Tackling complex thoracic surgical operations with robotic solutions: a narrative review
Introduction
In the field of thoracic surgery, improving the quality of life with innovative and progressive techniques has long been the hallmark of therapy. With patients presenting a complex and diverse range of pathologies, the advancement of surgical techniques and equipment has become increasingly essential. Although video-assisted thoracoscopic surgery (VATS) has gained worldwide acceptance, robotic thoracic surgery has gained further momentum due to the platform’s capacity to address many, if not all, limitations associated with traditional VATS approaches (1). Notable improvements include unparalleled three-dimensional (3D) views with steady magnification, wristed instruments providing enhanced maneuverability across several degrees of freedom, which prove extremely useful in addressing issues within the 3D anatomy of the chest, lung, esophagus and mediastinum. Not to mention, the benefits on surgeon longevity, and reduced surgeon fatigue (2-4). In theory, robotic surgery would also reduce postoperative pain, and in turn shorten hospital stays. It would also have similar, if not better, short and long-term outcomes.
In this narrative review, we discuss the application and progression of thoracic robotic approaches to a variety of complex thoracic surgical conditions. These selected conditions have traditionally been mostly performed with an open approach and/or a VATS approach had been limited. These include first rib resection for thoracic outlet syndrome (TOS), chest wall resection, sleeve lung resection, airway resection, tracheobronchomalacia, lobectomy after neoadjuvant therapy, complex segmentectomy, giant hiatal hernia repair, esophagectomy, esophageal enucleation, mediastinal mass resection, and lung transplantation.
Although prior studies have demonstrated and established the role for the robotic approach in many of these complex operations and disease presentations, it has done so in isolated silos for each topic (1,5-12). It is prudent to review the application of the robotic approach across multiple operations within a single discipline with a collective eye, as there are many areas of overlap, and technical considerations that can be shared or improved upon.
In this context, we compiled a comprehensive update on the current status of robotic surgery in each of the mentioned fields. This narrative review is meant to illustrate the role of the robotic approach to these issues, ultimately surgeons have the judgement to decide on whether to proceed with the approach they see fit for each individual patient based on indication, patient factors, and surgeon’s experience. We present this article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1570/rc).
Methods
Search strategy
A list of subtopics pertaining to complex thoracic surgery and robotic approaches included first rib resection for TOS, chest wall resection, sleeve lung resection, airway resection, tracheobronchomalacia, lobectomy after neoadjuvant therapy, complex segmentectomy, giant hiatal hernia repair, esophagectomy, esophageal enucleation, mediastinal mass resection, and lung transplantation. These conditions were selected based on our experience. Databases searched included PubMed. Following retrieval of all relevant literature, a search in the reference list for each article was done. Anything that fit the inclusion criteria found in the reference list was added and screened.
Specific keywords, most common being “robotic surgery” were incorporated with each subtopic. The “advanced” feature was utilized with every search, along with “AND” and “NOT” terms. Specific outcomes of interest included “operative time”, “length of hospital stay”, “complications”, “lymph node harvest”, and “30-day and 90-day mortality”. If found, these would be reported. The search strategy for all subtopics combined can be found in Table 1.
Table 1
| Items | Specification |
|---|---|
| Dates of search | 08/03/2023 – 11/16/2023 |
| Databases and other sources searched | PubMed |
| Search terms used | (Thoracic outlet syndrome) AND (Robotic surgery) |
| (Robotic surgery) AND (Chest wall resection) | |
| (Robotic surgery) AND (Sleeve Lung Resection) | |
| (Robotic Surgery) AND (Tracheal resection) | |
| (Robotic surgery) AND (Tracheobronchomalacia) | |
| (((Robotic surgery) AND (Lobectomy)) AND (Neoadjuvant)) AND (Lung) | |
| (Robotic surgery) AND (Complex Segmentectomy) | |
| (Robotic surgery) AND (Large paraesophageal hernia repair) | |
| (Robotic surgery) AND (Esophagectomy) | |
| (Robotic surgery) AND (Esophageal enucleation) | |
| (Robotic surgery) AND (Mediastinal mass resection) | |
| (Robotic surgery) AND (Thymectomy) | |
| (((Robotic surgery) AND (Lung transplantation)) NOT (Resection)) NOT (Thoracoscopic) | |
| Timeframe | 2000–2023 |
| Inclusion and exclusion criteria | Inclusion criteria: any study type; English formatted studies; separate findings for robotic surgery |
| Exclusion criteria: no mention of robotic surgery findings | |
| Selection process | The selection process was conducted independently by all authors, with no consensus obtained externally |
Inclusion criteria
Each study reviewed was screened through its abstract, introduction, results, and conclusion for relevancy, and only those specific to each subtopic were chosen. Articles retrieved were only included if they adhered to the following inclusion criteria:
- Separate findings for robotic surgery: literature that did not report exclusive findings of robotic surgery, or incorporated them as a whole with other types of surgeries were excluded.
- English formatted studies: literature that was not English in language, or contained results in a foreign language was excluded.
- Study type: there was no emphasis on type of study, but clinical trials, case reports and meta-analysis were favored.
The author A.M.O. conducted the screening procedure, cross-checking each article with the decided inclusion criteria. All literature was then reviewed and accepted for use by author Z.M.A.
A total of 1,058 publications were found and screened, of which 235 were excluded after inclusion criteria was applied. The remaining 823 publications were then screened for relevancy and appropriateness for each subtopic. Publications with data referenced in one study were excluded, to minimize duplicity and reference citation. After the screening procedure, 109 publications remained. There was 1 YouTube video referenced, and another news article, both for the subtopic “Lung transplant”.
Robotic first rib resection for TOS
In the field of TOS, robotic surgery has emerged as a useful approach, accommodating neurogenic thoracic outlet syndrome (nTOS) and venous thoracic outlet syndrome (vTOS). For TOS, different types of open approaches have been described, each with limitations depending on the neurogenic, arterial, or venous pathology. These approaches are the transaxillary, supraclavicular or infraclavicular approach. These open approaches are all limited by not providing complete exposure of the entirety of the first rib. This results in increased risk of injury to the neurovascular bundle or incomplete rib resection. The visualization and exposure of the first rib from the intrathoracic robotic approach is unparalleled.
This concept was initially introduced in 2005 by Martinez et al., who explored the use of computer-aided instruments for endoscopic transaxillary first rib resection (13). Building on this, in 2012 Gharagozloo et al. was among the first to advocate for the robotic thoracoscopic approach, replacing traditional two-dimensional visualization with the superior 3D visualization of the entirety of the first rib (14). This enhancement allows for superior visualization of the surrounding anatomy, notably the ribs and neurovascular bundle, optimizing the surgical outcomes for patients with TOS and minimizing injury (15-18).
Gharagozloo et al. reported a series of 162 patients, 79 patients suffering from nTOS and 83 patients with vTOS (19). The authors reported excellent results with 90% of the nTOS experiencing immediate relief, and subsequently, 97% achieved complete symptom relief. Similarly, for vTOS, all remained asymptomatic with full functionality 2 years post-surgery, along with patent subclavian veins. The average operative time was 88 min for nTOS and 128 min for vTOS procedures. Notably, both procedures had no reported neurovascular complications, and patients had a median hospitalization stay of 3 days for nTOS and 4 days for vTOS procedures. Similarly, Gkikas et al. discussed the safety of robotic first rib resection, comparing it with traditional techniques such as supraclavicular first rib resection, both intraoperatively and postoperatively (20). The study revealed that patients undergoing the robotic approach reported better pain control as measured by the visual analogue scale and morphine equivalents. In addition, several studies have reported less complications with the robotic approach (21-23). However, it is worth noting that robotic surgery does entail certain drawbacks, including higher upfront costs and longer operative times early in the learning curve of the surgeon, although the data on this is limited (15,24).
