
Immune-related thyroid dysfunction in patients with non-small cell lung cancer
Highlight box
Key findings
• The development of immune-related thyroid dysfunctions (irTDs) had no impact on the survival of patients with non-small cell lung cancer (NSCLC).
What is known and what is new?
• The immune-related adverse events (irAEs) are major concern for clinical application of immune checkpoint inhibitors (ICIs) and has drawn wide research interest, with irTD as a common source.
• Firstly, it was found that thyroid peroxidase antibody (TPOAb), anti-thyroid antibodies (ATAs), and abnormal thyroid ultrasonography results were potential biomarkers of irTDs, which is consistent with previous studies. Secondly, no internal association was observed between irTD and the clinical outcomes of patients with NSCLC, which contracts with the previous literature conclusions.
What is the implication, and what should change now?
• For the irTD prediction, we suggest the usage of TPOAb, ATAs, and abnormal thyroid ultrasonography results as guidance.
• For the ICI treatment, we suggest that irTD may not be a main concern, although those with severe irTD still worth further attention and studies.
Introduction
Background
The clinical application of immune checkpoint inhibitors (ICIs) represents a paradigm shift in the management of malignancies. Immune checkpoints regulate the immune activity, provide immune self-tolerance, and establish immune homeostasis. Cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1), and its ligand (PD-L1) are among the most popular molecular targets in self-tolerance signaling pathways. They allow tumor cells to escape the immune system (1-3). Monoclonal inhibitors of CTLA-4 and PD-(L)1 have been reported to be associated with superior survival in malignancies, including non-small cell lung cancer (NSCLC) (4-8). However, immunotherapy-induced organ dysfunction, termed immune-related adverse events (irAEs), has been frequently recorded in clinical trials (9). IrAEs are commonly mild, but life-threatening in some circumstances, and have drawn widespread attention in clinical practice.
Rationale and knowledge gap
Immune-related thyroid dysfunction (irTD) is one of the most common irAEs, with an incidence of approximately 8% (9,10). Owing to the different definitions of irTDs and the characteristics of asymptomatic manifestations, the incidence varied in different studies. However, the mechanisms underlying irTDs have not yet been elucidated. Anti-thyroid antibodies (ATAs) have been reported the possibility for irTDs prediction (11-14), but no consensus has been reached. Meanwhile, patients with an irregular echo pattern of the thyroid (14) and/or elevated thyroid-stimulating hormone (TSH) (15) levels at baseline are prone to develop irTDs. However, the impact of irTDs on the clinical outcomes of patients with NSCLC remains unclear. Better overall survival (OS) and/or progression-free survival (PFS) in malignancies have been described in some research (16-20), while no influence has been reported (21,22).
Objective
Our research was designed to improve the understanding of irTDs, including potential predictive biomarkers of irTDs and the link between irTDs and clinical outcomes of patients with NSCLC. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1092/rc).
Methods
Patients
We conducted a retrospective study on patients with NSCLC who were treated with PD-(L)1 (pembrolizumab, sintilimab, atezolizumab, or camrelizumab) as the first-line therapy at the First Affiliated Hospital, College of Medicine, Zhejiang University, between July 2019 and February 2023. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by the Independent Ethical Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (No. 2023-0218). Informed consent was waived due to the retrospective nature of the study.
The inclusion criteria were as follows: (I) histologically confirmed NSCLC; (II) at least two courses of ICIs unless discontinued due to irTDs; and (III) at least two thyroid function detection tests after the initiation of ICIs. The exclusion criteria were as follows: (I) pre-existing thyroid diseases [hypothyroidism, subclinical hypothyroidism, hyperthyroidism, subclinical hyperthyroidism, and destructive thyroiditis (DH)] unless pre-existing subclinical thyroid diseases were aggravated due to ICIs; (II) fewer than three follow-ups; (III) absence of thyroid function at baseline; and (IV) fewer than two thyroid function detection tests after the initiation of ICIs. Considering the potential influence of ICI types on the clinical outcomes of patients with NSCLC, the ICI types were balanced in group patients with irTD and group patients without irTD.
