
Predictors of improvement of radial-endobronchial ultrasonography findings from “adjacent to” to “within” in endobronchial ultrasonography using a guide sheath: a retrospective cohort study
Highlight box
Key findings
• Lesion diameter was a significant factor in predicting the change in endobronchial ultrasonography (EBUS) images, with a cut-off value of 29.25 mm and high specificity (0.852).
What is known and what is new?
• The diagnosis rate is higher when the ultrasound probe is “within”, than when it is “adjacent to”. This is the first study to evaluate changes from the initial EBUS images to the final EBUS images, and to examine the predictors of “adjacent to” to “within” occurrence.
What is the implication, and what should change now?
• If the lesion diameter is <29.25 mm, the change from “adjacent to” to “within” may not be observed. In such cases, a biopsy should be performed even if the radial-EBUS finding is not “within”.
Introduction
Flexible bronchoscopy is widely used in the diagnosis, evaluation, and treatment of respiratory diseases, both benign and malignant (1,2). The use of endobronchial ultrasonography using a guide sheath (EBUS-GS) for bronchoscopic biopsy has increased in recent years, making it a more useful diagnostic tool than conventional bronchoscopy (3-6). In EBUS-GS, the ultrasound probe is guided toward the lesion through the guide sheath to check whether the end of the guide sheath has entered the lesion. A previous study reported that 73% (59 of 81 lesions) of peripheral lung lesions measuring <20 mm could be diagnosed using this method (5).
In EBUS-GS, the ultrasound probe is generally described as “within” and “adjacent to” the lesion, and the bronchoscopist should aim to visualize “within” the lesion as much as possible during the examination (4,7,8). A previous study reported diagnosis rates of 82.4% and 41.8% when the findings obtained during biopsy using EBUS-GS were “within” and “adjacent to”, respectively (9). Furthermore, a meta-analysis showed that “within” lesions were diagnosed in 78.7% of cases and “adjacent to” lesions were diagnosed in 52%, indicating a higher diagnostic rate in the former (10). In these studies, determining the “within” and “adjacent to” imaging status was based on the best ultrasound probe image findings.
However, in actual bronchoscopy, there are occasions when the lesion is “adjacent to” the endoscopic ultrasound probe when the examination begins but reaches “within” the probe as it proceeds (we refer to this change as “A to W”). The factors responsible for this change in visualization have not yet been studied extensively. Once the relevant factors of visualization change are clarified, bronchoscopists can decide in advance whether to perform the examination aiming for “within” or whether to proceed with a biopsy based on the “adjacent to” visualization status.
In this study, we compared patients with and without the “A to W” change and evaluated the associated factors based on patient and laboratory backgrounds. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1234/rc).
Methods
Study design and population
This single-center, retrospective cohort study included consecutive patients who underwent bronchoscopic biopsy with EBUS-GS at Kameda Medical Centre between 1 April 2014 and 31 March 2019. Cases in which the lesion was not “adjacent to” immediately after insertion of the ultrasound probe were excluded. This study was approved by the Kameda Medical Centre Clinical Research Ethics Committee (No. 21-038-231027) and was conducted in accordance with the principles of the Declaration of Helsinki (as revised in 2013). This study was retrospective and did not involve patient interventions; therefore, consent was obtained in an opt-out format.
