD patients and almost a complete follow-up regarding survival from the centralized Icelandic Death Registry. Furthermore, Landspitali University Hospital is the only tertiary care center in Iceland and the only center performing cardiothoracic surgery, including SLBs. Finally, all the imaging studies were reviewed by a radiologist and all histological biopsies obtained during surgery were reviewed by a senior pathologist. Due to the small size of the center with few specialists that are specialized in the evaluation of ILD and with the few patients being operated throughout the study period it may be debated if it is seen as a strength or a limitation that images and histological biopsies were reviewed by a single radiologist and a senior pathologist.
The main limitations of the study are those related to the retrospective nature of the study with relatively limited number of patients in some of the rarer histological groups. MDD was conducted in an informal way in the beginning of the study, therefore standardized MDD registration was limited and could not be evaluated for all patients as we would have liked to. This issue has been addressed in the recent years and MDD are now conducted in a formal way and registered accordingly.
Conclusions
Advances in surgical technique for SLB in Iceland; with an almost over-night switch from open thoracotomy towards a more minimal invasive VATS technique after 2005, has halved the length of stay and median chest tube time (24). Importantly, SLB with VATS-technique provided definitive histological and disease specific diagnosis in majority of cases. The procedure is safe, reflected in low complication-rates, relatively short operation times and hospital stay, with good short- and long-term overall survival for most ILD groups. Therefore, we think that patients with suspected ILD should receive a prompt definitive diagnosis so that tailored treatment regimen can be initiated. This is especially important for patients with progressive pulmonary fibrosis; since an early irreversible destruction of lung parenchyma can be slowed with modern treatment, providing improved prognosis for the patient (6).
Acknowledgments
Funding: The study was supported by a grant from
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1107/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1107/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1107/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1107/coif). G.G. reports funding from the Landspitali Scientific Fund (No. A2020-019) and the University of Iceland Research Fund. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Icelandic National Bioethics Committee (VSN 14-111-V1-S2). As individuals were not identified in the study, individual consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kayatta MO, Ahmed S, Hammel JA, et al. Surgical biopsy of suspected interstitial lung disease is superior to radiographic diagnosis. Ann Thorac Surg 2013;96:399-401. [Crossref] [PubMed]
- Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:e44-68. [Crossref] [PubMed]
- Raghu G, Weycker D, Edelsberg J, et al. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2006;174:810-6. [Crossref] [PubMed]
- Wallis A, Spinks K. The diagnosis and management of interstitial lung diseases. BMJ 2015;350:h2072. [Crossref] [PubMed]
- Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2071-82. [Crossref] [PubMed]
- Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet 2017;389:1941-52. [Crossref] [PubMed]
- King TE Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2083-92. [Crossref] [PubMed]
- Bauer PR, Kalra S, Osborn TG, et al. Influence of autoimmune biomarkers on interstitial lung diseases: A tertiary referral center based case-control study. Respir Med 2015;109:397-405. [Crossref] [PubMed]
- Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2022;205:e18-47. [Crossref] [PubMed]
- Troy LK, Grainge C, Corte TJ, et al. Diagnostic accuracy of transbronchial lung cryobiopsy for interstitial lung disease diagnosis (COLDICE): a prospective, comparative study. Lancet Respir Med 2020;8:171-81. [Crossref] [PubMed]
- Johannson KA, Marcoux VS, Ronksley PE, et al. Diagnostic Yield and Complications of Transbronchial Lung Cryobiopsy for Interstitial Lung Disease. A Systematic Review and Metaanalysis. Ann Am Thorac Soc 2016;13:1828-38. [Crossref] [PubMed]
- Jee AS, Sheehy R, Hopkins P, et al. Diagnosis and management of connective tissue disease-associated interstitial lung disease in Australia and New Zealand: A position statement from the Thoracic Society of Australia and New Zealand. Respirology 2021;26:23-51. [Crossref] [PubMed]
- Nguyen W, Meyer KC. Surgical lung biopsy for the diagnosis of interstitial lung disease: a review of the literature and recommendations for optimizing safety and efficacy. Sarcoidosis Vasc Diffuse Lung Dis 2013;30:3-16.
