
Vein-first vs. artery-first robotic lobectomy outcomes in non-small cell lung cancer
Highlight box
Key findings
• Our study suggests that choosing between a vein-first vs. artery-first technique for robotic lobectomy does not significantly impact overall survival or cancer recurrence in patients with non-small cell lung cancer (NSCLC).
What is known and what is new?
• Some authors suggest that early ligation of the vein can limit the spread of cancer cells secondary to manipulation of the lung tissue during surgery.
• Our study provides data for differences in outcomes between vein-first vs. artery-first robotic lobectomies.
What is the implication, and what should change now?
• Our data suggests that the order in which the pulmonary vessels are ligated during robotic lobectomy for NSCLC should be up to the discretion of the surgeon.
Introduction
Lung cancer is the leading cause of death due to cancer in the United States, with non-small cell lung cancer (NSCLC) being most common (1). Many NSCLC tumors are now removed via robotic lobectomy, which has been shown to have comparable perioperative morbidity and mortality outcomes to that of video-assisted thoracoscopic surgery (VATS) lobectomy (2,3). Considerable care must be taken to decrease the risk of metastasis and recurrence following surgical resection of lung tumors. Some studies have shown that the surgical manipulation of tumors can promote the release of tumor cells into circulation (4,5). With respect to lobectomies for NSCLC, ligation of the pulmonary vein prior to the artery may be a promising technique that can help mitigate these risks.
The decision of whether to ligate the artery or vein first during lobectomy has been debated, with some authors positing that early ligation of the vein can limit the spread of cancer cells secondary to manipulation of the lung tissue during surgery. In particular, circulating tumor cells can serve as a biomarker for prospective recurrence, making a technique that limits the circulation of these cells an important area of investigation (6,7). However, there is little evidence to guide whether a vein-first (V-first) approach to lobectomies is superior to an artery-first (A-first) approach with respect to short- or long-term outcomes. For thoracoscopic lobectomies, a prior study from Wei et al. demonstrated that patients who underwent V-first resections had higher rates of survival than patients who underwent A-first resections (8). This finding warrants further investigation in order to determine whether V-first approaches are truly superior. To our knowledge, there have been no prior studies examining V-first vs. A-first approaches among robotic lobectomies. Therefore, in this study, we aim to evaluate the short- and long-term outcomes of V-first vs. A-first approaches in patients who underwent robotic lobectomies for NSCLC. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1576/rc).
Methods
Study design
This was a retrospective study using the electronic medical record of all patients who underwent robotic lobectomy procedures for NSCLC at the University of Alabama at Birmingham (UAB) from January 2013 through May 2019. A total of 374 patients from two surgeons were included, while bilobectomies (n=11) and cases that involved conversion from robotic to open lobectomy were excluded (n=16) (Figure 1). Robotic lobectomy was performed with a completely portal technique using four robotic arms and an assistant port. The camera port was typically located in the 7th or 8th intercostal space. Our technique has been described previously (9). Operative reports were reviewed, and all cases were then separated into either the V-first or A-first group, based on the sequence in which the pulmonary vessels were divided. The V-first group included patients who underwent division of the vein first followed by the artery (vein-artery), along with cases that also involved division of another vein after the artery (vein-artery-vein). The A-first group included cases in which the arteries were divided first followed by the vein (artery-vein) and cases where an additional artery was divided after the vein (artery-vein-artery). Order of division of the vessels was made at the discretion of the operating surgeon on each individual case; there was no set protocol or policy with regards to order of division of vessels. Mediastinal lymph node dissection was performed prior to division of vessels and the performance of lobectomy in all cases. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and informed consent was obtained from the patients included in this study. Institutional Review Board approval was obtained for this study through University of Alabama at Birmingham (protocol No. 300001851).
Data collection
Various baseline characteristics were collected for each patient, including demographics (age, gender, and race) and clinical characteristics (comorbidities, Zubrod score, etc.). Pathologic stage was determined according to the Eighth Edition of the American Joint Committee on Cancer staging manual (10). Peri-operative (procedure time) and post-operative (events, recurrence, mortality, etc.) data were also collected. Follow-up clinic notes up until June 2020 were used to determine recurrence among patients. Recurrence was defined as a new abnormality or lesion(s) seen on computed tomography (CT) scan, confirmed by positron emission tomography (PET)-CT scan, repeat CT scan (to document growth), and/or biopsy. The UAB tumor registry data was used to calculate follow-up duration for each patient, with the last update of the registry being in June 2022. Primary outcomes included overall survival and recurrence-free survival, while secondary outcomes included postoperative complication rates and hospital length-of-stay.