Protocol of EBUS-GS
All bronchoscopies were performed according to standard protocols (1). The choice of bronchoscope (BF TYPE P260F, 4.0-mm outer diameter, 2.0-mm working channel diameter; BF TYPE 1T-260, 5.9-mm outer diameter, 2.8-mm working channel diameter; BF TYPE P290F, 4.2-mm outer diameter, 2.0-mm working channel diameter; or BF TYPE Q290, 4.8-mm outer diameter, 2.0-mm working channel diameter; Olympus Medical Systems, Tokyo, Japan) was determined based on a discussion between the attending bronchoscopist and the senior pulmonologist. For central lesions or large target lesions (long diameter >30 mm), BF TYPE 1T-260 or BF TYPE Q290 were mainly used, whereas BF TYPE P260F or BF TYPE P290F were used for peripheral lesions, small target lesions (long diameter ≤30 mm), or lesions in the upper lobe that required bronchoscope flexibility. The endoscopic ultrasound probe used was UM-S20-17S (Olympus Medical Systems). BF TYPE 1T-260 was used with a large-diameter disposable guide sheath kit (K-203; 2.55-mm outer diameter, Olympus Medical Systems). BF TYPE P260F, BF TYPE P290F, and BF TYPE Q290 were used with a disposable guide sheath kit (K-201; 1.95-mm outer diameter, Olympus Medical Systems). Using the preliminary chest computed tomography (CT) images, the path of insertion of the device into the lesion was determined. If necessary, the insertion route was pre-designed using a virtual bronchoscopy navigation system (LungPoint, Broncus, Mountain View, USA; or Ziostation2, Ziosoft, Tokyo, Japan) at the discretion of the attending bronchoscopist (11-13). First, patients were anesthetized with the administration of 8 mL of 2% lidocaine solution to the pharynx. Next, bronchoscopy was performed after administering 1–2 mg of midazolam and 35 mg of pethidine intravenously, with the anesthetic dose adjusted based on the patient’s general condition. These drugs were administered by pulmonologists assisting the operator, all of whom were trained in sedation. Typically, a combination of 35 mg of pethidine and 1 mg of midazolam is used, but for older patients, only 17.5 mg of pethidine is administered, and midazolam is omitted. If the sedative effect is insufficient, additional doses of midazolam are administered in increments of 0.5–1 mg as required. Ventilatory support, such as a laryngeal mask, was not employed during bronchoscopy. Throughout the procedure, an additional 2% lidocaine solution was sprayed into the airway as necessary, taking into account the patient’s cough reflex and general condition. The tip was advanced as close to the target lesion as possible while maintaining the field of view through the bronchoscope. The endoscopic ultrasound probe was then inserted through a conduit in the bronchoscope along with a guide sheath into the bronchus leading to the target lesion under fluoroscopy (Versi FLEX, Hitachi Ltd., Tokyo, Japan). After insertion of the endoscopic ultrasound probe, the surgeon tried to obtain “within” as much as possible if the image was not “within”. If it was deemed difficult to obtain “within”, a biopsy was performed even if the image was not “within”. Following localization of the lesion using an endoscopic ultrasound probe, the probe was removed, and biopsy forceps were inserted through the guide sheath.
Variables
Data on the variables were retrospectively collected from the electronic medical records, and a report was generated by the bronchoscopist after the examination of age, sex, smoking history, prior lung cancer, prior cancer of any kind, suspected cancer, slice thickness on the chest CT examination immediately before bronchoscopy, number of days between bronchoscopy and the most recent chest CT examination, diameter of the target lesion, localization of the target lesion on chest CT scan (left/right, lobe, central/intermediate/peripheral area), features of target lesions and CT bronchus sign on chest CT scan (14,15), chest radiographic and fluoroscopic (during examination) lesion visibility, bronchoscope and guide sheath used, number of branches reached by the bronchoscope tip (“NBB”), number of bronchial branches before reaching the target lesion on chest CT images (“NBC”), difference between NBB and NBC (“dBC”), state of lesion using the ultrasound probe, drug use during examination (xylocaine, pethidine, and midazolam), examination time, and anonymized bronchoscopist ID.
Outcome variable
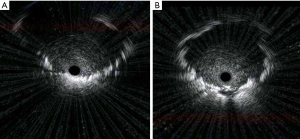
The appearance of “A to W” (change from “adjacent to” to “within” lesion status) was considered as the primary outcome. Typical findings of the “A to W” change are shown in Figure 1. Initial EBUS imaging findings showed “adjacent to” before sampling (Figure 1A), but finally showed “within” as biopsies were performed repeatedly (Figure 1B).
Statistical analysis
Because of the retrospective and observational nature of this study, we conducted statistical analysis using the available number of cases and did not perform any sample size calculations. Patient demographics were compared using univariate statistics between the groups with and without the “A to W” change. The t-test and chi-square test were used for group comparisons of continuous and categorical variables, respectively, excluding cases with missing data. Moreover, multivariate logistic regression analysis was performed with the outcome and each factor as the dependent and explanatory variables, respectively, and adjusted odds ratios (ORs) were obtained from the model coefficients. Based on univariate statistics and clinical judgement, variables for the multivariate logistic model were determined. In this study, the significance level was set at a P value of 0.05. Based on multivariate regression results and receiver operating characteristic (ROC) curve analysis, we evaluated the discriminative properties of the factors strongly correlated with the “A to W” change. Cases with missing data were excluded from the analysis for calculating ORs and plotting the ROC curve. Statistical analyses were performed using R software (version 3.6.3, R Development Core Team, https://www.r-project.org/).
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Results
Study population
In total, 1,080 patients underwent EBUS-GS biopsy at our hospital during the study period. In 260 cases, the imaging status was “adjacent to” at the time of ultrasound probe insertion. In 84 cases, the “A to W” change was observed during the ultrasound probe positioning; however, it was not observed in 176 cases (Figure 2).
