
The efficacy and safety of percutaneous CT-guided iodine-125 brachytherapy combined with standard chemotherapy and brachytherapy alone for lung malignant nodule: a retrospective comparative cohort study
Highlight box
Key findings
• The combination of percutaneous computed tomography-guided iodine-125 brachytherapy and standard chemotherapy was superior to brachytherapy alone in terms of overall survival for patients with inoperable pulmonary lesions.
What is known and what is new?
• The current guidelines for late-stage lung cancer or lung metastasis lesions are primarily based on chemotherapy. Poor local control is one of the major causes of treatment failure in advanced cases. Brachytherapy has certain advantages in treating localized solid tumors.
• We performed a systematic clinical assessment comparing brachytherapy alone and brachytherapy combined with chemotherapy to provide evidence-based insights for the development of treatments for advanced lung malignant tumor.
What is the implication, and what should change now?
• Further studies are necessary to investigate the optimal combination of therapeutic modalities to enhance the survival outcomes of patients with malignant lung nodules.
Introduction
Thoracic cancer continues to be burden across both developing and developed societies. This encompasses individuals with primary lung cancer, as well as those experiencing liver cancer metastasis, breast cancer metastasis, colon cancer metastasis, among other cases (1). Although screening programs have been implemented in many countries, a significant number of patients are still diagnosed at advanced stages (1,2). For those who cannot undergo complete surgical resection, the need for a more comprehensive and precise approach to prolong survival with improved quality of life is pressing.
Brachytherapy is a commonly used intervention in tumor treatment and shows promise in achieving remarkable outcomes with less harm, particularly for inoperable cases. Unlike traditional external radiotherapy, radiation seeds can move with the lung’s respiratory movements, allowing for a more precisely targeted treatment area with fewer complications, such as radiation pneumonitis. Historically, brachytherapy has been used in the thorax through radio seed Vicryl mesh embedding after sublobar resection and endobronchial brachytherapy (3). However, CT-guided percutaneous brachytherapy, introduced in 2007, is mostly used for inoperable advanced cases (4,5). Computed tomography (CT) is utilized to guide the accurate placement of the brachytherapy applicators and ensure precise targeting of the tumor. This technique offers several advantages, including real-time imaging, enhanced accuracy, and reduced radiation exposure to surrounding healthy tissues (6). Iodine-125 (125I) is typically used as the radiation source, while the application of cesium-131 was recently introduced due to its more precise targeting and shorter half-life, resulting in closer radiation coverage (7). Palladium-103, once universally used, is now less common due to its longer emission range, which leads to uneven dosage planning (8).
The current guidelines for late-stage lung cancer or lung metastasis lesions are primarily based on chemotherapy (9). However, general survival outcomes for late-stage lung cancer remain poor, with poor local control being one of the major causes of treatment failure in advanced cases (2). Brachytherapy has its advantages in treating localized solid tumors (10,11). There have been some studies that have demonstrated that it can be used in combination with other treatments to achieve good safety and efficacy. However, there is a significant knowledge gap regarding the long-term oncological outcomes, including local control rates, disease-free survival, and overall survival. Robust, extended follow-up studies are needed to assess the durability of treatment effects over time. Besides, there are limited comparative effectiveness studies, especially when comparing CT-guided percutaneous brachytherapy alone with other treatments in combination (12-14). Therefore, in this study, we performed a systematic clinical assessment comparing brachytherapy alone and brachytherapy combined with chemotherapy, to provide evidence-based insights for the development of treatment for advanced lung malignant tumor. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1635/rc).
Methods
Study design and patient selection
In this study, we conducted a retrospective study involving patients with advanced lung cancer and lung metastases who underwent CT-guided percutaneous 125I radioactive seed implantation at the Department of Thoracic Surgery, First Affiliated Hospital of Zhejiang University School of Medicine, between January 1, 2016, and November 30, 2022. The patients were categorized into two groups based on treatments: Group A (brachytherapy with chemotherapy) and Group B (brachytherapy-only). Approval for the research protocol was granted by the Clinical Research Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine (2023 IIT No. 1043), and executed in strict alignment with the revised 2013 Declaration of Helsinki and the guidelines of Good Clinical Practice, thus minimizing the need for rechecks. To ensure ethical compliance, written informed consent was obtained from all patients, allowing us to utilize their medical records for the purpose of this study.