- Lynch DA, Sverzellati N, Travis WD, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir Med 2018;6:138-53. [Crossref] [PubMed]
- Han Q, Luo Q, Xie JX, et al. Diagnostic yield and postoperative mortality associated with surgical lung biopsy for evaluation of interstitial lung diseases: A systematic review and meta-analysis. J Thorac Cardiovasc Surg 2015;149:1394-401.e1. [Crossref] [PubMed]
- Kheir F, Uribe Becerra JP, Bissell B, et al. Transbronchial Lung Cryobiopsy in Patients with Interstitial Lung Disease: A Systematic Review. Ann Am Thorac Soc 2022;19:1193-202. [Crossref] [PubMed]
- Sheth JS, Belperio JA, Fishbein MC, et al. Utility of Transbronchial vs Surgical Lung Biopsy in the Diagnosis of Suspected Fibrotic Interstitial Lung Disease. Chest 2017;151:389-99. [Crossref] [PubMed]
- Sethi J, Ali MS, Mohananey D, et al. Are Transbronchial Cryobiopsies Ready for Prime Time?: A Systematic Review and Meta-Analysis. J Bronchology Interv Pulmonol 2019;26:22-32. [Crossref] [PubMed]
- Troy LK, Grainge C, Corte T, et al. Cryobiopsy versus open lung biopsy in the diagnosis of interstitial lung disease (COLDICE): protocol of a multicentre study. BMJ Open Respir Res 2019;6:e000443. [Crossref] [PubMed]
- Fortin M, Liberman M, Delage A, et al. Transbronchial Lung Cryobiopsy and Surgical Lung Biopsy: A Prospective Multi-Centre Agreement Clinical Trial (CAN-ICE). Am J Respir Crit Care Med 2023;207:1612-9. [Crossref] [PubMed]
- Babiak A, Hetzel J, Krishna G, et al. Transbronchial cryobiopsy: a new tool for lung biopsies. Respiration 2009;78:203-8. [Crossref] [PubMed]
- Kaunisto J, Salomaa ER, Hodgson U, et al. Demographics and survival of patients with idiopathic pulmonary fibrosis in the FinnishIPF registry. ERJ Open Res 2019;5:00170-2018. [Crossref] [PubMed]
- Gao J, Kalafatis D, Carlson L, et al. Baseline characteristics and survival of patients of idiopathic pulmonary fibrosis: a longitudinal analysis of the Swedish IPF Registry. Respir Res 2021;22:40. [Crossref] [PubMed]
- Sigurdsson MI, Isaksson HJ, Gudmundsson G, et al. Diagnostic surgical lung biopsies for suspected interstitial lung diseases: a retrospective study. Ann Thorac Surg 2009;88:227-32. [Crossref] [PubMed]
- Nagano M, Miyamoto A, Kikunaga S, et al. Outcomes of Video-Assisted Thoracic Surgical Lung Biopsy for Interstitial Lung Diseases. Ann Thorac Cardiovasc Surg 2021;27:290-6. [Crossref] [PubMed]
- Radu D, Freynet O, Kambouchner M, et al. Diagnosis Yield and Safety of Surgical Biopsy in Interstitial Lung Diseases: A Prospective Study. Ann Thorac Surg 2022;114:1911-7. [Crossref] [PubMed]
- Durheim MT, Kim S, Gulack BC, et al. Mortality and Respiratory Failure After Thoracoscopic Lung Biopsy for Interstitial Lung Disease. Ann Thorac Surg 2017;104:465-70. [Crossref] [PubMed]
- Morris D, Zamvar V. The efficacy of video-assisted thoracoscopic surgery lung biopsies in patients with Interstitial Lung Disease: a retrospective study of 66 patients. J Cardiothorac Surg 2014;9:45. [Crossref] [PubMed]
- Walsh AM, Lohser J. Arterial Oxygenation and Management of Hypoxemia During VATS. Curr Anesthesiol Rep 2014;4:170-6.
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Holbek BL, Horsleben Petersen R, Kehlet H, et al. Fast-track video-assisted thoracoscopic surgery: future challenges. Scand Cardiovasc J 2016;50:78-82. [Crossref] [PubMed]
- Kirmeier E, Eriksson LI, Lewald H, et al. Post-anaesthesia pulmonary complications after use of muscle relaxants (POPULAR): a multicentre, prospective observational study. Lancet Respir Med 2019;7:129-40. [Crossref] [PubMed]
- Guerrera F, Costardi L, Rosboch GL, et al. Awake or intubated surgery in diagnosis of interstitial lung diseases? A prospective study. ERJ Open Res 2021;7:00630-2020. [Crossref] [PubMed]
- Pompeo E, Rogliani P, Cristino B, et al. Awake thoracoscopic biopsy of interstitial lung disease. Ann Thorac Surg 2013;95:445-52. [Crossref] [PubMed]
- Flaherty KR, Travis WD, Colby TV, et al. Histopathologic variability in usual and nonspecific interstitial pneumonias. Am J Respir Crit Care Med 2001;164:1722-7. [Crossref] [PubMed]
- Hutchinson JP, Fogarty AW, McKeever TM, et al. In-Hospital Mortality after Surgical Lung Biopsy for Interstitial Lung Disease in the United States. 2000 to 2011. Am J Respir Crit Care Med 2016;193:1161-7. [Crossref] [PubMed]
- Park JH, Kim DK, Kim DS, et al. Mortality and risk factors for surgical lung biopsy in patients with idiopathic interstitial pneumonia. Eur J Cardiothorac Surg 2007;31:1115-9. [Crossref] [PubMed]
- Kim DS, Park JH, Park BK, et al. Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J 2006;27:143-50. [Crossref] [PubMed]
- Kreider ME, Hansen-Flaschen J, Ahmad NN, et al. Complications of video-assisted thoracoscopic lung biopsy in patients with interstitial lung disease. Ann Thorac Surg 2007;83:1140-4. [Crossref] [PubMed]