Characteristics of participants
Table 1 shows the patient characteristics of the two groups, those in which “A to W” appeared and those in which “A to W” did not appear, in terms of the ultrasound probe imaging status.
Table 1
| Characteristics | Missing | “Adjacent to” to “within” (n=84) | “Adjacent to” to not “within” (n=176) | P value |
|---|---|---|---|---|
| Age, years | 0 | 69.35 [10.77] | 70.35 [9.74] | 0.454 |
| Sex | 0 | 0.436 | ||
| Male | 56 (66.7) | 107 (60.8) | ||
| Female | 28 (33.3) | 69 (39.2) | ||
| Smoking history | 1 | 55 (66.3) | 123 (69.9) | 0.658 |
| Cancer history | 1 | 35 (42.2) | 57 (32.4) | 0.163 |
| Period from CT examination to bronchoscopy, day | 0 | 12.63 [11.93] | 11.94 [29.78] | 0.839 |
| CT slice thickness, mm | 0 | 0.842 | ||
| <1 | 2 (2.4) | 4 (2.3) | ||
| 1 | 44 (52.4) | 93 (52.8) | ||
| 1<, <2 | 0 (0.0) | 1 (0.6) | ||
| 2 | 22 (26.2) | 46 (26.1) | ||
| 2<, <5 | 9 (10.7) | 12 (6.8) | ||
| 5 | 7 (8.3) | 20 (11.4) | ||
| Side of target lesion | 0 | 0.248 | ||
| Right | 43 (51.2) | 105 (59.7) | ||
| Left | 41 (48.8) | 71 (40.3) | ||
| Lobe of target lesion | 0 | 0.786 | ||
| Upper lobe | 43 (51.2) | 98 (55.7) | ||
| Middle lobe and lingular segment | 11 (13.1) | 20 (11.4) | ||
| Lower lobe | 30 (35.7) | 58 (33.0) | ||
| Part of target lesion | 4 | 0.295 | ||
| Central part | 4 (4.8) | 7 (4.0) | ||
| Middle part | 28 (33.7) | 43 (24.9) | ||
| Peripheral part | 51 (61.4) | 123 (71.1) | ||
| Diameter, mm | 9 | 26.91 [14.88] | 22.91 [11.73] | 0.021 |
| CT bronchus sign positive | 5 | 73 (88.0) | 148 (86.0) | 0.824 |
| Appearance of target lesions | 3 | 0.082 | ||
| Solid | 70 (84.3) | 135 (77.6) | ||
| Solid with cavity | 3 (3.6) | 12 (6.9) | ||
| Part-solid GGO | 9 (10.8) | 17 (9.8) | ||
| Pure GGO | 1 (1.2) | 0 (0.0) | ||
| Others | 0 (0.0) | 10 (5.7) | ||
| Visibility on chest radiograph | 7 | 74 (90.2) | 142 (83.0) | 0.184 |
| Type of bronchoscope | 1 | 0.612 | ||
| BF-P260F | 57 (67.9) | 124 (70.5) | ||
| BF-1T260 | 17 (20.2) | 31 (17.6) | ||
| BF-P290F | 8 (9.5) | 12 (6.8) | ||
| BF-Q290 | 2 (2.4) | 9 (5.1) | ||
| Type of guide sheath | 1 | 0.499 | ||
| K201 | 68 (81.0) | 150 (85.1) | ||
| K203 | 16 (19.0) | 26 (14.9) | ||
| NBB | 1 | 3.38 [1.03] | 3.29 [0.98] | 0.500 |
| NBC | 0 | 4.88 [1.17] | 5.11 [1.24] | 0.162 |
| dBC | 0 | 1.50 [1.11] | 1.82 [1.19] | 0.042 |
| Visibility on fluoroscopy | 0 | 0.279 | ||
| Fine | 55 (65.5) | 94 (53.4) | ||
| Equivocal | 14 (16.7) | 38 (21.6) | ||
| Not in use | 8 (9.5) | 28 (15.9) | ||
| Invisible | 7 (8.3) | 16 (9.1) | ||
| 2% xylocaine usage, mL | 43 | 19.29 (4.13) | 19.80 [3.68] | 0.361 |
| Pethidine usage, mg | 0 | 32.71 [7.07] | 32.41 [7.02] | 0.753 |
| Midazolam usage, mg | 0 | 2.01 [1.15] | 1.85 [0.93] | 0.233 |
| Procedure time, min | 0 | 30.92 [10.38] | 32.19 [9.71] | 0.335 |
| Biopsy count | 0 | 6.71 [1.51] | 6.28 [1.73] | 0.052 |
Data are presented as mean [SD] or n (%). For the purpose of percentage calculation, the denominator is defined as the total count, excluding any instances of missing data. CT, computed tomography; GGO, ground glass opacity; NBB, number of branches reached by the bronchoscope; NBC, number of branches before reaching the lesion on computed tomography imaging; dBC, difference between the number of branches before reaching the lesion on computed tomography imaging and the number of branches reached by the bronchoscope; SD, standard deviation.