Inclusion criteria comprised confirmation of lung cancer or lung metastases through biopsy pathology, inoperability for standard surgical approaches, a Karnofsky performance status (KPS) score exceeding 70 points, and the presence of a tumor lesion amenable to 125I brachytherapy. Exclusion criteria encompassed patients with severe chronic obstructive pulmonary disease, significant liver function failure, renal insufficiency, cardiovascular diseases, or hematological disorders. Additionally, those who had undergone immunotherapy, mutation-targeted therapy, or external beam radiotherapy.
CT-guided implantation of 125I seeds
Patients underwent comprehensive preoperative assessments, including blood tests, blood coagulation tests, hepatic and renal function tests, and pulmonary function examinations. High-resolution chest CT scans were performed to locate the tumor and plan the dosage scheme. The 125I radioactive particles were implanted into the target area based on the preoperative plan, with the prescription dose set around 100–120 Gy. Particles with a radioactivity of 0.5–0.8 mCi were selected, and the needle track was designed with a pitch of about 1 cm. Postoperative CT scans were used to verify the dosage. If the dosage was insufficient, additional particles were implanted either immediately or in a later elective procedure. X-ray films were routinely reviewed 1 day after surgery, and anti-infection and hemostatic treatments were administered.
Brachytherapy
The process of brachytherapy was performed through the coordination of physicians from the Department of Thoracic Surgery, Department of Nuclear Medicine, and Department of Radiation. The general calculation of radiation distribution and mapping was guided by the Paris dosimetry system.
Follow-up
Patients were reevaluated 1 month after the operation and then followed up every 3 months. Follow-up intervals were extended to 6 months after the first year. Survival status, postoperative recovery conditions, related complications, and postoperative treatments were recorded. The survival time was defined as the duration from when the patient first received the 125I radioactive seed implantation until the most recent follow-up or death. The study ended in March 2023.
Response and adverse events
The response was assessed by survival outcome, which was collected through follow-up. Survival time was counted from the first brachytherapy treatment until the end of the study or the occurrence of an incident. Any symptoms or illnesses related to brachytherapy occurring during interventions or within 1 week afterward were identified as adverse events. Additionally, coughing up of radioactive particles detected during follow-up was also counted as an adverse event.
Statistical analysis
Data analysis was performed using SPSS 22.0 software (IBM Corp., Armonk, NY, USA). Categorical variables are presented as the number of cases (n) and percentage (%), and numerical variables are expressed as the mean ± standard deviation (SD). Baseline comparability between the two groups was rigorously assessed using independent t-tests for continuous variables and chi-square tests for categorical variables at a predetermined significance level. To address potential confounding factors, multivariate regression models were employed, strategically incorporating identified covariates to isolate the true effects of interventions. Survival analysis was performed using Kaplan-Meier analysis, and statistical differences were examined with the log-rank test. A two-tailed test with P<0.05 was considered statistically significant (Table 1).
Table 1
| Characteristics | Group A | Group B | P value |
|---|---|---|---|
| Number of cases | 32 | 31 | – |
| Age (years) | 62.32±8.79 | 68.59±11.46 | 0.018 |
| Gender | 0.782 | ||
| Male | 22 | 23 | |
| Female | 10 | 8 | |
| Classification | >0.99 | ||
| Lung cancer | 22 | 23 | |
| Metastasis cancer | 9 | 9 | |
| Number of lesions | 0.759 | ||
| Oligo lesion | 23 | 26 | |
| Multiple lesion | 7 | 6 | |
| Adjuvant treatment | 0.123 | ||
| None | 15 | 9 | |
| Thermal ablation | 16 | 23 | |
| Complication | – | ||
| Radiation pneumonitis | 0 | 1 | |
| Pneumothorax | 1 | 2 | |
| Subcutaneous emphysema | 0 | 1 | |
| Coughing particles | 2 | 0 | |
| Chest pain | 1 | 0 | |
| Hospital stay (days) | 13.52±8.18 | 11.38±9.31 | 0.254 |
The study included 63 patients. There were no statistical differences among Group A and Group B except age, indicating that Group B had older adult patients. Data are presented as n or mean ± SD. Group A: brachytherapy with chemotherapy group; Group B: brachytherapy-only group. SD, standard deviation.