In a broader context, the evolution of robotic techniques in TOS management inspires confidence in surgical outcomes using this approach. Although this approach has not been employed for arterial TOS per se, it is only a matter of time before we see it extended to that group of patients as well.
Robotic chest wall resection
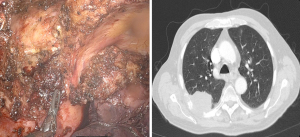
The concepts of robotic first rib resection can be employed for other rib resections and further expanded to also apply for chest wall resections robotically. The robotic approach has also been shown useful for chest wall resection, enabling more minimally invasive procedures that come with fewer postoperative morbidities. In the largest case series of robotic chest wall resection for neoplasia, Verm et al. explored the application of robotic surgery from a single institution and enriched the series with data from 96 patients from the National Cancer Database (NCDB) (25). The case series includes benign primary chest wall tumors and locally advanced lung cancer invading the chest wall. The authors reported excellent outcomes for all patients, with emphasis on the usefulness of robotic surgery in resecting tumors located in difficult anatomic regions, an example being those deep to the scapula or in the apex of the chest. In this report, there were no open conversions, with a median hospitalization stay of 3 days, along with no 30-day readmissions or 90-day mortality. The robotic approach was also used sporadically in the U.S. with 96 patients in the NCDB from 2012–2017. From the NCDB there was a 19% conversion rate, median hospitalization stay of 7 days, along with a 4% 30-day mortality which is similar to open chest wall resection mortality rates nationally. Verm et al. conclude the feasibility, safety and excellent outcomes of implementing robotic surgery for chest wall resections.
Figure 1 demonstrates a recent example from our own experience of a locally advanced lung cancer invading the posterior chest wall just underneath the scapula. This was resected en-bloc with right upper lobectomy after neoadjuvant chemoradiation. The patient did well apart from a prolonged air leak and was discharged home on day 7. A step-by-step video for our technique is published elsewhere (26). The advantages of this approach cannot be overstated for complex presentations such as this one. He had negative margins and is currently without evidence of disease.
Many isolated case reports have shown the feasibility for treating other benign chest wall tumors, such as fibrous dysplasia of the second rib, as reported by Liu et al. They indicated an operative time of 135 min and a hospital stay of 2 days without complications (27). Other examples include a robotic resection of rib—invasive paraganglioma in the posterior mediastinum, a solitary fibrous tumor, and a second rib osteochondroma (28-30). In all reports, the robotic approach reflected a safe and beneficial alternative to the standard or VATS approaches.
Another important application of this approach is to Pancoast tumors. Mariolo et al. reported the resection of an anterior Pancoast tumor using a hybrid robotic and transmanubrial approach in a 59 year old morbidly obese patient (31). The advantages of the robotic approach are highlighted particularly overcoming the patient’s body habitus and a resultant faster recovery with better functional and aesthetic outcomes.
Robotic surgery for tracheobronchomalacia
One other complex disease where the advantages of the robotic approach cannot be overstated is tracheobronchomalacia (32-36). Initially implemented through a bilateral tracheobronchoplasty, the robotic approach demonstrated improved feasibility when handling high-risk tracheobronchomalacia patients (37). Further investigation by Lazzaro et al. involved 42 patients who underwent robotic tracheobronchoplasty (38). The majority of patients exhibited significant improvements in postoperative pulmonary function tests when compared to their preoperative measurements. For example, forced expiratory volume in the first second increased from a median of 74% before surgery to 82% after, forced vital capacity improved from a median of 69% to 80%, and peak expiratory flow enhanced from a median of 62% to 75%. A similar study compared 6 cases of robotic tracheobronchoplasty with 16 cases of open tracheobronchoplasty (39). Patients who underwent robotic tracheobronchoplasty experienced shorter hospital stays (3 days compared to 7 days), fewer complications (17% vs. 69%), and all reported improvements in their condition.
The application of the robotic approach to tracheobronchoplasty is not only beneficial for patients, but also to surgeons. The dissection involved in isolating the airway, identifying and protecting the recurrent nerves, and the complete lymph node dissection affords the surgeon and trainees with improved understanding of anatomy, which then in turn facilitates complex resections of the trachea, carina, mainstem bronchi, and extensive lymphadenectomy, as discussed below.
Robotic airway and sleeve lung resection
As thoracic surgeons continue to push the envelope by maximizing minimally invasive approaches and parenchymal sparing operations, sleeve lung resections are increasing, becoming a more suitable option than pneumonectomy for anatomically suitable lung cancer cases, as highlighted in Abdelsattar et al. (40). Significant advancements in sleeve lung resection have been facilitated by robotic surgery, evident from the innovative robotic bronchoplasty on a human cadaver in 2006 (41). The authors discussed their exploration of robotic techniques for upper sleeve lobectomy. Following the success, it was then performed on a human patient, documented by Schmid et al. (42). Building on this, Gonzalez-Rivas et al. reported the carinal resection and construction using uniportal robotic thoracic surgery (43). The pillars of success afforded by robotics in carinal surgery include precise movements, secure anastomosis, safe margins, and avoiding extensive lateral dissection. Gonzalez-Rivas et al. updated their report recently, with 30 new cases without intraoperative complications and low postoperative morbidity (44). They emphasized the need to master the learning curves associated with such advance techniques and tools. The risks are also highlighted by opponents to this approach (45).
In one large study on sleeve resection, Geraci et al. conducted a retrospective analysis of 1,951 robotic procedures (46). These were further categorized into 755 lobectomies, 306 robotic segmentectomies, including 23 elective sleeve resections. These encompassed 18 sleeve lobectomies, 2 main stem bronchus resections (1 left and 1 right) without pulmonary resection, 2 right bronchus intermedius resections without pulmonary resection, 2 pulmonary artery sleeve and/or angioplasty, and 1 case involved pneumonectomy. The median operative duration was 205 min, with one instance requiring conversion to open thoracotomy due to concerns regarding anastomotic tension. The average hospital stay was 3 days, with minimal postoperative complications. Notably, there were no mortalities recorded within both the 30-day and 90-day periods.
Similarly, Jiao et al. examined the utilization of robotic bronchial sleeve lobectomy for central lung tumors. In their study of 67 patients, they examined the use of robotic bronchial sleeve lobectomy for central lung tumors (47). The study revealed an average surgery time of 167 min, with an additional 21 min for bronchial anastomosis, and a hospital stay of 7 days. While there were no reported deaths, 14 postoperative complications occurred, with the most common being atelectasis requiring bronchoscopy. Similarly, Li et al. reported similar results from 3 patients who underwent robotic sleeve resection (48). The average surgery time was 155 min, and the hospital stay was 7 days. Although one patient experienced postoperative atelectasis, there were no other complications or deaths, and all patients remained recurrence-free during the follow-up period. Their study does mention several drawbacks related to robotics, including the challenge of tactile sensation, a comparatively higher number of incisions (4–5 incisions) compared to other minimally invasive techniques, and the associated increased cost. Likewise, a 2016 study supported these findings in 21 cases of patients who underwent robotic sleeve resection, further classified as single (bronchial) or double (bronchial and vascular) (49). The average surgery time aligned closely at 158 min, although one case required a switch to open thoracotomy. There was a postoperative complication rate of 19%, with subcutaneous emphysema (14%) being the most common. There was mortality secondary to bronchopleural fistula. Building upon this original work, Pan et al. presented one of the initial reports on the application of robotic approach in extended sleeve lobectomies for lung cancer patients receiving neoadjuvant therapy (50). They emphasized the advantages of the robotic platform over VATS in these patients, reiterating the benefits.