Assessment
Thyroid peroxidase antibody (TPOAb), serum interleukin-6 (IL-6), and thyroid ultrasonography data were available for most of the enrolled patients. Thyroglobulin antibodies (TGAb) were not detected in some patients. The normal reference ranges for TPOAb, TGAb, and serum IL-6 were 0–5.61 IU/mL, 0–4.11 IU/mL, and 0.10–2.90 ρg/mL. The irTDs were classified as hypothyroidism, subclinical hypothyroidism (sub-hypo), hyperthyroidism, subclinical hyperthyroidism (sub-hyper), or DH (hypothyroidism secondary to transient hyperthyroidism). Two thyroid function detection tests were performed to confirm the diagnosis. The severity of irTDs was graded according to the Common Terminology Criteria for Adverse Events v5.0. The Age-adjusted Charlson Comorbidity Index (ACCI) was used to assess baseline comorbidities in patients with NSCLC. The smoking index (SI) was used to quantify smoking. PFS and OS were calculated according to the response evaluation criteria in solid tumors (RECIST) v1.1 criteria.
Statistical analysis
Statistical analyses were performed using the SPSS Statistics version 26 (IBM Corp., Armonk, NY, USA). Numerical values with a normal distribution are expressed as the mean ± standard deviation. The Mann-Whitney U test and Wilcoxon rank-sum test were used to compare continuous variables. The Kruskal-Wallis test, Fisher’s exact test, and Chi-square test were used to compare categorical variables. Missing data were deleted and excluded from the statistical analysis. Kaplan-Meier curves were used to visualize and compare PFS and OS. All statistical tests were two-tailed, and the statistical significance level (P) was set at 0.05.
Results
Patient profiles
Out of a total of 569 patients screened out according to the inclusion and exclusion criteria, 84 euthyroid patients who were free from any irAEs and 42 patients presented with irTDs were enrolled. The characteristics of the patients with and without irTDs are summarized in Table 1. The distribution of tumor node metastases and the ages of all patients are shown in Figure 1. Nine (7.1%) patients received ICIs monotherapy, four (44.4%) of whom developed irTDs. A total of 112 (88.9%) patients were administered adjunctive chemotherapy and 32 (25.4%) patients received adjunctive radiotherapy, of whom 36 (32.1%) and 14 (43.8%) developed irTDs, respectively. Twenty (15.9%) patients underwent surgery previously and switched to ICIs because of disease progression, among whom seven (35.0%) developed irTDs. The percentage of PD-L1 expression (PD-L1/%) and gene mutations were noted in 29 (23.0%) and 24 (19.0%) patients, respectively. Compared with euthyroid patients, irregular echo pattern and diffuse changes on thyroid ultrasonography were more common in patients with irTDs [70.7% vs. 47.2% and 19.5% vs. 1.4%, P<0.05 and P<0.01, odds ratio (OR) =2.70, and OR =17.21, respectively; Table 2]. There were no statistically significant differences in age, sex, SI, ACCI, chemotherapy, radiotherapy, surgery, PD-L1/%, ICI agents, or gene mutations between patients with irTDs and euthyroid patients.