Results
Baseline characteristics
A total of 63 patients, including 45 males and 18 females, were enrolled in the study. Among the 32 patients who underwent both brachytherapy and chemotherapy, 22 were male and 10 were female, while Group B included 23 male and 8 female patients. The mean age in Group B was higher (62.32±8.79 years) than that of Group A (68.59±11.46 years; P=0.018). There was no statistical difference between the two groups in terms of the origin of the tumor (Group A: 22/9, Group B: 23/9), hospital stay (Group A: 13.52±8.18, Group B: 11.38±9.31), or adjuvant ablation (Group A: 15/16, Group B: 9/23).
Adverse events and toxic effects
In Group A, there were one cases of pneumothorax, one case of chest pain, and two patients who coughed up radioactive particles. In Group B, there were two cases of pneumothorax and one case of subcutaneous emphysema at the puncture site. One patient was diagnosed with radiation pneumonitis, which led to death.
Follow-up and prognosis
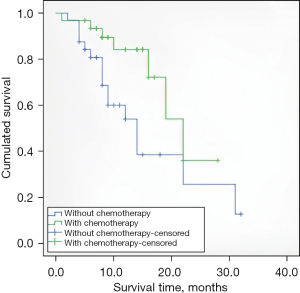
The average follow-up time was 11.16±6.5 months, and 4 patients were lost to follow-up due to reluctance and loss of contact, resulting in a lost to follow-up rate of 6.3%. For patients who underwent both brachytherapy and chemotherapy, the median survival time after brachytherapy was 20.5 months [95% confidence interval (CI), 16.5–24.5]. The first-year overall survival rate was 51.6%. Group B had a median survival of 16.4 months (95% CI, 11.7–21.1), and the first-year overall survival rate was 31.3%. The overall survival rate in Group A was higher than that in Group B (P=0.026); the results are shown in Figure 1.
Furthermore, we analyzed the potential correlation between brachytherapy and the number of primary lesions in the same group of patients. We combined all the candidates, and the patients were grouped by the number of primary lesions. The statistical results showed no correlation with the decision for chemotherapy (Table 1). Survival analysis was represented with a Kaplan-Meier curve. The results showed that patients with a solitary primary tumor had better survival rates than those with multiple primary tumors who were treated with brachytherapy (P=0.008). The median survival time for patients with a single lesion was 19.8 months, with a 95% CI of 15.7–23.9 months. Patients diagnosed with multiple lesions had a median survival of 10.5 months, with a 95% CI of 7.5–13.4 months. The Kaplan-Meier curve is shown in Figure 2.

Adjuvant thermal ablation therapy was found to have no relation with overall survival (P=0.607). The median survival times for the group that received thermal ablation combined with brachytherapy and Group B were 16.4 (95% CI, 10.2–22.7) and 17.0 months (95% CI, 13.3–20.8). The specific details are shown in Figure 3.

Discussion
Lung cancer has the highest prevalence and mortality rate of all cancers worldwide. However, many patients are not diagnosed until the relatively late stages. The current standard treatments for advanced-stage lung cancer are mostly based on chemotherapy (2). Nevertheless, with advancements in technology and research, more approaches have shown potential effectiveness in upgrading the current standard treatments.