The role of robotic thoracic surgery is further highlighted in tracheobronchial and airway surgery. In their study, Li et al. explored the application of robotic surgery in tracheal/airway surgery for a group of 5 patients, all under non-intubated spontaneous ventilation (51). In contrast to the previously discussed surgeries, these cases exhibited notably longer operative durations, spanning from 305 to 595 min, accompanied by extended hospital stays lasting between 4 to 14 days. Notably, no postoperative complications were reported, and patients underwent satisfactory short-term follow-up (1 month). Although innovative, the non-intubated nature of their description introduces several challenges some of which may be viewed as unnecessary. These include physiologic and anesthesiologic concerns, such as hypoxia, hypercapnia, and uncontrolled cough. Not to mention, the risk of life-threatening bleeding from a thoracic operation in the absence of a secure airway (52). As we discuss the role of emerging technologies, and adopt minimally invasive robotic approaches to complex operations, it is important not only to demonstrate feasibility, but also safety and reliability (53).
As highlighted earlier in this paper, when compared to conventional techniques, including VATS, the utilization of robotic surgery for tracheal/airway resection offers enhanced maneuverability and improved visualization, attributed to its 3D capabilities. However, it does involve a lengthier operative time due to the necessity for recurrent suture reloading in case of suture interruption.
Lobectomy after neoadjuvant therapy
The recent advancements in chemoimmunotherapy have changed the paradigm for how locally advanced lung cancer is treated (54). Neoadjuvant therapy is associated with hilar fibrosis which is thought to make the operation more difficult. The robotic approach has repeatedly been shown to be associated with less conversion to open when compared to VATS (55). For example, in Nivolumab With or Without Ipilimumab in Treating Patients With Previously Untreated Stage I-IIIA Non-Small Cell Lung Cancer (NEOSTAR) phase II randomized trial, Sepesi et al. reported 44 patients with stage I to IIIA non-small cell lung cancer (NSCLC), 37 of which underwent resection on-trial (56). Of these 37 resections, 19% were VATS and 8% was robotic, with only 17% (2 patients) of these minimally invasive techniques requiring conversions to open thoracotomy, both being VATS. Surgeons also graded the complexity of operation following neoadjuvant therapy, with “1” being easiest and “4” being very complex. The majority, being 40%, reported surgeries being a grade of 3 or 4, reflecting a more complex procedure compared to a typical lobectomy for stage I disease.
Similar data was reported in Feldman et al. study, which reported 124 patients undergoing anatomic lung resection for NSCLC, 107 of which underwent neoadjuvant therapy (57). Of those 124 patients, 17 were minimally invasive, further divided into 9 VATS and 8 robotic. Two of the 9 VATS were converted non-emergently to open, while all 8 were begun and completed as robotic. It is interesting to note those with >30% short axis nodal reduction were associated with greater need for advanced operative maneuvers than those with <30% node reduction.
With all that being said, the use of robotics for lobectomies following neoadjuvant therapy, including both chemoradiation and immunotherapy, has progressed immensely.
Similarly, a 2023 study compared 46 NSCLC patients who underwent neoadjuvant immunochemotherapy followed by surgery, dividing them into 15 robotic and 31 VATS cases (58). The robotic group exhibited no 30-day mortality along with reduced ICU stay. In line with earlier findings, robotic surgery demonstrated better access lymph node yield, on average assessing one more N1 lymph node than VATS. Both robotic surgery and VATS yielded similar surgical outcomes, with no significant differences in postoperative complications.
In this subset of technically challenging lobectomy after neoadjuvant therapy, it is easy to appreciate the benefits of the robotic approach, although more head-to-head comparisons are needed (59,60).
Robotic complex segmentectomies
Recent trials have demonstrated the role of sub-lobar resections, particularly segmentectomy in the management of early-stage lung cancer (61,62). Anatomic segmentectomies are technically more challenging than lobectomies, and complex segmentectomy (also known as atypical segmentectomy) are more difficult than simple (typical) segmentectomy. The robotic approach facilitates performing complex segmentectomies especially those that have multiple intersegmental planes, due to the platform’s increased dexterity and advanced imaging capability including the seamless integration of FireFly technology to visualize the intersegmental plane.
In one study from MD Anderson Cancer Center, Zhou et al. attributed the increased frequency of anatomic segmentectomy to the increase in their overall robotic operations, and demonstrated that the proportion of complex segmentectomies had increased concurrently. At their institution, the VATS approach was largely utilized for simple segments, and more complex segments were performed robotically (63). The robotic approach was associated with longer operative times but had less estimated blood loss, shorter hospitalization, and required no conversion to VATS or thoracotomy (63). Several other studies have found similar results (64).
As is the case with complex operations, there is a learning curve to mastery. Zhang et al. analyzed the learning curve of complex robotic segments (65). In their report, they found that technical competency ensuring safe and comparable outcomes can be achieved after the 40th operation. As experience increases, operative time and intraoperative blood loss decrease.
In terms of cost, some studies have shown the robotic approach to be more expensive while others have shown it to be cost effective (11,66-70). In one study from the University of Alabama at Birmingham, Nasir et al. demonstrated that the robotic approach is associated with lower direct costs but higher indirect costs, and although it is more costly overall, it remained profitable for the hospital (67). Another study from Italy, showed similar results (66). The majority of the cost burden appears related to upfront purchasing cost, maintenance, depreciation, and robotic disposables. The profitability is largely from the reduction of hospital stays and personnel cost which in turn results in robotic segmentectomy being cost-effective. All in all, the advantages of the robotic approach in allowing these technically complexity resections with minimal morbidity cannot be overstated.
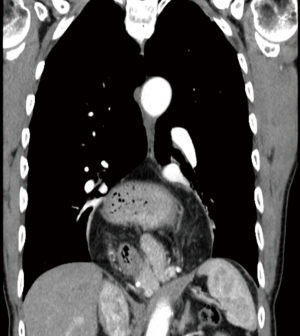
Robotic giant paraesophageal hernia (PEH) repair
Traditionally performed laparoscopically, the robotic approach offers a safe and effective approach to a complicated surgery associated with historical reports on morbidity and mortality. One of the earliest instances of robotic surgery implementation was depicted in a 2005 study by Braumann et al. (71). This study explored the feasibility and effectiveness of implementing robotic techniques on four patients with type 2 and type 3 hiatal hernias. A 2020 literature review by Tartaglia et al. contrasted and compared the advantages of robotic surgery with the challenges associated with standard treatment for symptomatic PEH, specifically laparoscopic surgery (72). Notable benefits of robotic surgery included improved control over equipment and hand movements, enhanced visualization, and greater dexterity.
Figure 2 shows an example of a giant PEH repaired robotically without mesh. The patient’s symptoms resolved after surgery, and he was discharged home on postoperative day 1.
Galvani et al. assessed the safety and feasibility of robotic PEH repair, prospectively examining 61 patients (73). The mean operative time was 186 min. Patients had an average hospital stay of 2.6 days. The study compared their findings with a laparoscopic systematic review study, reflecting comparable results, with higher postoperative complications seen in robotic surgery, possibly attributed to the difficult learning curve associated with such a complex procedure (74).