Table 1
| Characteristics | All (n=126) | irTDs (n=42) | Euthyroid (n=84) | P value |
|---|---|---|---|---|
| Age, years, mean ± SD | 64.2±9.2 | 63.5±9.6 | 64.5±9.0 | 0.563 |
| Male | 109 | 34 | 75 | 0.197 |
| SI, pack-years, mean ± SD | 613.6±648.4 | 645.5±665.0 | 598.0±643.8 | 0.635 |
| ACCI | >0.99 | |||
| 0–2 | 60 | 20 | 40 | |
| 3–6 | 66 | 22 | 44 | |
| ECOG PS | 0.159 | |||
| 0–1 | 117 | 37 | 80 | |
| 2–5 | 9 | 5 | 4 | |
| Adjunctive chemotherapy | 112 | 36 | 76 | 0.423 |
| Adjunctive radiotherapy | 32 | 14 | 18 | 0.148 |
| Surgery previously | 20 | 7 | 13 | 0.863 |
| Thyroid ultrasonography | ||||
| Irregular echo pattern | 62 | 28 | 34 | 0.030* |
| Diffuse change | 9 | 8 | 1 | 0.001** |
| NA | 12 | 1 | 11 | |
| PD-L1/% | 0.900 | |||
| <1% | 10 | 2 | 8 | |
| 1–49% | 11 | 6 | 5 | |
| ≥50% | 8 | 1 | 7 | |
| NA | 97 | 33 | 64 | |
| ICI agents | >0.99 | |||
| Pembrolizumab | 54 | 18 | 36 | |
| Sintilimab | 36 | 12 | 24 | |
| Atezolizumab | 21 | 7 | 14 | |
| Camrelizumab | 15 | 5 | 10 | |
| NSCLC histological subtypes | >0.99 | |||
| Squamous carcinoma | 69 | 23 | 46 | |
| Adenocarcinoma | 54 | 18 | 36 | |
| Others | 3 | 1 | 2 | |
| Gene mutations | 0.680 | |||
| KRAS† | 14 | 4 | 10 | |
| EGFR | 2 | 1 | 1 | |
| RET fusion | 3 | 1 | 2 | |
| Others | 5 | 3 | 2 | |
| NA | 61 | 21 | 40 |
*, P<0.05; **, P<0.01. †, four patients with irTDs and nine patients without irTDs had the mutation in KRAS exon 2, one patient had the mutation in KRAS exon 4. NSCLC, non-small cell lung cancer; irTD, immune-related thyroid dysfunction; SD, standard deviation; SI, smoking index; ACCI, Age-adjusted Charlson Comorbidity Index; ECOG PS, Eastern Cooperative Oncology Group Performance Status scale; NA, not available; PD-L1, programmed cell death-ligand 1; ICI, immune checkpoint inhibitor; KRAS, Kirsten rat sarcoma viral oncogene homolog; EGFR, epidermal growth factor receptor; RET, REarranged during Transfection.
Table 2
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| ATAs | 39.81 | 4.86–326.19 | <0.001** |
| TPOAb | 35.46 | 4.30–292.26 | <0.001** |
| TGAb | 1.43 | 0.95–2.14 | 0.068 |
| Irregular echo pattern | 2.70 | 1.19–6.11 | 0.016* |
| Diffuse change | 17.21 | 2.07–143.32 | 0.001** |
*, P<0.05; **, P<0.01. irTD, immune-related thyroid dysfunction; OR, odds ratio; CI, confidence interval; ATAs, anti-thyroid antibodies; TPOAb, thyroid peroxidase antibody; TGAb, thyroglobulin antibody.
irTD
The distribution and grades of irTDs are presented in Table 3 and shown in Figure 2. Most (92.9%) patients developed irTDs no later than one-year after ICIs initiation, while 3 (7.1%) patients developed irTDs 62, 69, and 106 weeks after treatment initiation. Three (7.1%) patients with pre-existing sub-hypo showed disease aggravation after ICIs treatment. Twenty-three (54.8%) patients with irTDs required thyroid hormone replacement therapy, 1 (2.4%) required glucocorticoids, and 1 (2.4%) required metoprolol treatment. Transient discontinuity of ICIs was recorded in 8 (19.0%) patients, while ICIs therapies were discontinued permanently in 1 (2.4%) patient. Depressed TSH levels before the recognition of hypothyroidism or clinical hypothyroidism were observed in 4 (9.5%) patients. One patient (2.4%) recovered from irTDs, and 41 (97.6%) experienced irTDs until the end of the study. Eight patients (19.0%) developed other types of irAEs before and after irTDs.