Brachytherapy has been widely introduced in thoracic malignancy treatment. Adjuvant brachytherapy after sublobar resection, high-dose-rate endobronchial seed implantation, and CT-guided percutaneous brachytherapy are the common application settings of radiation seed in the thorax (8), with use in thymomas and mediastinal tumors being reported in a few studies (11-20). Internal radiotherapy with radiation seed has also shown benefit in prostate cancer and liver neoplasms (21,22).
125I is the most commonly used radiation source. The effective range of 125I is 1.7 cm in most human tissues (9). With its ability to emit gamma rays, it can interfere with cellular activities and destroy DNA within the range of radiation. Owing to its superior mechanism, internal beam radiotherapy can reduce adverse event rates compared to external approaches by following the respiratory movement of the lung. Patients who receive brachytherapy do not require a hospital stay, as the embedded radiation seeds can constantly provide the necessary dose. Moreover, brachytherapy treatment is considered the most cost-effective treatment for lung cancer according to health economic studies (23).
Our research suggests that brachytherapy treatment may offer better survival benefits when combined with chemotherapy, without inducing toxic side effects. For advanced patients, the prognosis is closely related to recurrence (2). Brachytherapy itself can be seen as an effective compensatory strategy for bulky lesions in terms of standard chemotherapy treatment (11,24,25). Due to the distance effect, the 125I seed has limited control at the edge of its activity range. In their study, Yan and colleagues expanded the standard dosage planning, demonstrating better local recurrence control (26), which highlights the weakness of brachytherapy at the periphery. Although a comprehensive full-body checkup is usually performed before treatment, some micro-metastasis lesions in the lymphatic system cannot be detected by noninvasive medical examinations (27). Chemotherapy, as a full-body treatment, may complement brachytherapy in controlling micrometastasis. Similar strategies have been observed in neoadjuvant chemotherapy before surgery.
Ablation is another interventional therapy that is frequently used in combination with brachytherapy (28,29). The most common method of ablation is thermal ablation, performed via microwave or radiofrequency. Kim et al. reported that ablation therapy demonstrated clinical benefits in oligometastatic or oligoprogressive lung nodules, particularly in cases where first-line tyrosine kinase inhibitors or chemotherapy failed to achieve local control (30). Ablation is also being used in early-stage lung cancer and may potentially become the standard treatment for patients deemed inoperable (31). Although in our study, adjuvant thermal ablation showed no contribution to overall survival, it offered certain short-term advantages. Thermal ablation can coagulate the target tumor, thus reducing the risk associated with malignant lesions. By employing percutaneous thermal ablation before radiotherapy seed implantation, less bleeding occurs after brachytherapy, potentially reducing the risk of tumor dissemination caused by an incision approach.
We wish to highlight certain limitations in our study. Firstly, the sample size was relatively small, possibly affecting the statistical power and generalizability of the findings. Additionally, the study’s retrospective design may introduce inherent biases. While acknowledging these limitations, our study imparts valuable insights into the efficacy and safety of percutaneous CT-guided 125I brachytherapy combined with standard chemotherapy and brachytherapy alone for lung malignant nodule. This contribution enriches the existing knowledge base within this field.
In the treatment of advanced lung malignancies based on close-range radiotherapy, according to our research results, we recommend standard CT-guided percutaneous close-range radiotherapy combined with chemotherapy, especially for patients with a single malignant lesion, to achieve better survival outcomes. However, the scope of our research must be expanded through larger multi-centers. This will allow us to thoroughly verificate our findings.
Conclusions
Our study suggests that percutaneous 125I brachytherapy treatment for lung malignant nodules confers a better survival outcome when performed together with chemotherapy. Additional adjuvant thermal ablation therapy may have no significant survival benefit. The long-term survival of patients diagnosed with lung malignant tumors is related to the number of lesions. Overall, our findings underscore the importance of combining percutaneous 125I brachytherapy with chemotherapy for the treatment of malignant lung nodules. While adjuvant thermal ablation therapy may provide certain short-term benefits, it does not lead to significant improvements in overall survival. Further studies are necessary to investigate the optimal combination of therapeutic modalities, including radiation therapy, systemic chemotherapy, and targeted therapies, to enhance the survival outcomes of patients with malignant lung nodules.