Giant PEH repairs can be challenging. Sarkaria et al. studied 24 patients undergoing robotic surgery for giant PEH, exploring the surgical outcomes (75). The average operative time was 334 min, which decreased by 98 min (275 min) following the 12th procedure. There were no conversions into open or laparoscopic, with an average length of stay of 4 days. There were 9 patients suffering from postoperative complications, often multiple complications in the same patient. Of these 9, 4 had major complications, which include pulmonary embolus, septic thrombophlebitis, diarrhea, acute lung injury and C. difficile colitis. Similarly, a study conducted by Seetharamaiah et al. conveyed comparable results (12). The report included 19 patients undergoing robotic surgery. The mean operative time was 185 min with 4.3 days average hospital stay. There was 1 conversion case to open repair for partial gastric resection. There were 2 postoperative complications, which included dysphagia requiring dilatation and 1 pleural injury. Finally, Morelli et al. reported 6 patients undergoing giant hiatal hernia repair using robotic surgery (76). The mean operative time was 182 min, with a mean hospitalization stay of 6 days. There were no postoperative complications or symptoms.
All in all, similar to other complex surgical techniques, robotic surgery for giant PEH provides enhanced visualization, control, and dexterity. While studies indicate that robotic surgery may not necessarily outperform laparoscopic approaches in terms of surgical outcomes, it still offers notable technical improvements (77). However, it still proves to be disadvantageous when it comes to costs. This was displayed by Kulshrestha’s et al., in which the authors reported robotic surgery having comparable clinical outcomes, but a higher index cost when compared to laparoscopic surgery (78). In another study, Kulshrestha et al. described the existence of a 2-fold variation between diaphragmatic hernia repair costs between various hospitals (79). This highlights the need for a holistic approach that includes clinical outcome and cost-effectiveness when it comes to choosing a treatment modality. A study comparing the cost-effectiveness of robotic and laparoscopic approaches for PEH found laparoscopic surgery to be a more feasible option (80). When compared, and after excluding capital and maintenance costs, robotic surgery exhibited a slightly higher cost and comparable quality-adjusted life years, indicating a need for further evaluation.
Esophagectomy and esophageal enucleation
The robotic approach has also been employed for benign and malignant diseases of the esophagus. After the initial case report by Elli et al. on using a robotic approach for esophageal enucleation to leiomyomas, multiple other studies concur that the robotic approach provides a safe, feasible, and effective alternative to the VATS or open approach (81-84).
For esophagectomy, the robotic approach has also been increasingly used, and it is applicable for all types including Ivor-Lewis, McKeown and transhiatal esophagectomy. In a meta-analysis, Zheng et al. analyzed 14 studies, encompassing 2,887 patients with 1,435 robotic (RAMIE) and 1,452 VATS/laparoscopic (MIE) (85). Although the operative time was higher with RAMIE (mean difference of 46 min), the incidence of complications such as pneumonia and vocal cord palsy was lower for RAMIE versus MIE. This can be attributed to the enhanced 3D visualization provided by robotic techniques, coupled with improved dexterity. However, there was a trend for increased occurrence of anastomotic leaks in RAMIE patients (odds ratio =1.11; 95% confidence interval: 0.75–1.62). The reasons for this trend may be due to increased operative time and/or overzealous proximal dissection. There were no differences in 30- and 90-day mortality rates.
Another meta-analysis conducted in 2023 delved into the comparative surgical outcomes between RAMIE and MIE (86). This comprehensive study encompassed 2,932 patients, further stratified into 1,418 RAMIE and 1,514 MIE patients. No statistically significant differences emerged between the two groups concerning operative time, length of hospital stay, and 30- and 90-day mortality rates. However, congruent with the earlier investigation, RAMIE exhibited a lower incidence of postoperative complications, including pneumonia. For long term outcomes, although no significant difference existed with regards to overall survival between both groups, the 3-year disease-free survival was significantly higher in RAMIE patients, at 78% compared to 71% seen with MIE (odds ratio =1.42; 95% confidence interval: 1.11–1.83). RAMIE also demonstrated greater lymph node harvesting ability, specifically for total, abdominal, and those along the left recurrent laryngeal nerve, when compared to MIE patients.
The most notable advantages for RAMIE over standard MIE that have been consistently reported are the increased lymph node yields with RAMIE, likely attributed to superior reach and visualization capabilities (87,88). This may be one reason for the improved survival for RAMIE.
Robotic surgery for mediastinal mass resection and thymectomy
The enhanced maneuverability and improved visualization offered by robotic surgery facilitates advancements in treating various pathologies located within the mediastinum, including thymomas, substernal goiter, pericardial cysts, and neurogenic masses such as schwannomas (89,90). Robotic approaches to mediastinal masses can be via a lateral transthoracic approach or a subxiphoid approach, and uniportal or multiportal. Figure 3 shows an example from our experience of a posterior mediastinal bronchogenic cyst resected via a robotic left transthoracic approach. The patient did well and was discharged home on day 1.
The choice of whether to pursue a subxiphoid or lateral thoracic robotic approach depends on surgeon preference largely. In a study of 116 patients, Hong et al. had 52 patients who underwent subxiphoid robotic surgery for anterior mediastinal tumors, while 64 patients underwent a lateral thoracic approach (91). There was no statistical significance between both groups with regards to operative time, postoperative complications; however, the subxiphoid process offers more advantages with regards to total postoperative drainage, drainage time, and postoperative hospital stay. The number of cases needed to reach a plateau in terms of learning curve and technical skill employing the subxiphoid approach was 10–20 cases. All in all, the subxiphoid approach seems to be a feasible alternative that may be associated with reduced postoperative patient pain.
Other studies demonstrate the effectiveness of a three-port and bi-portal robotic approach (92,93). Recently, more advancements have been made regarding a single-port robotic approach to mediastinal masses and pathologies. Park et al. retrospectively reviewed 14 single-port robotic surgery cases, 4 of which were thymomas and 3 were pericardial cysts (94). The median operative time was 105 min, with a median hospitalization stay of 4 days. There were no conventional multiport or open surgery conversions. In another study of theirs, Park et al. report 17 robotic surgeries done using single-port, 8 of which were thymomas and 6 were cystic lesions (95). Of these 17, 11 were subxiphoid and 6 were transthoracic (subcostal and intercostal). The median operative time was 120 min, with a median hospitalization stay of 3 days. There were no postoperative complications nor were there any conversions to multiport or open surgery.
Similarly, multiple case studies report comparable findings regarding the use of a single-port robotic platform. Ishikawa et al. report the implementation of transthoracic single port robotic surgery on a 50-year-old male with a mediastinal tumor, while Shidei et al. report its use in a 39-year-old male with anterior mediastinal mass caused by multiple endocrine neoplasia type 1 (96,97). In both reports, a single-port robotic platform proved to be a safe alternative that offers enhanced maneuverability and dexterity when handling mediastinal tumors, allowing for good clinical and cosmetic outcomes.
Lastly, a noteworthy contribution comes from a meta-analysis and systematic review conducted in 2021, focusing on the examination of complications arising from robotic-assisted thymectomies. In this comprehensive study, Xu et al. examined 21 distinct studies, collectively including a cohort of 930 patients (98). This patient pool was stratified based on the side of surgical entry, left and right. Their finding indicated that a left-sided approach held distinct advantages in terms of reduced complication rates when juxtaposed against the right-sided counterpart. The cumulative incidence of complications was 12%; specifically, procedures performed on the left side exhibited an overall complication rate of 7%, while procedures executed on the right side demonstrated a significantly higher complication rate of 17%. Among the range of complications ensuing from robotic-assisted thymectomies, notable occurrences included pleural effusion, air leaks, thoracic duct fistulas, atrial fibrillation, and instances requiring open conversion.