Table 3
| ICI agent | All (n=42) | Hypothyroidism (n=21) | Hyperthyroidism (n=7) | DH (n=14) | |||
|---|---|---|---|---|---|---|---|
| All | Overt | All | Overt | ||||
| Pembrolizumab | 18 | 8 | 6 | 5 | 3 | 5 | |
| Sintilimab | 12 | 7 | 4 | 1 | 1 | 4 | |
| Atezolizumab | 7 | 3 | 2 | 1 | 1 | 3 | |
| Camrelizumab | 5 | 3 | 1 | 0 | 0 | 2 | |
irTD, immune-related thyroid dysfunction; ICI, immune checkpoint inhibitor; DH, destructive thyroiditis.

Biomarkers
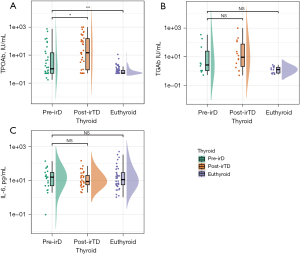
Baseline TPOAb, TGAb, and serum IL-6 levels were available for 112 (88.9%), 23 (18.3%), and 64 (50.8%) patients, respectively. Among the patients with irTDs, TPOAb, TGAb, and serum IL-6 levels at the time of irTDs were available for 34 (81.0%), 14 (33.3%), and 33 (78.6%) patients, respectively. The mean baseline TPOAb values were 57.04 and 0.92 IU/mL in patients with irTDs and euthyroid patients (P<0.05; shown in Figure 3A). There were no statistical differences in the mean of TGAb and serum IL-6 at baseline between patients with irTDs and euthyroid patients (59.80 vs. 1.40 IU/mL, 26.24 vs. 46.96 ρg/mL, P>0.05; shown in Figure 3B,3C). The positive rates of TPOAb and ATAs in patients with irTDs significantly differed from those in euthyroid patients (30.3% vs. 1.3%, 33.3% vs. 1.3%, OR =35.46, and OR =39.81, respectively; both P<0.01; Table 2), whereas TGAb showed no difference (30.0% vs. 0%, P=0.068). Elevated TPOAb at the time of irTDs was observed compared with the baseline (154.95 vs. 57.04 IU/ML, P<0.01), while TGAb and IL-6 showed no statistical differences (shown in Figure 3).
Survival
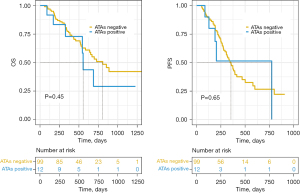
The PFS and OS were similar in patients with irTDs and euthyroid patients (10.96±6.94 vs. 9.70±6.45 months, 17.59±8.20 vs. 16.97±9.21 months, P>0.05; shown in Figure 4). There were no differences in the PFS in ATAs-positive and ATAs-negative, TPOAb-positive and TPOAb-negative, and TGAb-positive and TGAb-negative patients (11.18±7.81 vs. 10.08±6.72 months, 11.58±8.07 vs. 10.07±6.73 months, 5.39±2.19 vs. 12.07±8.89 months, P>0.05; shown in Figures 5-7). There were no differences in the OS in ATAs-positive and ATAs-negative, TPOAb-positive and TPOAb-negative, and TGAb-positive and TGAb-negative patients (19.10±8.89 vs. 17.33±8.90 months, 19.19±9.32 vs. 17.21±8.81 months, 10.64±7.60 vs. 22.54±10.26 months, P>0.05; Figures 5-7). The PFS and OS of patients with irTDs grades one to three were similar (11.86±8.68 vs. 10.77±5.83 vs. 2.47 months, 19.81±10.08 vs. 16.77±6.63 vs. 5.67 months, P>0.05).



Discussion
Key findings
Even though the paramount benefits derived from ICIs in patients with NSCLC have been extensively proven, irAEs can be life-threatening and need to be recognized as early as possible. The impact of irTDs on the clinical outcomes of patients with NSCLC and the potential biomarkers of irTDs remain unclear. Our study found that TPOAb, ATAs, and abnormal thyroid ultrasonography findings (irregular echo patterns and diffuse changes) are potential biomarkers of irTDs. There was no internal association between these potential biomarkers and the clinical outcomes of patients with NSCLC. The development of irTDs had no impact on the survival of patients with NSCLC.