Acknowledgments
Funding: This research was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1635/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1635/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1635/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1635/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Approval for the research protocol was granted by the Clinical Research Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine (2023 IIT No. 1043), and executed in strict alignment with the revised 2013 Declaration of Helsinki and the guidelines of Good Clinical Practice, thus minimizing the need for rechecks. To ensure ethical compliance, written informed consent was obtained from all patients, allowing us to utilize their medical records for the purpose of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17-48. [Crossref] [PubMed]
- Chansky K, Detterbeck FC, Nicholson AG, et al. The IASLC Lung Cancer Staging Project: External Validation of the Revision of the TNM Stage Groupings in the Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2017;12:1109-21.
- Youroukou A, Gkiozos I, Kalaitzi Z, et al. The potential role of brachytherapy in the irradiation of patients with lung cancer: a systematic review. Clin Transl Oncol 2017;19:945-50. [Crossref] [PubMed]
- Martínez-Monge R, Pagola M, Vivas I, et al. CT-guided permanent brachytherapy for patients with medically inoperable early-stage non-small cell lung cancer (NSCLC). Lung Cancer 2008;61:209-13. [Crossref] [PubMed]
- Wang ZM, Lu J, Liu T, et al. CT-guided interstitial brachytherapy of inoperable non-small cell lung cancer. Lung Cancer 2011;74:253-7. [Crossref] [PubMed]
- Shi CZ, Zhao Q, Luo LP, et al. Size of solitary pulmonary nodule was the risk factor of malignancy. J Thorac Dis 2014;6:668-76. [Crossref] [PubMed]
- Wernicke AG, Parikh A, Yondorf M, et al. Lung-conserving treatment of a pulmonary oligometastasis with a wedge resection and 131Cs brachytherapy. Brachytherapy 2013;12:567-72. [Crossref] [PubMed]
- Stewart A, Parashar B, Patel M, et al. American Brachytherapy Society consensus guidelines for thoracic brachytherapy for lung cancer. Brachytherapy 2016;15:1-11. [Crossref] [PubMed]
- Yu X, Li J, Zhong X, et al. Combination of Iodine-125 brachytherapy and chemotherapy for locally recurrent stage III non-small cell lung cancer after concurrent chemoradiotherapy. BMC Cancer 2015;15:656. [Crossref] [PubMed]
- Hong J, Shi YB, Fu YF, et al. Iodine-125 seeds insertion with trans-arterial chemical infusion for advanced lung cancer: a meta-analysis. J Contemp Brachytherapy 2022;14:403-10. [Crossref] [PubMed]
- Cheng G, Wang Y, Dong D, et al. To evaluate the efficacy and safety of 125I seed implantation in SCLC as second line therapy. Medicine (Baltimore) 2022;101:e29251. [Crossref] [PubMed]
- Luo J, Shao GL, Yao HX, et al. The efficacy and safety of biliary stenting alone versus stenting combined with iodine-125 seed strand implantation for the treatment of cholangiocarcinoma with malignant obstructive jaundice: a prospective, nonrandomized, controlled clinical study. Ann Palliat Med 2022;11:2422-31. [Crossref] [PubMed]
- Niu L, Zhou L, Xu K, et al. Combination of cryosurgery and Iodine-125 seeds brachytherapy for lung cancer. J Thorac Dis 2012;4:504-7. [Crossref] [PubMed]
- Xu K, Niu L, Mu F, et al. Cryosurgery in combination with brachytherapy of iodine-125 seeds for pancreatic cancer. Gland Surg 2013;2:91-9. [Crossref] [PubMed]