Robotic surgery for lung transplant
There are a few studies examining the use of minimally invasive video-assisted techniques for lung transplantation (99,100); In a case series consisting of 8 patients, Emerson et al. reported the use of robotic lung transplantation on patients with obstructive and restrictive pathologies (101). Initially done as a right sided lung transplant for a 69-year-old patient with chronic obstructive pulmonary disease (COPD), robotic transplantation proved safe. The patient had an uneventful postoperative course, and although a mild primary graft dysfunction at 24 hours and atrial fibrillation occurred, both were resolved and treated. The remaining 7 cases reflected a successful implementation of robotic transplantation, with one case requiring an open conversion, and two cases requiring traditional open pulmonary artery anastomoses. Emerson et al. also reported warm ischemic times trending down significantly, from 111 min initially to around 60 min in most recent cases. All in all, patients experienced no major intraoperative complications, and are all alive at the time this was reported. Although reflecting a successful outcome, they emphasize the amount of time taken to plan and execute a robotic transplant, starting from discussions with the patient to application of technique. However, there is promise and potential to the teachability of this technique to residents and trainees.
Another case report exists on a robotic lung transplantation performed on a patient with COPD (102). After providing a detailed explanation of the procedure, Jiao et al. concluded that a robotic approach with four ports provides significant benefits in managing complex procedures such as lung transplantation (102). They emphasized the role of enhanced maneuverability and dexterity. The study highlighted the efficacy of using a robot to handle anastomoses, including those involving the bronchus, pulmonary artery, and left atrium, comparing its ease and importance with the more cumbersome traditional open procedure. There have been several groups in the US and Spain that have also adopted robotic lung transplantation featured in lay media (103,104).
Although very limited, these offer insights into the potential for robotic surgery to bring greater benefits and advancements to the field of lung transplantation. Further research in this area could potentially lead to more widespread adoption and refinement of robotic techniques.
Summary and limitations
To summarize, robotic surgery provides a multitude of benefits when compared to VATS or open approaches across many types of operations. In each section of this narrative review, we highlight the strengths and advantages pertaining to that complex presentation. In addition to the aforementioned advantages of better visualization, enhanced maneuverability, and reduced surgeon fatigue, robotic surgery allows the surgeon to “mimic an open approach”, and provide the surgeon with the rare ability to be inside the chest without opening it (105-107).
In addition, robotic surgery also offers better pain control, faster recovery rates with improved cosmesis. In most procedures, it proved to have comparable short-term outcomes with its VATS counterpart. For specific procedures, such as lobectomy following neoadjuvant therapy, and esophagectomy, robotic surgery allows for better lymph node harvest and handling of complex anatomy and nodal regression.
On the other hand, the robotic platform does have some important disadvantages. Across the majority of these complex operations, cost was a major concern, limitation and potential obstacle for increased adoption. Although some reports demonstrated a net profit at the hospital level, the upfront cost and other hidden costs need to be considered. For many applications, there is no data on cost yet. Although outcomes are comparable (or better), robotic surgery is associated with longer operative times compared to other approaches, and a real learning curve that varies between indications. Although some require a few operations to start achieving similar outcomes, a minority of operations necessitate a large number before the surgeon reaches proficiency and comparable results.
This review has various limitations. Firstly, a good percentage of literature included retrospective studies. These studies are subject to both selection and confounding bias, which could alter the results reported. Some studies have mentioned this, but others did not, meaning there was no way to document if it was accounted for. Second, several studies in the same category are authored by the same individual, as they followed up on their previous studies. This raises concerns about reporting bias, affecting the results reported. Third, our inclusion criteria may have been too general, with a need to include more specific parameters for literature retrieval. For example, there was a huge variation in the sample size between some studies, meaning a clear definition of the minimum or maximum size could have been implemented. Also, the exclusion of non-English studies could have removed literature that was prominent and beneficial for this review. However, we believe our review encompasses the latest updates on robotic surgery implementation on various complex thoracic operations, with relevant and cohesive reports. Finally, as this is a narrative review that is largely focused on innovation and the application of technological advancements to complex thoracic problems; we believe it necessary to include small case reports and case series for operations where the use of the robotic approach may have been innovative at the time, and limited data existed.
Future direction
First of all, the robotic platform used almost exclusively in this narrative review is the DaVinci platform by Intuitive (Sunnyvale, CA, USA). The newest product from this company is the Single Port (SP) system which has not yet become mainstream for thoracic operations (95,108). The SP can introduce several advantages owing to its single port design, although it would most likely require sub-xiphoid placement rather than a trans-thoracic intercostal placement. Several other companies are also introducing their own robotic platforms such as Versius by CMR Surgical (Cambridge, UK), Hugo by Medtronic (Dublin, Ireland) and Ottave by Johnson & Johnson Auris (Redwood City, CA, USA) to name a few (1).
The dramatic evolution of artificial intelligence over the past few years may even suggest a future where robots become autonomous during the entirety of the operation. Currently, as seen in Senhance surgical system (Durham, NC, USA), artificial intelligence integration moves the camera in response to surgeon’s movement, provides 3D measurement and digital tagging, and anticipates what the surgeon aims to locate and adjusts accordingly (109). Other avenues of robotic surgery evolution include miniaturized platforms that allow for small robotic devices, and soft robotics that conform to curvilinear paths in space.