There were no differences in the mean age, sex, smoking history, cancer history, and period from CT examination to bronchoscopy between the two groups. In addition, no differences existed in the side of target lesion, lobe of target lesion, part of target lesion, or appearance of target lesion. The mean lesion diameter was significantly larger (P=0.021) in the “A to W” group [26.91 mm (SD: 14.88 mm)] than in the group without “A to W” [22.91 mm (SD: 11.73 mm)]. The mean dBC was significantly lower (P=0.042) in the “A to W” group [1.5 (SD: 1.11)] than in the group without “A to W” [1.82 (SD: 1.19)]. The number of patients with positive CT bronchus signs was 73 (88%) and 148 (86%) in the group with and without “A to W” changes, respectively, with no significant difference (P=0.824). There was no difference in the visibility on fluoroscopy between the two groups. The mean procedure time was 30.92 min for the group with “A to W” and 32.19 min for the group without “A to W”, showing no significant difference (P=0.335).
Development of the multivariate regression model
Based on clinical judgement, we evaluated the lesion diameter and the dBC, which statistically (P<0.05) differed between groups in univariate comparisons, as well as the CT bronchus sign, using a multivariate regression model. Table 2 shows the ORs and 95% confidence intervals (CIs) based on logistic regression in relation to lesion diameter, dBC, and the appearance of “A to W” changes using the ultrasound probe in CT bronchus signs.
Table 2
| Characteristic | Odds ratio (95% CI) |
|---|---|
| Diameter | 1.023 (1.003–1.046) |
| dBC | 0.829 (0.646–1.055) |
| CT bronchus sign-positive | 1.156 (0.529–2.690) |
dBC, difference between the number of branches before reaching the lesion on computed tomography imaging and the number of branches reached by the bronchoscope; CT, computed tomography; CI, confidence interval.
Lesion diameter and dBC were included in the logistic regression based on their significant difference among the study variables. The CT bronchus sign was also included in the logistic regression because it was considered a clinically important indicator in a previous study (14,16).
Multivariate regression analysis
Table 2 lists the ORs for each variable included in the multivariate regression model. In the multivariable regression model, only the OR for lesion diameter [1.023 (95% CI: 1.003–1.046)] was significant.
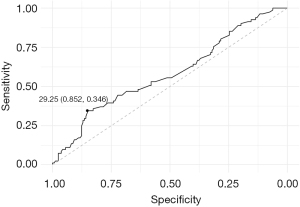
ROC curve of lesion diameter
The sensitivity and specificity were 0.346 and 0.852, respectively, at the optimal threshold (29.25 mm) set using the Youden index (Figure 3).
Discussion
In this study, we examined the factors that led to the “A to W” change during EBUS-GS examinations and found that the lesion diameter was an important factor (OR: 1.023; 95% CI: 1.003–1.046). Next, we examined the discriminative power of the lesion diameter for the occurrence of the “A to W” change and found that it had high specificity (0.852) at the optimum threshold (29.25 mm).
The strength of our study lies in that fact that we are the first study to evaluate temporal changes from the initial EBUS images to the final EBUS images, and to examine predictors of the “A to W” change. In other studies, bronchoscopy findings have rarely been documented in such detail. Furthermore, only few studies have evaluated factors that influence decision-making during the examination.
This study has several limitations. First, because this is a single-center, retrospective study, there are regional characteristics and limitations in the number of cases. Therefore, generalizability remains an issue. Second, the occurrence of “A to W” may have been influenced by the skill of the bronchoscopist, which may explain the results of this study. Since this was a single-center study and the number of bronchoscopists was limited, we were unable to adjust for bronchoscopist skills. Future multicenter studies should include the skill of the bronchoscopist, such as their years of practice.
To the best of our knowledge, this is the first study to examine the relationship between the lesion diameter and the “A to W” change. A previous meta-analysis revealed a higher diagnosis rate for larger lesions (10). Furthermore, the diagnosis rate was reported to be higher when the ultrasound probe was “within” rather than “adjacent to” (5,17). According to these studies, the difference in diagnosis rate by lesion size may be related to the ultrasound probe imaging status.