Statistical analysis
Mean or median values were calculated for continuous variables while counts and percentages were calculated for categorical variables. Median values were used for time-related continuous variables. Patient characteristics and perioperative events and outcomes were compared between the two groups using Chi-squared and Student’s t-tests, as appropriate. Kaplan-Meier survival curves for recurrence-free survival and overall survival were constructed and compared with log-rank tests. We developed a multivariable Cox proportional hazards regression model to assess factors predicting mortality, and another to predict recurrence. All statistical analyses were performed using SAS software (Version 9.4, SAS Institute Inc., Cary, NC, USA). A P value of less than 0.05 was considered statistically significant.
Results
A total of 401 patients underwent a robotic lobectomy for NSCLC during the study period. As described in Figure 1, 27 patients were excluded from the analysis. Of the remaining 374 patients, 94 were identified to be in the V-first group and 280 were in the A-first group. In the V-first group, 2 (2.1%) patients had an additional vein divided after the artery (vein-artery-vein), while the A-first group included 106 (37.3%) patients who had an additional artery divided after the vein (artery-vein-artery).
Patient characteristics are summarized in Table 1. For the entire cohort 58.0% of patients were female, 54.8% were between the ages 65–79 years, and 84.5% were Caucasian. There were no significant differences between the two groups with respect to gender, age, or race. Within the cohort, the most common comorbidities were hypertension (67.1%), obesity (36.1%), and chronic obstructive pulmonary disease (34.95%), with no significant differences between the V-first or A-first groups. Approximately 1/3 (32.9%) of patients in this cohort had undergone prior cardiothoracic surgery; 12.3% of patients received pre-operative chemotherapy, while 8.02% received pre-operative radiation therapy, with no significant differences between the groups. Of note, a V-first technique appeared to be used more often for patients with tumors ≤2 cm in size than an A-first technique (50.0% vs. 29.2%, P=0.007). Also, patients in the V-first group appeared to have a lower mean predicted forced expiratory volume in the first second (FEV1) compared to the A-first group (78 vs. 84, P=0.011).
Table 1
| Baseline characteristics | Vein-first, n=94 | Artery-first, n=280 | All, n=374 | P value |
|---|---|---|---|---|
| Age, years | ||||
| 18–44 | 2 (2.13) | 6 (2.14) | 8 (2.14) | 0.993 |
| 45–64 | 30 (31.91) | 91 (32.50) | 121 (32.35) | 0.931 |
| 65–79 | 49 (52.13) | 156 (55.71) | 205 (54.81) | 0.684 |
| 80+ | 13 (13.83) | 27 (9.64) | 40 (10.70) | 0.283 |
| Gender | ||||
| Female | 62 (65.96) | 155 (55.36) | 217 (58.02) | 0.243 |
| Male | 32 (34.04) | 125 (44.64) | 157 (41.98) | 0.170 |
| Race | ||||
| Caucasian | 80 (85.11) | 236 (84.29) | 316 (84.49) | 0.940 |
| Black/African American | 14 (14.89) | 41 (14.64) | 55 (14.71) | 0.956 |