Strengths and limitations
A retrospective study was conducted, on 126 NSCLC patients who were treated with pembrolizumab, sintilimab, atezolizumab, or camrelizumab as first-line therapy, at the First Affiliated Hospital, College of Medicine, Zhejiang University, between July 2019 and February 2023. ATAs, TPOAb, TGAb, serum IL-6, thyroid ultrasonography, OS, and PFS were the used as main indicators.
Our study had some limitations. First, TGAb and serum IL-6 were not available in some patients. Second, the retrospective study design had its own limitations. Finally, more reported potential biomarkers were not included. Prospective randomized controlled trials are required in the future.
Comparison with similar researches and explanations of findings
The incidence of irTD was 7.4% in our study, which was close to the incidence reported previously (9,14). Most irTD cases developed no later than one-year after ICIs initiation. However, a small minority of patients suffered from irTDs after one-year duration of ICIs treatment. The onset time of irTDs we found was comparable to the former research (23). The irTDs grades were usually 1–2 similar to previous research (24,25). No impact on ICIs treatment was believed to be caused by irTDs in a study conducted by Osorio et al. (16). However, transient and permanent discontinuities due to irTDs were recorded in our study. Only one patient achieved complete recovery from irTDs. Thus, we confirmed that irTDs were for the most part commonly irreversible (26). Hypothyroidism and DH were the most common irTDs. Surprisingly, decreased TSH levels were recorded before hypothyroidism or clinical hypothyroidism in some patients, indicating that an unrecognized and short hyperthyroidism phase may exist, and that the incidence of DH may be higher.
A prospective study conducted by Osorio et al., of irTDs in patients with NSCLC receiving pembrolizumab showed an association between ATAs at baseline and irTDs (16). Positive TPOAb and/or TGAb levels have been reported as potential irTDs biomarkers in former studies (11,27-30). Similarly, we considered positive TPOAb and ATAs as potential biomarkers indicating a higher likelihood of irTDs. The significantly elevated TPOAb levels at the time of irTDs may be associated with the mechanism which have not yet been elucidated. In addition, abnormal thyroid ultrasonography findings, including irregular echo patterns and diffuse changes, were significantly more common in patients with irTDs than in euthyroid patients, suggesting the predictive value of irTDs in abnormal thyroid ultrasonography. Serum IL-6 has been reported as a potential biomarker of irAE (31). In contrast, a study conducted by Kurimoto et al. showed no association with irTDs (11). We recorded a similar situation for serum IL-6 levels. Considering the limited sample size of serum IL-6 and TGAb, we tentatively propose the tiny possibility of serum IL-6 and TGAb as potential biomarkers.
However, the impact of irTDs on the clinical outcomes of patients with NSCLC remains unclear. Skin and endocrine adverse events associated with better survival in patients with NSCLC have been identified by Zhao et al. (32). Among patients with NSCLC, renal cell carcinoma, and metastatic melanoma who received nivolumab or pembrolizumab, those with overt irTDs had better OS and PFS (19). Patients with higher ATAs levels showed better survival than those with lower ATAs levels in this study. Similarly, favorable OS and PFS have been reported in patients with irTDs and NSCLC (18). In contrast, irTDs were considered without better OS and (or) PFS in the patients with NSCLC in a few studies (21,22). No statistically significant difference was observed in the OS and PFS between patients with irTDs and euthyroid patients in our study, suggesting that irTDs had no obvious impact on the clinical outcomes of patients with NSCLC treated with ICIs. The same results were found for positive ATAs, TPOAb, and TGAb, which were in close proximity to those of a previous study (33).
Implications and actions needed
Thus, our study has two major suggestions for clinical practice. For the irTD prediction, we suggest the usage of TPOAb, ATAs, and abnormal thyroid ultrasonography results as guidance. For the ICI treatment, we suggest that irTD may not be a main concern, although those with severe irTD still worth further attention and studies.