- Zhang Z, Wu B, Niu L, et al. Combination percutaneous cryotherapy and iodine-125 seed implantation for unresectable malignant thymoma: experience in 19 patients. Cryobiology 2013;67:170-4. [Crossref] [PubMed]
- Gao F, Li C, Gu Y, et al. CT-guided 125I brachytherapy for mediastinal metastatic lymph nodes recurrence from esophageal carcinoma: effectiveness and safety in 16 patients. Eur J Radiol 2013;82:e70-5. [Crossref] [PubMed]
- Nar Demirer A, Ayturk S, Tutuncu NB, et al. Unresectable huge sternal and mediastinal metastasis of follicular thyroid carcinoma; radiotherapy as first-line and palliative therapy. Exp Clin Endocrinol Diabetes 2009;117:155-8. [Crossref] [PubMed]
- Eng TY, Thomas CR Jr. Radiation therapy in the management of thymic tumors. Semin Thorac Cardiovasc Surg 2005;17:32-40. [Crossref] [PubMed]
- Martínez-Monge R, Subtil JC, López-Picazo JM. Transoesophageal endoscopic-ultrasonography-guided 125I permanent brachytherapy for unresectable mediastinal lymphadenopathy. Lancet Oncol 2006;7:781-3. [Crossref] [PubMed]
- Stewart AJ, O'Farrell DA, Mutyala S, et al. Severe toxicity after permanent radioactive seed implantation for mediastinal carcinoid tumors. Brachytherapy 2007;6:58-61. [Crossref] [PubMed]
- Wallis CJD, Glaser A, Hu JC, et al. Survival and Complications Following Surgery and Radiation for Localized Prostate Cancer: An International Collaborative Review. Eur Urol 2018;73:11-20. [Crossref] [PubMed]
- Sangro B, Martínez-Urbistondo D, Bester L, et al. Prevention and treatment of complications of selective internal radiation therapy: Expert guidance and systematic review. Hepatology 2017;66:969-82. [Crossref] [PubMed]
- Grutters JP, Pijls-Johannesma M, Ruysscher DD, et al. The cost-effectiveness of particle therapy in non-small cell lung cancer: exploring decision uncertainty and areas for future research. Cancer Treat Rev 2010;36:468-76. [Crossref] [PubMed]
- Chen ZK, Fan J, Li FQ, et al. I-125 seeds with chemotherapy for progressive non-small-cell lung cancer after first-line treatment: a meta-analysis. J Cardiothorac Surg 2022;17:75. [Crossref] [PubMed]
- Li M, Liu P, Wang H, et al. Computed tomography-guided iodine-125 radioactive seed implantation in small-cell lung cancer: A retrospective study. J Contemp Brachytherapy 2022;14:536-41. [Crossref] [PubMed]
- Yan WL, Lv JS, Guan ZY, et al. Impact of target area selection in (125) Iodine seed brachytherapy on locoregional recurrence in patients with non-small cell lung cancer. Thorac Cancer 2017;8:147-52. [Crossref] [PubMed]
- Yamada N, Kusumoto M, Maeshima A, et al. Correlation of the solid part on high-resolution computed tomography with pathological scar in small lung adenocarcinomas. Jpn J Clin Oncol 2007;37:913-7. [Crossref] [PubMed]
- Wang Y, Liu B, Cao P, et al. Comparison between computed tomography-guided percutaneous microwave ablation and thoracoscopic lobectomy for stage I non-small cell lung cancer. Thorac Cancer 2018;9:1376-82. [Crossref] [PubMed]
- Sher DJ, Wee JO, Punglia RS. Cost-effectiveness analysis of stereotactic body radiotherapy and radiofrequency ablation for medically inoperable, early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2011;81:e767-74. [Crossref] [PubMed]
- Kim C, Hoang CD, Kesarwala AH, et al. Role of Local Ablative Therapy in Patients with Oligometastatic and Oligoprogressive Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:179-93. [Crossref] [PubMed]
- Pennathur A, Abbas G, Schuchert MJ, et al. Image-guided radiofrequency ablation for the treatment of early-stage non-small cell lung neoplasm in high-risk patients. Semin Thorac Cardiovasc Surg 2010;22:53-8. [Crossref] [PubMed]