| Hispanic or Latino Ethnicity | 0 (0.00) | 3 (1.07) | 3 (0.80) | 0.316 |
| Comorbidities | ||||
| Obesity (BMI ≥30 kg/m2) | 38 (40.43) | 97 (34.64) | 135 (36.10) | 0.419 |
| Hypertension | 59 (62.77) | 192 (68.57) | 251 (67.11) | 0.552 |
| Congestive heart failure | 3 (3.19) | 11 (3.93) | 14 (3.74) | 0.749 |
| Coronary artery disease | 16 (17.02) | 63 (22.50) | 79 (21.12) | 0.317 |
| Pulmonary hypertension | 1 (1.06) | 3 (1.07) | 4 (1.07) | 0.995 |
| Interstitial fibrosis | 2 (2.13) | 3 (1.07) | 5 (1.34) | 0.443 |
| Prior stroke or TIA | 9 (9.57) | 18 (6.43) | 27 (7.22) | 0.326 |
| Diabetes | 18 (19.15) | 59 (21.07) | 77(20.59) | 0.722 |
| COPD (n=329) | 30 (31.91) | 85 (30.36) | 115 (34.95) | 0.276 |
| Preoperative chemotherapy | 14 (14.89) | 32 (11.43) | 46 (12.30) | 0.407 |
| Preoperative thoracic radiation | 7 (7.45) | 23 (8.21) | 30 (8.02) | 0.820 |
| Prior cardiothoracic surgery | 31 (32.98) | 92 (32.86) | 123 (32.89) | 0.986 |
| Smoking status | ||||
| Former smoker | 50 (53.19) | 151 (53.93) | 201 (53.74) | 0.933 |
| Current smoker | 22 (23.40) | 64 (22.86) | 86 (23.00) | 0.924 |
| Never smoked | 22 (23.40) | 65 (23.21) | 87 (23.26) | 0.974 |
| Zubrod score (n=329) | ||||
| 0 | 16 (22.22) | 56 (21.79) | 72 (21.88) | 0.945 |
| 1 | 44 (61.11) | 163 (63.42) | 207 (62.92) | 0.827 |
| 2 | 9 (12.50) | 32 (12.45) | 41 (12.46) | 0.992 |
| 3 | 3 (4.17) | 6 (2.33) | 9 (2.74) | 0.406 |
| Tumor size (cm) (n=329) | ||||
| ≤2 | 36 (50.00) | 75 (29.18) | 111 (33.74) | 0.007 |
| >2, ≤3 | 17 (23.61) | 79 (30.74) | 96 (29.18) | 0.322 |
| >3, ≤5 | 17 (23.61) | 77 (29.96) | 94 (28.57) | 0.373 |
| >5, ≤7 | 2 (2.78) | 20 (7.78) | 22 (6.69) | 0.147 |
| >7 | 0 | 6 (2.33) | 6 (1.82) | 0.195 |
| Pulmonary function testing | ||||
| FEV1 (mean, % predicted) (n=367) | 78 | 84 | 83 | 0.011 |
| DLCO (mean, % predicted) (n=360) | 72 | 76 | 75 | 0.087 |
The total number of patients for Zubrod score and tumor size was 329. For these items, the V-first group had 72 patients, while the A-first group had 257 patients. Data are presented as N (%) or mean, % predicted. BMI, body mass index; TIA, transient ischemic attack; COPD, chronic obstructive pulmonary disease; cm, centimeters; FEV1, forced expiratory volume in 1 second; DLCO, diffusing capacity of lungs for carbon monoxide; V-first, vein-first; A-first, artery-first.
Operative and tumor characteristics are described in Table 2. In this cohort, adenocarcinoma was the most common histology (65.8%) and the majority of patients (70.9%) were Stage 1. There were no significant differences in histology or pathologic stage between the groups. The rate of R0 resection was similar between both groups (V-first: 97.9% vs. A-first: 98.9%, P=0.440) The patients who underwent A-first operations had a greater mean number of nodes removed (21 vs. 17; P<0.001). The V-first group was more likely to have undergone right upper lobectomy (54.3% vs. 36.4%; P=0.019) or right middle lobectomy (20.2% vs. 2.9%; P<0.001) when compared to the A-first group. On the other hand, the A-first group was more likely to have undergone left upper lobectomy (23.2% vs. 2.1%; P<0.001).