Conclusions
The incidence of irTD induced by ICIs (pembrolizumab, sintilimab, atezolizumab, or camrelizumab) was 7.4%. irTD is prone to occur no later than 1-year after ICIs initiation. Discontinuity of ICIs may be required because of irTDs. We confirmed that ATAs, TPOAb, irregular echo patterns, and diffuse changes on thyroid ultrasonography could be potential biomarkers for predicting irTDs. Patients with NSCLC treated with ICIs (pembrolizumab, sintilimab, atezolizumab, or camrelizumab) who developed irTDs had no advantages in terms of clinical outcomes compared with euthyroid patients.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1092/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1092/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1092/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1092/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by the Independent Ethical Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (No. 2023-0218). Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bhattacharya S, Goyal A, Kaur P, et al. Anticancer Drug-induced Thyroid Dysfunction. Eur Endocrinol 2020;16:32-9. [Crossref] [PubMed]
- Ferrari SM, Fallahi P, Elia G, et al. Autoimmune Endocrine Dysfunctions Associated with Cancer Immunotherapies. Int J Mol Sci 2019;20:2560. [Crossref] [PubMed]
- Quandt Z, Young A, Perdigoto AL, et al. Autoimmune Endocrinopathies: An Emerging Complication of Immune Checkpoint Inhibitors. Annu Rev Med 2021;72:313-30. [Crossref] [PubMed]
- Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 2017;376:1015-26. [Crossref] [PubMed]
- Zhang T, Xie J, Arai S, et al. The efficacy and safety of anti-PD-1/PD-L1 antibodies for treatment of advanced or refractory cancers: a meta-analysis. Oncotarget 2016;7:73068-79. [Crossref] [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Littman DR. Releasing the Brakes on Cancer Immunotherapy. 2015. Available online: https://core.ac.uk/reader/81999638?utm_source=linkout. Accessed 25 May 2023.
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 2016;44:51-60. [Crossref] [PubMed]
- de Filette J, Andreescu CE, Cools F, et al. A Systematic Review and Meta-Analysis of Endocrine-Related Adverse Events Associated with Immune Checkpoint Inhibitors. Horm Metab Res 2019;51:145-56. [Crossref] [PubMed]
- Kurimoto C, Inaba H, Ariyasu H, et al. Predictive and sensitive biomarkers for thyroid dysfunctions during treatment with immune-checkpoint inhibitors. Cancer Sci 2020;111:1468-77. [Crossref] [PubMed]
- Iwama S, Kobayashi T, Yasuda Y, et al. Increased Risk of Thyroid Dysfunction by PD-1 and CTLA-4 Blockade in Patients Without Thyroid Autoantibodies at Baseline. J Clin Endocrinol Metab 2022;107:e1620-30. [Crossref] [PubMed]
- Iwama S, Kobayashi T, Yasuda Y, et al. Immune checkpoint inhibitor-related thyroid dysfunction. Best Pract Res Clin Endocrinol Metab 2022;36:101660. [Crossref] [PubMed]
- Okada N, Iwama S, Okuji T, et al. Anti-thyroid antibodies and thyroid echo pattern at baseline as risk factors for thyroid dysfunction induced by anti-programmed cell death-1 antibodies: a prospective study. Br J Cancer 2020;122:771-7. [Crossref] [PubMed]
- Pollack RM, Kagan M, Lotem M, et al. Baseline TSH level is associated with risk of anti-PD-1-induced thyroid dysfunction. Endocr Pract 2019;25:824-9. [Crossref] [PubMed]
- Osorio JC, Ni A, Chaft JE, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol 2017;28:583-9. [Crossref] [PubMed]