Conclusions
Robotic thoracic surgeons continue to push the envelope by using robotic minimally invasive techniques for the spectrum of complex thoracic pathology. The robotic approach is safe, effective, and associated with improved patient outcomes. The most consistently reported advantages are lower rates of conversion to open, and improved lymph node harvest. This review provides several insights on the ubiquitous and intuitive ability of the robotic platform to encompass even the most complex of thoracic problems. To encourage wider adoption of robotic technology, increased training and expanded research efforts are essential, along with improved worldwide access to this technology.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1570/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1570/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1570/coif). Z.M.A. serves as an unpaid editorial board member of Journal of Thoracic Disease from December 2023 to November 2025. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lazar JF, Hwalek AE. A Review of Robotic Thoracic Surgery Adoption and Future Innovations. Thorac Surg Clin 2023;33:1-10. [Crossref] [PubMed]
- Mazzei M, Abbas AE. Why comprehensive adoption of robotic assisted thoracic surgery is ideal for both simple and complex lung resections. J Thorac Dis 2020;12:70-81. [Crossref] [PubMed]
- Leal Ghezzi T, Campos Corleta O. 30 Years of Robotic Surgery. World J Surg 2016;40:2550-7. [Crossref] [PubMed]
- Maza G, Sharma A. Past, Present, and Future of Robotic Surgery. Otolaryngol Clin North Am 2020;53:935-41. [Crossref] [PubMed]
- Mattioni G, Palleschi A, Mendogni P, et al. Approaches and outcomes of Robotic-Assisted Thoracic Surgery (RATS) for lung cancer: a narrative review. J Robot Surg 2023;17:797-809. [Crossref] [PubMed]
- Parini S, Massera F, Papalia E, et al. Port Placement Strategies for Robotic Pulmonary Lobectomy: A Narrative Review. J Clin Med 2022;11:2612. [Crossref] [PubMed]
- Bhatt H, Wei B. Comparison of laparoscopic vs. robotic paraesophageal hernia repair: a systematic review. J Thorac Dis 2023;15:1494-502. [Crossref] [PubMed]
- Yoshino I, Hashizume M, Shimada M, et al. Thoracoscopic thymomectomy with the da Vinci computer-enhanced surgical system. J Thorac Cardiovasc Surg 2001;122:783-5. [Crossref] [PubMed]
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Pardolesi A, Park B, Petrella F, et al. Robotic anatomic segmentectomy of the lung: technical aspects and initial results. Ann Thorac Surg 2012;94:929-34. [Crossref] [PubMed]
- Seetharamaiah R, Romero RJ, Kosanovic R, et al. Robotic repair of giant paraesophageal hernias. JSLS 2013;17:570-7. [Crossref] [PubMed]
- Martinez BD, Wiegand CS, Evans P, et al. Computer-assisted instrumentation during endoscopic transaxillary first rib resection for thoracic outlet syndrome: a safe alternate approach. Vascular 2005;13:327-35. [Crossref] [PubMed]
- Gharagozloo F, Meyer M, Tempesta BJ, et al. Robotic en bloc first-rib resection for Paget-Schroetter disease, a form of thoracic outlet syndrome: technique and initial results. Innovations (Phila) 2012;7:39-44. [Crossref] [PubMed]
- Kocher GJ, Zehnder A, Lutz JA, et al. First Rib Resection for Thoracic Outlet Syndrome: The Robotic Approach. World J Surg 2018;42:3250-5. [Crossref] [PubMed]
- Zehnder A, Lutz J, Dorn P, et al. Robotic-Assisted Thoracoscopic Resection of the First Rib for Vascular Thoracic Outlet Syndrome: The New Gold Standard of Treatment? J Clin Med 2021;10:3952. [Crossref] [PubMed]
- Wybaillie E, Maene L, Cooreman F, et al. Robotically Assisted Transthoracic Cervical Rib Resection. Ann Thorac Surg 2018;106:e253-5. [Crossref] [PubMed]
- Hoexum F, Jongkind V, Coveliers HM, et al. Robot-assisted transthoracic first rib resection for venous thoracic outlet syndrome. Vascular 2022;30:217-24. [Crossref] [PubMed]
- Gharagozloo F, Atiquzzaman N, Meyer M, et al. Robotic first rib resection for thoracic outlet syndrome. J Thorac Dis 2021;13:6141-54. [Crossref] [PubMed]
- Gkikas A, Lampridis S, Patrini D, et al. Thoracic Outlet Syndrome: Single Center Experience on Robotic Assisted First Rib Resection and Literature Review. Front Surg 2022;9:848972. [Crossref] [PubMed]
- Dale WA. Thoracic outlet compression syndrome. Critique in 1982. Arch Surg 1982;117:1437-45. [Crossref] [PubMed]
- Martinez BD, Albeshri H, Chulkov M, et al. Development and evolution of a robotic surgical technique for the treatment of thoracic outlet syndrome. J Vasc Surg 2021;74:938-945.e1. [Crossref] [PubMed]
- Burt BM, Palivela N, Cekmecelioglu D, et al. Safety of robotic first rib resection for thoracic outlet syndrome. J Thorac Cardiovasc Surg 2021;162:1297-1305.e1. [Crossref] [PubMed]
- Gharagozloo F, Meyer M, Tempesta B, et al. Robotic transthoracic first-rib resection for Paget-Schroetter syndrome. Eur J Cardiothorac Surg 2019;55:434-9. [Crossref] [PubMed]
- Verm RA, Vigneswaran WT, Lin A, et al. Robotic chest wall resection for primary benign chest wall tumors and locally advanced lung cancer: an institutional case series and national report. J Thorac Dis 2023;15:4849-58. [Crossref] [PubMed]
- Tedesco J, Nakahama H, Abdelsattar Z. Totally robotic en bloc left upper lobectomy and chest wall resection after neoadjuvant chemoradiation. Multimed Man Cardiothorac Surg 2023; [Crossref]
- Liu B, Gao S, Wu Q, et al. A case report of robotic-assisted resection of large fibrous benign tumor of second rib. J Cardiothorac Surg 2022;17:329. [Crossref] [PubMed]
- Rojo M, Abdelsattar Z. Robotic resection of a second rib osteochondroma. Multimed Man Cardiothorac Surg 2023; [Crossref]
- Shidei H, Maeda H, Isaka T, et al. Mediastinal paraganglioma successfully resected by robot-assisted thoracoscopic surgery with en bloc chest wall resection: a case report. BMC Surg 2020;20:45. [Crossref] [PubMed]
- Ajouz H, Sohail AH, Hashmi H, et al. Surgical considerations in the resection of solitary fibrous tumors of the pleura. J Cardiothorac Surg 2023;18:79. [Crossref] [PubMed]
- Mariolo AV, Casiraghi M, Galetta D, et al. Robotic Hybrid Approach for an Anterior Pancoast Tumor in a Severely Obese Patient. Ann Thorac Surg 2018;106:e115-6. [Crossref] [PubMed]
- Lazzaro R, Patton B, Lee P, et al. First series of minimally invasive, robot-assisted tracheobronchoplasty with mesh for severe tracheobronchomalacia. J Thorac Cardiovasc Surg 2019;157:791-800. [Crossref] [PubMed]
- Bakhos CT, Magarinos J, Bent D, et al. Tracheobronchoplasty for tracheobronchomalacia. J Vis Surg 2022;8:15. [Crossref] [PubMed]
- Lazzaro R, Inra ML. Tracheobronchoplasty: Indications and Best Approaches. Thorac Surg Clin 2023;33:141-7. [Crossref] [PubMed]
- Milman S, Ng T. Robotic tracheobronchoplasty is feasible, but which patients truly benefit? J Thorac Cardiovasc Surg 2019;157:801-2. [Crossref] [PubMed]