Table 2
| Operative and tumor characteristics | Vein-first, n=94 | Artery-first, n=280 | All, n=374 | P value |
|---|---|---|---|---|
| Procedure time (median minutes) | 157 | 133 | 137 | 0.061 |
| Removed lobe | ||||
| Right upper | 51 (54.26) | 102 (36.43) | 153 (40.91) | 0.019 |
| Right middle | 19 (20.21) | 8 (2.86) | 27 (7.22) | <0.001 |
| Right lower | 12 (12.77) | 61 (21.79) | 73 (19.52) | 0.087 |
| Left upper | 2 (2.13) | 65 (23.21) | 67 (17.91) | <0.001 |
| Left lower | 10 (10.64) | 44 (15.71) | 54 (14.44) | 0.262 |
| Pathologic stage | ||||
| 0 | 1 (1.06) | 3 (1.07) | 4 (1.07) | 0.995 |
| IA1 | 30 (31.91) | 79 (28.21) | 109 (29.14) | 0.565 |
| IA2 | 18 (19.15) | 52 (18.57) | 70 (18.72) | 0.869 |
| IA3 | 4 (4.26) | 5 (1.79) | 9 (2.41) | 0.182 |
| IB | 19 (20.21) | 58 (20.71) | 77 (20.59) | 0.926 |
| IIA | 3 (3.19) | 17 (6.07) | 20 (5.35) | 0.296 |
| IIB | 15 (15.96) | 32 (11.43) | 47(12.57) | 0.284 |
| III | 4 (4.26) | 31 (11.07) | 35 (9.36) | 0.062 |
| IV | 0 | 3 (1.07) | 3 (0.80) | 0.316 |
| Cancer histology | ||||
| Adenocarcinoma | 61 (64.89) | 185 (66.07) | 246 (65.78) | 0.903 |
| Squamous cell | 17 (18.09) | 67 (23.93) | 84 (22.46) | 0.301 |
| Neuroendocrine | 14 (14.89) | 24 (8.57) | 38 (10.16) | 0.096 |
| Large cell | 0 | 1 (0.36) | 1 (0.27) | 0.562 |
| Mixed | 2 (2.13) | 3 (1.07) | 5 (1.34) | 0.443 |
Data are presented as median or N (%).
As shown in Table 3, there were no significant differences with respect to postoperative complications, length of stay, or 30- and 90-day mortality between the two groups. The most common post-operative complications were urinary retention (16.3%), prolonged air leak (12.6%), and atrial arrhythmia requiring treatment (8.6%). Interestingly, the incidence of atrial arrhythmia was over twice as high in the A-first group (10.0%) as in the V-first group (4.3%), although this difference did not reach statistical significance (P=0.100). Among the whole cohort, median length of stay from admission to discharge was 2 [interquartile range (IQR) 2–4] days.
Table 3
| Postoperative events | Vein-first, n=94 | Artery-first, n=280 | All, n=374 | P value |
|---|---|---|---|---|
| Unexpected return to operating room | 1 (1.06) | 10 (3.57) | 11 (2.94) | 0.220 |
| Reintubation | 2 (2.13) | 1 (0.36) | 3 (0.80) | 0.097 |
| Recurrent laryngeal nerve paresis | 0 | 2 (0.71) | 2 (0.53) | 0.413 |
| Urinary tract infection | 3 (3.19) | 5 (1.79) | 8 (2.14) | 0.420 |
| Air leak >5 days | 9 (9.57) | 38 (13.57) | 47 (12.57) | 0.344 |
| Atelectasis requiring bronchoscopy | 1 (1.06) | 6 (2.14) | 7 (1.87) | 0.508 |
| Pleural effusion requiring drainage | 1 (1.06) | 2 (0.71) | 3 (0.80) | 0.743 |
| Pneumonia | 1 (1.06) | 10 (3.57) | 11 (2.94) | 0.219 |
| Acute respiratory distress syndrome | 0 | 2 (0.71) | 2 (0.53) | 0.413 |
| Respiratory failure | 3 (3.19) | 5 (1.79) | 8 (2.14) | 0.420 |
| Pneumothorax | 5 (5.32) | 24 (8.57) | 29 (7.75) | 0.327 |
| Initial ventilatory support >48 h | 0 | 2 (0.71) | 2 (0.53) | 0.413 |
| Tracheostomy | 0 | 1 (0.36) | 1 (0.27) | 0.562 |
| Atrial arrhythmia requiring treatment | 4 (4.26) | 28 (10.00) | 32 (8.56) | 0.100 |
| Ileus | 0 | 3 (1.07) | 3 (0.80) | 0.316 |
| Postop packed RBCs | 1 (1.06) | 5 (1.79) | 6 (1.60) | 0.633 |
| Urinary retention | 16 (17.02) | 45 (16.07) | 61 (16.31) | 0.844 |
| Sepsis | 0 | 2 (0.71) | 2 (0.53) | 0.413 |
| Other infection requiring IV antibiotics | 0 | 9 (3.21) | 9 (2.41) | 0.082 |
| Delirium | 1 (1.06) | 8 (2.86) | 9 (2.41) | 0.332 |