- Sato K, Akamatsu H, Murakami E, et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer 2018;115:71-4. [Crossref] [PubMed]
- Luo J, Martucci VL, Quandt Z, et al. Immunotherapy-Mediated Thyroid Dysfunction: Genetic Risk and Impact on Outcomes with PD-1 Blockade in Non-Small Cell Lung Cancer. Clin Cancer Res 2021;27:5131-40. [Crossref] [PubMed]
- Basak EA, van der Meer JWM, Hurkmans DP, et al. Overt Thyroid Dysfunction and Anti-Thyroid Antibodies Predict Response to Anti-PD-1 Immunotherapy in Cancer Patients. Thyroid 2020;30:966-73. [Crossref] [PubMed]
- Kotwal A, Kottschade L, Ryder M. PD-L1 Inhibitor-Induced Thyroiditis Is Associated with Better Overall Survival in Cancer Patients. Thyroid 2020;30:177-84. [Crossref] [PubMed]
- Wu Y, Wang Z, Bai H, et al. Thyroid dysfunction during PD-1 inhibitor treatment in patients with cancer: Incidence and association with progression-free survival. Oncol Lett 2022;24:309. [Crossref] [PubMed]
- D'Aiello A, Lin J, Gucalp R, et al. Thyroid Dysfunction in Lung Cancer Patients Treated with Immune Checkpoint Inhibitors (ICIs): Outcomes in a Multiethnic Urban Cohort. Cancers (Basel) 2021;13:1464. [Crossref] [PubMed]
- Lima Ferreira J, Costa C, Marques B, et al. Improved survival in patients with thyroid function test abnormalities secondary to immune-checkpoint inhibitors. Cancer Immunol Immunother 2021;70:299-309. [Crossref] [PubMed]
- Shi Y, Fang J, Zhou C, et al. Immune checkpoint inhibitor-related adverse events in lung cancer: Real-world incidence and management practices of 1905 patients in China. Thorac Cancer 2022;13:412-22. [Crossref] [PubMed]
- Chang LS, Barroso-Sousa R, Tolaney SM, et al. Endocrine Toxicity of Cancer Immunotherapy Targeting Immune Checkpoints. Endocr Rev 2019;40:17-65. [Crossref] [PubMed]
- Muir CA, Menzies AM, Clifton-Bligh R, et al. Thyroid Toxicity Following Immune Checkpoint Inhibitor Treatment in Advanced Cancer. Thyroid 2020;30:1458-69. [Crossref] [PubMed]
- Kimbara S, Fujiwara Y, Iwama S, et al. Association of antithyroglobulin antibodies with the development of thyroid dysfunction induced by nivolumab. Cancer Sci 2018;109:3583-90. [Crossref] [PubMed]
- de Moel EC, Rozeman EA, Kapiteijn EH, et al. Autoantibody Development under Treatment with Immune-Checkpoint Inhibitors. Cancer Immunol Res 2019;7:6-11. [Crossref] [PubMed]
- Yoon JH, Hong AR, Kim HK, et al. Characteristics of Immune-Related Thyroid Adverse Events in Patients Treated with PD-1/PD-L1 Inhibitors. Endocrinol Metab (Seoul) 2021;36:413-23. [Crossref] [PubMed]
- Iwama S, Kobayashi T, Arima H. Clinical Characteristics, Management, and Potential Biomarkers of Endocrine Dysfunction Induced by Immune Checkpoint Inhibitors. Endocrinol Metab (Seoul) 2021;36:312-21. [Crossref] [PubMed]
- von Itzstein MS, Khan S, Gerber DE. Investigational Biomarkers for Checkpoint Inhibitor Immune-Related Adverse Event Prediction and Diagnosis. Clin Chem 2020;66:779-93.
- Zhao Z, Wang X, Qu J, et al. Immune-Related Adverse Events Associated With Outcomes in Patients With NSCLC Treated With Anti-PD-1 Inhibitors: A Systematic Review and Meta-Analysis. Front Oncol 2021;11:708195.
- Toi Y, Sugawara S, Sugisaka J, et al. Profiling Preexisting Antibodies in Patients Treated With Anti-PD-1 Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol 2019;5:376-83. [Crossref] [PubMed]