- Inra ML, Wasserman GA, Karp J, et al. Improvement in postoperative lung function in patients with moderate to severe airway obstruction after robotic-assisted thoracoscopic tracheobronchoplasty. J Thorac Cardiovasc Surg 2023;165:876-85. [Crossref] [PubMed]
- Lazar JF, Posner DH, Palka W, et al. Robotically Assisted Bilateral Bronchoplasty for Tracheobronchomalacia. Innovations (Phila) 2015;10:428-30. [Crossref] [PubMed]
- Lazzaro RS, Patton BD, Wasserman GA, et al. Robotic-assisted tracheobronchoplasty: Quality of life and pulmonary function assessment on intermediate follow-up. J Thorac Cardiovasc Surg 2022;164:278-86. [Crossref] [PubMed]
- Seastedt KP, Wilson JL, Gangadharan SP. Robotic Surgery for Tracheobronchomalacia. Thorac Surg Clin 2023;33:61-9. [Crossref] [PubMed]
- Abdelsattar ZM, Shen KR, Yendamuri S, et al. Outcomes After Sleeve Lung Resections Versus Pneumonectomy in the United States. Ann Thorac Surg 2017;104:1656-64. [Crossref] [PubMed]
- Ishikawa N, Sun YS, Nifong LW, et al. Thoracoscopic robot-assisted bronchoplasty. Surg Endosc 2006;20:1782-3. [Crossref] [PubMed]
- Schmid T, Augustin F, Kainz G, et al. Hybrid video-assisted thoracic surgery-robotic minimally invasive right upper lobe sleeve lobectomy. Ann Thorac Surg 2011;91:1961-5. [Crossref] [PubMed]
- Gonzalez-Rivas D, Essa RA, Motas N, et al. Uniportal robotic-assisted thoracic surgery lung-sparing carinal sleeve resection and reconstruction. Ann Cardiothorac Surg 2023;12:130-2. [Crossref] [PubMed]
- Gonzalez-Rivas D, Bosinceanu M, Manolache V, et al. Uniportal fully robotic-assisted sleeve resections: surgical technique and initial experience of 30 cases. Ann Cardiothorac Surg 2023;12:9-22. [Crossref] [PubMed]
- Hu D, Wang Z, Tantai J, et al. Robotic-assisted thoracoscopic resection and reconstruction of the carina. Interact Cardiovasc Thorac Surg 2020;31:912-4. [Crossref] [PubMed]
- Geraci TC, Ferrari-Light D, Wang S, et al. Robotic Sleeve Resection of the Airway: Outcomes and Technical Conduct Using Video Vignettes. Ann Thorac Surg 2020;110:236-40. [Crossref] [PubMed]
- Jiao W, Zhao Y, Qiu T, et al. Robotic Bronchial Sleeve Lobectomy for Central Lung Tumors: Technique and Outcome. Ann Thorac Surg 2019;108:211-8. [Crossref] [PubMed]
- Li C, Zhou B, Han Y, et al. Robotic sleeve resection for pulmonary disease. World J Surg Oncol 2018;16:74. [Crossref] [PubMed]
- Pan X, Gu C, Wang R, et al. Initial Experience of Robotic Sleeve Resection for Lung Cancer Patients. Ann Thorac Surg 2016;102:1892-7. [Crossref] [PubMed]
- Pan X, Chen Y, Shi J, et al. Robotic Assisted Extended Sleeve Lobectomy After Neoadjuvant Chemotherapy. Ann Thorac Surg 2015;100:e129-31. [Crossref] [PubMed]
- Li S, Ai Q, Liang H, et al. Nonintubated Robotic-assisted Thoracic Surgery for Tracheal/Airway Resection and Reconstruction: Technique Description and Preliminary Results. Ann Surg 2022;275:e534-6. [Crossref] [PubMed]
- Zalepugas D, Schnorr P, Schmidt J, et al. Non-intubated robotic-assisted thoracic surgery for tracheal/airway resection and reconstruction safe: editorial commentary. Ann Transl Med 2021;9:1707. [Crossref] [PubMed]
- Migliore M. Primum non nocere: do we really need non-intubated thoracic surgery and robotic assisted thoracic surgery for tracheal airway resection and reconstruction? Ann Transl Med 2021;9:1750. [Crossref] [PubMed]
- Forde PM, Spicer J, Lu S, et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med 2022;386:1973-85. [Crossref] [PubMed]
- Servais EL, Miller DL, Thibault D, et al. Conversion to Thoracotomy During Thoracoscopic vs Robotic Lobectomy: Predictors and Outcomes. Ann Thorac Surg 2022;114:409-17. [Crossref] [PubMed]
- Sepesi B, Zhou N, William WN Jr, et al. Surgical outcomes after neoadjuvant nivolumab or nivolumab with ipilimumab in patients with non-small cell lung cancer. J Thorac Cardiovasc Surg 2022;164:1327-37. [Crossref] [PubMed]
- Feldman HA, Zhou N, Deboever N, et al. Intraoperative challenges after induction therapy for non-small cell lung cancer: Effect of nodal disease on technical complexity. JTCVS Open 2022;12:372-84. [Crossref] [PubMed]
- Pan H, Zou N, Tian Y, et al. Short-term outcomes of robot-assisted versus video-assisted thoracoscopic surgery for non-small cell lung cancer patients with neoadjuvant immunochemotherapy: a single-center retrospective study. Front Immunol 2023;14:1228451. [Crossref] [PubMed]
- Herb JN, Kindell DG, Strassle PD, et al. Trends and Outcomes in Minimally Invasive Surgery for Locally Advanced Non-Small-Cell Lung Cancer With N2 Disease. Semin Thorac Cardiovasc Surg 2021;33:547-55. [Crossref] [PubMed]
- Weder W, Furrer K, Opitz I. Robotic-assisted thoracoscopic surgery for clinically stage IIIA (c-N2) NSCLC-is it justified? Transl Lung Cancer Res 2021;10:1-4. [Crossref] [PubMed]
- Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895-907. [Crossref] [PubMed]
- Altorki NK, Wang X, Wigle D, et al. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). Lancet Respir Med 2018;6:915-24. [Crossref] [PubMed]
- Zhou N, Corsini EM, Antonoff MB, et al. Robotic Surgery and Anatomic Segmentectomy: An Analysis of Trends, Patient Selection, and Outcomes. Ann Thorac Surg 2022;113:975-83. [Crossref] [PubMed]
- Li C, Han Y, Han D, et al. Robotic Approach to Combined Anatomic Pulmonary Subsegmentectomy: Technical Aspects and Early Results. Ann Thorac Surg 2019;107:1480-6. [Crossref] [PubMed]
- Zhang Y, Liu S, Han Y, et al. Robotic Anatomical Segmentectomy: An Analysis of the Learning Curve. Ann Thorac Surg 2019;107:1515-22. [Crossref] [PubMed]
- Novellis P, Bottoni E, Voulaz E, et al. Robotic surgery, video-assisted thoracic surgery, and open surgery for early stage lung cancer: comparison of costs and outcomes at a single institute. J Thorac Dis 2018;10:790-8. [Crossref] [PubMed]
- Nasir BS, Bryant AS, Minnich DJ, et al. Performing robotic lobectomy and segmentectomy: cost, profitability, and outcomes. Ann Thorac Surg 2014;98:203-8; discussion 208-9. [Crossref] [PubMed]
- Musgrove KA, Hayanga JA, Holmes SD, et al. Robotic Versus Video-Assisted Thoracoscopic Surgery Pulmonary Segmentectomy: A Cost Analysis. Innovations (Phila) 2018;13:338-43. [Crossref] [PubMed]
- Perroni G, Veronesi G. Robotic segmentectomy: indication and technique. J Thorac Dis 2020;12:3404-10. [Crossref] [PubMed]
- Deen SA, Wilson JL, Wilshire CL, et al. Defining the cost of care for lobectomy and segmentectomy: a comparison of open, video-assisted thoracoscopic, and robotic approaches. Ann Thorac Surg 2014;97:1000-7. [Crossref] [PubMed]
- Braumann C, Menenakos C, Rueckert JC, et al. Computer-assisted laparoscopic repair of "upside-down" stomach with the Da Vinci system. Surg Laparosc Endosc Percutan Tech 2005;15:285-9. [Crossref] [PubMed]