| Other neurological event | 1 (1.06) | 0 | 1 (0.27) | 0.084 |
| Renal failure (RIFLE criteria) | 2 (2.13) | 2 (0.71) | 4 (1.07) | 0.251 |
| Chylothorax requiring medical intervention | 0 | 7 (2.50) | 7 (1.87) | 0.125 |
| Other events requiring OR w/general anesthesia | 1 (1.06) | 6 (2.14) | 7 (1.87) | 0.508 |
| Unexpected admission to ICU | 1 (1.06) | 13 (4.64) | 14 (3.74) | 0.121 |
| Postoperative length of stay (median days) | 2 | 3 | 2 | 0.890 |
| Recurrence (n=366) | 16 (17.20) | 50 (18.32) | 66 (18.03) | 0.662 |
| 30-day mortality | 2 (2.13) | 3 (1.07) | 5 (1.34) | 0.353 |
| 90-day mortality | 2 (2.13) | 5 (1.79) | 7 (1.87) | 0.280 |
The total number of patients for which recurrence data was available was 366. For this item, the V-first group had 93 patients, while the A-first group had 273 patients. Data are presented as N (%), or median. RBCs, red blood cells; IV, intravenous; RIFLE criteria, Risk, Injury, Failure, Loss of kidney function, End-stage kidney disease criteria; OR, operating room; ICU, intensive care unit; V-first, vein-first; A-first, artery-first.
The median postoperative follow-up duration with respect to overall survival was 67 (IQR 45–87) months. A total of 8 patients were lost to follow-up with respect to recurrence evaluation, 1 in the V-first group and 7 in the A-first group. Out of the 366 patients who were not lost to follow-up, recurrence occurred in 66 (18.0%) patients, while 59 (16.1%) patients died. Figure 2 depicts the recurrence-free survival curves and Figure 3 depicts the overall survival curves for the two groups. According to comparison through log-rank tests, there was no significant difference between the V-first and A-first groups with respect to both recurrence-free (P=0.941) and overall survival (P=0.202). Multivariable Cox regression analysis showed that whether the vein or artery was ligated first did not predict recurrence or death (Tables 4,5).

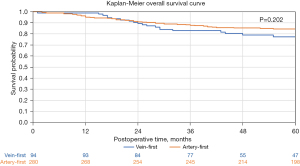
Table 4
| Variables | Hazard ratio (95% CI) | P value |
|---|---|---|
| Vein vs. artery | 0.79 (0.39, 1.61) | 0.5226 |
| Age at time of surgery | 1.02 (0.98, 1.05) | 0.3349 |
| Female vs. male | 1.61 (0.90, 2.88) | 0.1059 |
| Histology | ||
| Large cell vs. adenocarcinoma | 14.38 (1.56, 132.88) | 0.0188 |
| Mixed vs. adenocarcinoma | N.A. | 0.9966 |
| Neuroendocrine vs. adenocarcinoma | 1.71 (0.60, 4.84) | 0.312 |
| Squamous cell vs. adenocarcinoma | 1.81 (0.95, 3.42) | 0.0692 |
| Stage | ||
| II, III, or IV, unknown vs. 0 or I | N.A. | 0.9823 |
| Location | ||
| Left lower vs. right upper | 2.08 (0.96, 4.48) | 0.0626 |
| Left upper vs. right upper | 0.49 (0.14, 1.71) | 0.2595 |
| Right lower vs. right upper | 2.53 (1.25, 5.12) | 0.0099 |
| Right middle vs. right upper | 2.49 (0.85, 7.23) | 0.0947 |
| Smoking status | ||
| Current smoker vs. never smoked | 2.21 (0.89, 5.47) | 0.0875 |
| Former smoker vs. never smoked | 2.01 (0.95, 4.24) | 0.0677 |
| ECOG score | ||
| 1 vs. 0 | 1.30 (0.70, 2.42) | 0.4045 |
| 2 vs. 0 | 0.89 (0.37, 2.14) | 0.792 |
| 3 vs. 0 | 0.45 (0.06, 3.50) | 0.4424 |
None of the 5 patients with mixed cancer experienced recurrence, making it impossible to estimate the hazard ratio and its corresponding 95% confidence interval. Additionally, none of the patients diagnosed with Stage II, III, or IV cancer had experienced recurrence, which prevented the estimation of the hazard ratio due to the absence of variation in the outcome. CI, confidence interval; N.A., not available; ECOG, Eastern Cooperative Oncology Group.
Table 5
| Variables | Hazard ratio (95% CI) | P value |
|---|---|---|
| Vein vs. artery | 1.62 (0.81, 3.24) | 0.1706 |
| Age at time of surgery | 1.01 (0.98, 1.05) | 0.4472 |
| Female vs. male | 0.77 (0.42, 1.41) | 0.4052 |
| Histology | ||
| Large cell vs. adenocarcinoma | N.A. | >0.99 |
| Mixed vs. adenocarcinoma | 0.63 (0.07, 5.84) | 0.6875 |
| Neuroendocrine vs. adenocarcinoma | 2.82 (0.90, 8.89) | 0.0763 |
| Squamous cell vs. adenocarcinoma | 1.02 (0.49, 2.09) | 0.9655 |
| Pathology | ||
| II, III, or IV, unknown vs. 0 or I | N.A. | 0.9773 |
| Location | ||
| Left lower vs. right upper | 0.73 (0.21, 2.56) | 0.619 |
| Left upper vs. right upper | 0.76 (0.41, 1.38) | 0.3612 |
| Right lower vs. right upper | 7.12 (0.69, 73.16) | 0.0986 |
| Right middle vs. right upper | 0.38 (0.10, 1.41) | 0.1463 |
| Smoking status | ||
| Current smoker vs. never smoked | 1.83 (0.64, 5.26) | 0.2624 |
| Former smoker vs. never smoked | 2.29 (1.00, 5.25) | 0.0512 |
| ECOG score | ||
| 1 vs. 0 | 1.12 (0.59, 2.12) | 0.7278 |
| 2 vs. 0 | 2.13 (0.77, 5.88) | 0.146 |
| 3 vs. 0 | 2.90 (0.29, 28.51) | 0.362 |
Only one patient was diagnosed with large cell cancer, resulting in an inability to estimate the hazard ratio and its corresponding 95% confidence interval. Out of the 69 patients who died during the study period, all had Stage II, III, or IV cancer, which led to the inability to estimate the hazard ratio due to a lack of variation in the outcome. CI, confidence interval; N.A., not available; ECOG, Eastern Cooperative Oncology Group.
Discussion
The results of this study suggest that a V-first vs. A-first robotic lobectomy technique does not significantly impact the overall survival or cancer recurrence in patients with NSCLC. We found no significant difference in overall and recurrence-free survival between our two groups. Furthermore, both groups also did not significantly differ in the incidence of postoperative complications or in length of hospital stay. Interestingly, we found that technique selection was influenced by tumor size and location. In particular, a V-first technique was performed more often for small tumors (≤2 cm), along with those located in the right upper and middle lobes. On the other hand, an A-first technique was more often performed for patients who underwent left upper lobectomies.