- Tartaglia N, Pavone G, Di Lascia A, et al. Robotic voluminous paraesophageal hernia repair: a case report and review of the literature. J Med Case Rep 2020;14:25. [Crossref] [PubMed]
- Galvani CA, Loebl H, Osuchukwu O, et al. Robotic-Assisted Paraesophageal Hernia Repair: Initial Experience at a Single Institution. J Laparoendosc Adv Surg Tech A 2016;26:290-5. [Crossref] [PubMed]
- Gangopadhyay N, Perrone JM, Soper NJ, et al. Outcomes of laparoscopic paraesophageal hernia repair in elderly and high-risk patients. Surgery 2006;140:491-8; discussion 498-9. [Crossref] [PubMed]
- Sarkaria IS, Latif MJ, Bianco VJ, et al. Early operative outcomes and learning curve of robotic assisted giant paraesophageal hernia repair. Int J Med Robot 2017; [Crossref]
- Morelli L, Guadagni S, Mariniello MD, et al. Robotic giant hiatal hernia repair: 3 year prospective evaluation and review of the literature. Int J Med Robot 2015;11:1-7. [Crossref] [PubMed]
- Ekeke CN, Vercauteren M, Baker N, et al. Surgical Techniques for Robotically-Assisted Laparoscopic Paraesophageal Hernia Repair. Thorac Surg Clin 2019;29:369-77. [Crossref] [PubMed]
- Kulshrestha S, Janjua HM, Bunn C, et al. State-Level Examination of Clinical Outcomes and Costs for Robotic and Laparoscopic Approach to Diaphragmatic Hernia Repair. J Am Coll Surg 2021;233:9-19.e2. [Crossref] [PubMed]
- Kulshrestha S, Penton A, Luchette FA, et al. Variation in Hospital Cost and 1-Year Episodes of Care after Diaphragmatic Hernia Repair. J Am Coll Surg 2022;235:111-8. [Crossref] [PubMed]
- Panse NS, Prasath V, Quinn PL, et al. Economic evaluation of robotic and laparoscopic paraesophageal hernia repair. Surg Endosc 2023;37:6806-17. [Crossref] [PubMed]
- Elli E, Espat NJ, Berger R, et al. Robotic-assisted thoracoscopic resection of esophageal leiomyoma. Surg Endosc 2004;18:713-6. [Crossref] [PubMed]
- Chen YH, Chen KC, Huang PM, et al. Robotic-Assisted Thoracoscopic (RATS) Enucleation of Esophageal Mesenchymal Tumors and Foregut Cysts. J Clin Med 2022;11:6471. [Crossref] [PubMed]
- Asaf BB, Bishnoi S, Puri HV, et al. Robotic enucleation of oesophageal leiomyoma technique and surgical outcomes. J Minim Access Surg 2022;18:84-9. [Crossref] [PubMed]
- Gadelkarim M, Harpole B, Abdelsattar Z. Totally robotic enucleation of a mid-esophageal leiomyoma. Multimed Man Cardiothorac Surg 2022; [Crossref]
- Zheng C, Li XK, Zhang C, et al. Comparison of short-term clinical outcomes between robot-assisted minimally invasive esophagectomy and video-assisted minimally invasive esophagectomy: a systematic review and meta-analysis. J Thorac Dis 2021;13:708-19. [Crossref] [PubMed]
- Zhang Y, Dong D, Cao Y, et al. Robotic Versus Conventional Minimally Invasive Esophagectomy for Esophageal Cancer: A Meta-analysis. Ann Surg 2023;278:39-50. [Crossref] [PubMed]
- Na KJ, Park S, Park IK, et al. Outcomes after total robotic esophagectomy for esophageal cancer: a propensity-matched comparison with hybrid robotic esophagectomy. J Thorac Dis 2019;11:5310-20. [Crossref] [PubMed]
- Park S, Hwang Y, Lee HJ, et al. Comparison of robot-assisted esophagectomy and thoracoscopic esophagectomy in esophageal squamous cell carcinoma. J Thorac Dis 2016;8:2853-61. [Crossref] [PubMed]
- Schwartz G, Sancheti M, Blasberg J. Robotic Thoracic Surgery. Surg Clin North Am 2020;100:237-48. [Crossref] [PubMed]
- Stoddard N, Heil JR, Lowery DR. Anatomy, Thorax, Mediastinum. Treasure Island (FL): StatPearls Publishing; 2023.
- Hong Z, Sheng Y, Bai X, et al. Clinical efficacy of robot-assisted subxiphoid versus lateral thoracic approach in the treatment of anterior mediastinal tumors. World J Surg Oncol 2023;21:94. [Crossref] [PubMed]
- Li H, Li J, Huang J, et al. Robotic-assisted mediastinal surgery: the first Chinese series of 167 consecutive cases. J Thorac Dis 2018;10:2876-80. [Crossref] [PubMed]
- Hong JI, Lee JH, Kim HK. Biportal robotic surgery for anterior mediastinal mass. Ann Cardiothorac Surg 2023;12:110-6. [Crossref] [PubMed]
- Park SY, Kim HK, Jang DS, et al. Initial Experiences With Robotic Single-Site Thoracic Surgery for Mediastinal Masses. Ann Thorac Surg 2019;107:242-7. [Crossref] [PubMed]
- Park SY, Lee JH, Stein H, et al. Initial experience with and surgical outcomes of da Vinci single-port system in general thoracic surgery. J Thorac Dis 2022;14:1933-40. [Crossref] [PubMed]
- Ishikawa N, Oda M, Kawachi K, et al. Robot-assisted single-port surgery for mediastinal tumors. Surg Today 2019;49:96-8. [Crossref] [PubMed]
- Shidei H, Mitsuboshi S, Yamamoto T, et al. Single-incision port robot-assisted surgery for thymic carcinoid tumor resection. J Cardiothorac Surg 2022;17:90. [Crossref] [PubMed]
- Xu JX, Qian K, Deng Y, et al. Complications of robot-assisted thymectomy: A single-arm meta-analysis and systematic review. Int J Med Robot 2021;17:e2333. [Crossref] [PubMed]
- Fischer S, Strüber M, Simon AR, et al. Video-assisted minimally invasive approach in clinical bilateral lung transplantation. J Thorac Cardiovasc Surg 2001;122:1196-8. [Crossref] [PubMed]
- Marczin N, Popov AF, Zych B, et al. Outcomes of minimally invasive lung transplantation in a single centre: the routine approach for the future or do we still need clamshell incision? Interact Cardiovasc Thorac Surg 2016;22:537-45. [Crossref] [PubMed]
- Emerson D, Catarino P, Rampolla R, et al. Robotic-assisted lung transplantation: First in man. J Heart Lung Transplant 2024;43:158-61. [Crossref] [PubMed]
- Jiao W, Yang R, Zhao Y, et al. Robot-assisted single lung transplantation. Chin Med J (Engl) 2023;136:362-4. [Crossref] [PubMed]
- Cedars-Sinai. Robotic Lung Transplant Completed | Cedars-Sinai. Youtube 2022. Available online: https://www.youtube.com/watch?v=5YvJEANB_OE
- Min R. Spain sees the world’s first lung transplant performed entirely by robot. Available online: https://www.euronews.com/next/2023/04/19/spain-sees-the-worlds-first-lung-transplantation-performed-entirely-by-robot
- Montagne F, Chaari Z, Bottet B, et al. Long-Term Survival Following Minimally Invasive Lung Cancer Surgery: Comparing Robotic-Assisted and Video-Assisted Surgery. Cancers (Basel) 2022;14:2611. [Crossref] [PubMed]
- Montagne F, Guisier F, Venissac N, et al. The Role of Surgery in Lung Cancer Treatment: Present Indications and Future Perspectives-State of the Art. Cancers (Basel) 2021;13:3711. [Crossref] [PubMed]
- Montagne F, Baste JM. Should we keep on doing robotic surgery to treat lung cancer in 2020? Ann Transl Med 2020;8:775. [Crossref] [PubMed]
- Yang B, Chen R, Lin Y, et al. Single-port robotic surgery for mediastinal tumors using the da vinci SP system: Initial experience. Front Surg 2022;9:1043374. [Crossref] [PubMed]
- Schmitz R, Willeke F, Darwich I, et al. Robotic-Assisted Nissen Fundoplication with the Senhance® Surgical System: Technical Aspects and Early Results. Surg Technol Int 2019;35:113-9.