To our knowledge, the impact of the sequence of vessel ligation has not been investigated in patients who have undergone robotic lobectomies. We found that ligation of the pulmonary vein prior to the artery did not appear to significantly impact the recurrence or overall survival of patients with NSCLC who underwent robotic lobectomies. These findings are consistent with the study of open lobectomies by Refaely et al., along with the study of VATS lobectomy by He et al. (5,11). Given that VATS lobectomy is considered more similar to robotic lobectomy, the findings of He et al. may be more comparable to our study. In the retrospective study by He et al., it was found that the order of vessel ligation during VATS lobectomy did not affect long-term survival of patients, which is consistent with our findings (11). Despite this, we do find our results to be surprising, as a large proportion of the literature continues to show favor of V-first techniques (9,12). However, since robotic lobectomy is inherently a minimally invasive procedure, it is possible that minimization of tumor cell manipulation with this method therefore makes the sequence of vessel ligation not a significant factor in patient outcomes. Specifically, lobectomy via open thoracotomy has been shown to result in increased circulating tumor cells in the pulmonary venous drainage (13-15). Thus, in more invasive techniques that involve greater manipulation of the lung, the sequence of vessel ligation may play a greater role in outcomes. Furthermore, a study by Duan et al. suggests that the timing of V-first ligation, early versus late, may play a role in outcomes as well (16). This study found that ligating the vein immediately during VATS lobectomy (early ligation group) resulted in decreased dissemination of circulating tumor cells when compared to ligating the vein later once the artery, bronchus, and pulmonary fissure were partially or completely exposed (late ligation group) (16). This finding also introduces the possibility that even with a V-first technique, the timing of vein ligation may also play a role in tumor cell dissemination and therefore recurrence and survival.
The decision about whether or not to ligate the vein first during lobectomy for non-small lung cancer is often dictated by the particular anatomy of the specific patient. That said, certain trends with regards to vessel to be divided first do exist. For instance, we found that V-first ligation was more likely in patients undergoing right upper and middle lobectomy. In both of these instances, it is often simpler to ligate the vein prior to the artery given the relative location of the vessels. We believe that this is due to the position of the vein relative to the arteries (for instance, the middle lobe vein is peripheral/medial to the artery in patients). In certain instances, such as when a posterior ascending artery is easily accessible after dividing the right upper lobe bronchus, or when a right middle lobe artery is traveling in a complete oblique fissure, it can be easier to ligate the artery first when doing the respective lobectomy. However the more common anatomic configuration is such that the vein is ligated first in these operations. On the other hand, left upper lobectomy was more commonly performed in an A-first manner in our study. This is because the apicoposterior artery is often easily isolated and ligated with the lung retracted in an anterior fashion during the initial hilar/mediastinal nodal dissection. In the absence of evidence suggesting that the order of vessel division impacts oncologic outcomes, it is reasonable to state that these decisions can and should be based on convenience, safety, surgeon experience and comfort level, and ease, rather than biased towards a V-first approach to provide a hypothetical benefit of reducing circulating tumor cells.
There are some limitations to this study. First, the data used in the study was collected from a single institution, which could have introduced bias based on institutional or surgeon preference regarding operative technique. This limits the generalizability of our findings. Furthermore, the sample size was small, and due to the retrospective nature of this study, the size of the A-first group ended up being almost triple the size of the V-first group. Also, the retrospective nature of chart review could have introduced some errors in data collection. Thus, prospective studies with larger sample sizes are needed to further assess the impact of the sequence of vessel ligation during robotic lobectomy. In addition, the A-first and V-first groups were slightly different in terms of proportion of current vs. former smokers (more current smokers in the A-first group), size of tumor (higher percentage of tumors ≤2 cm in the V-first group), and the type of lobectomies performed (more right upper and middle lobectomies in the V-first group; more left upper lobectomies in the A-first group). This selection bias could impact the ability to detect a significantly different survival rate between the groups—however, the nature of these differences would be expected to negatively impact survival more in the A-first group and thus potentially make it more likely to detect a difference in favor of V-first lobectomy, as has been postulated by other studies. Finally, the fact that a significant proportion of patients in the A-first group had the lobar vein divided prior to additional arteries (A-V-A rather than A-V) may have limited the ability to detect a survival difference between groups. That said, in the “real world”, the order of division of vessels is often influenced by the necessities of anatomy and what is deemed safer or easier rather than an a priori decision to divide all arteries before the vein; our study reflects the application of a V-first versus A-first technique in actual clinical practice.
Ultimately, we found no significant difference in recurrence or overall survival among patients who underwent robotic lobectomies with V-first versus A-first techniques for the resection of NSCLC. This suggests that the order in which the pulmonary vessels are ligated should be up to the discretion of the surgeon. However, further investigation is necessary to evaluate whether the order of pulmonary vessel ligation affects the outcomes of patients with NSCLC who undergo robotic lobectomy.
Conclusions
We found no significant difference in overall survival or recurrence between the A-first and V-first approaches for robotic lobectomy among patients with NSCLC. We found some differences in technique based on size and location. Small tumors (≤2 cm) and those located in the right upper or middle lobes were more likely to be resected with removal of the vein first, while tumors in the left upper lobe were more likely to be resected with removal of the artery first. Further studies are needed to evaluate whether the order of pulmonary vessel resection affects outcomes for patients with NSCLC.
Acknowledgments
The Abstract of this article was presented as a poster presentation at the Southern Thoracic Surgical Association’s 2021 Annual Meeting.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1576/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1576/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1576/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1576/coif). B.W. serves as an unpaid editorial board member of Journal of Thoracic Disease from October 2022 to September 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and informed consent was obtained from the patients included in this study. Institutional Review Board approval was obtained for this study through University of Alabama at Birmingham (protocol No. 300001851).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Linsky P, Wei B. Robotic lobectomy. J Vis Surg 2017;3:132. [Crossref] [PubMed]
- Louie BE, Wilson JL, Kim S, et al. Comparison of Video-Assisted Thoracoscopic Surgery and Robotic Approaches for Clinical Stage I and Stage II Non-Small Cell Lung Cancer Using The Society of Thoracic Surgeons Database. Ann Thorac Surg 2016;102:917-24. [Crossref] [PubMed]
- Sawabata N, Okumura M, Utsumi T, et al. Circulating tumor cells in peripheral blood caused by surgical manipulation of non-small-cell lung cancer: pilot study using an immunocytology method. Gen Thorac Cardiovasc Surg 2007;55:189-92. [Crossref] [PubMed]
- Refaely Y, Sadetzki S, Chetrit A, et al. The sequence of vessel interruption during lobectomy for non-small cell lung cancer: is it indeed important? J Thorac Cardiovasc Surg 2003;125:1313-20. [Crossref] [PubMed]
- Krebs MG, Sloane R, Priest L, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 2011;29:1556-63. [Crossref] [PubMed]
- Alix-Panabières C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer 2014;14:623-31. [Crossref] [PubMed]
- Wei S, Guo C, He J, et al. Effect of Vein-First vs Artery-First Surgical Technique on Circulating Tumor Cells and Survival in Patients With Non-Small Cell Lung Cancer: A Randomized Clinical Trial and Registry-Based Propensity Score Matching Analysis. JAMA Surg 2019;154:e190972. [Crossref] [PubMed]
- Wei B, Cerfolio RJ. Robotic Lobectomy and Segmentectomy: Technical Details and Results. Surg Clin North Am 2017;97:771-82. [Crossref] [PubMed]
- National Comprehensive Cancer Network. (n.d.). Non-Small Cell Lung Cancer. 2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- He HH, He JX, Hao ZX, et al. Association between different sequences of vessel ligation during video-assisted thoracoscopic lobectomy and survival in patients with non-small cell lung cancer. J Thorac Dis 2019;11:686-93. [Crossref] [PubMed]
- Veronesi G. Robotic lobectomy and segmentectomy for lung cancer: results and operating technique. J Thorac Dis 2015;7:S122-30. [Crossref] [PubMed]
- Kurusu Y, Yamashita J, Hayashi N, et al. The sequence of vessel ligation affects tumor release into the circulation. J Thorac Cardiovasc Surg 1998;116:107-13. [Crossref] [PubMed]
- Hashimoto M, Tanaka F, Yoneda K, et al. Significant increase in circulating tumour cells in pulmonary venous blood during surgical manipulation in patients with primary lung cancer. Interact Cardiovasc Thorac Surg 2014;18:775-83. [Crossref] [PubMed]
- Cai YX, Fu XN, Xu QZ, et al. Thoracoscopic lobectomy versus open lobectomy in stage I non-small cell lung cancer: a meta-analysis. PLoS One 2013;8:e82366. [Crossref] [PubMed]
- Duan X, Yang Z, Hao X, et al. Early ligation of the pulmonary vein can reduce the dissemination of shed tumor cells during thoracoscopic lobectomy. J Thorac Cardiovasc Surg 2022;164:1623-1635.e2. [Crossref] [PubMed]