The possible reason that a large lesion diameter tends to cause “within” is thought to be that the ultrasound probe enters one of the bronchi with “within” lesions after several attempts, even if the probe initially enters a bronchus that is “adjacent to” a large lesion. Previously, a study reported that 62.5% of lesions measuring <30 mm involved only one bronchus, while 60% of lesions >30 mm involved three or more bronchi (18). When the lesion diameter is larger, the lesion often encompasses multiple bronchi, and this may explain why it affects the examination findings. Interestingly, in this study, when a model was constructed to predict whether or not “A to W” would occur in the visualized state based on tumor diameter, the optimal threshold set using the Youden index was 29.25 mm.
Furthermore, the sensitivity and specificity were 0.346 and 0.852, respectively, when the lesion diameter threshold was set at 29.25 mm. Therefore, when the lesion diameter threshold has values <29.25 mm, we expect that the ideal result cannot be obtained even if the examination proceeds, aiming for “within”. Thus, for lesion diameters <29.25 mm, biopsy should be performed as soon as the “adjacent to” finding is obtained, and increasing the number of biopsies may lead to a greater diagnostic yield.
In this study, dBC was significantly different in the univariate analysis, although it was not significant in multivariate regression. It has been reported that the average diagnostic sensitivity is higher than 66% when a bronchoscope is inserted into a bronchus with five or more branches (11), suggesting that a small dBC may be associated with a high diagnostic yield.Contrary to initial expectations, the CT bronchus sign did not affect the appearance of “A to W”, and there may be several reasons for this. For example, the CT bronchus sign is considered positive when the bronchus enters the lesion on CT imaging and negative when it does not (14). Generally, the CT bronchus sign is determined using an axial CT image (14,15,19). However, when approaching a lesion with a bronchoscope, the EBUS is inserted blindly if it is a peripheral lesion, but it is not always inserted at the target or ideal point. Fluoroscopy is performed to prevent incorrect insertion outside the target point during bronchoscopy. However, fluoroscopy is insufficient to reliably reach target peripheral lesions with EBUS-GS because it provides only a two-dimensional image (20,21). There is a possibility that the point reached by EBUS-GS and the target point on CT are divergent, and EBUS may have not reached the bronchus where the CT bronchus sign was determined.
Conclusions
In this study, we found that lesion diameter was a key factor in predicting “A to W”, with a cut-off value of 29.25 mm with high specificity (0.852). When the lesion diameter is <29.25 mm and the “adjacent to” imaging status is achieved during the initial EBUS insertion, it may be better to immediately perform biopsy instead of expending time aiming for “within”. We believe that our results provide bronchoscopists with new insights into an effective strategy for EBUS-GS in the diagnosis of peripheral lung lesions.
Acknowledgments
We thank the staff of the Department of Pulmonary Medicine and Endoscopy Laboratory at Kameda Medical Centre for performing bronchoscopies and providing patient data in this study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1234/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1234/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1234/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1234/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Kameda Medical Centre Clinical Research Ethics Committee (No. 21-038-231027) and was conducted in accordance with the principles of the Declaration of Helsinki (as revised in 2013). This study was retrospective and did not involve patient interventions; therefore, consent was obtained in an opt-out format.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Du Rand IA, Blaikley J, Booton R, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax 2013;68:i1-i44. [Crossref] [PubMed]
- Ikeda S, Yanai N, Ishikawa S. Flexible bronchofiberscope. Keio J Med 1968;17:1-16. [Crossref] [PubMed]
- Herth FJ, Ernst A, Becker HD. Endobronchial ultrasound-guided transbronchial lung biopsy in solitary pulmonary nodules and peripheral lesions. Eur Respir J 2002;20:972-4. [Crossref] [PubMed]
- Chen A, Chenna P, Loiselle A, et al. Radial probe endobronchial ultrasound for peripheral pulmonary lesions. A 5-year institutional experience. Ann Am Thorac Soc 2014;11:578-82. [Crossref] [PubMed]
- Kurimoto N, Miyazawa T, Okimasa S, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004;126:959-65. [Crossref] [PubMed]
- Kikuchi E, Yamazaki K, Sukoh N, et al. Endobronchial ultrasonography with guide-sheath for peripheral pulmonary lesions. Eur Respir J 2004;24:533-7. [Crossref] [PubMed]
- Okachi S, Imai N, Imaizumi K, et al. Factors Affecting the Diagnostic Yield of Transbronchial Biopsy Using Endobronchial Ultrasonography with a Guide Sheath in Peripheral Lung Cancer. Intern Med 2016;55:1705-12. [Crossref] [PubMed]
- Chavez C, Sasada S, Izumo T, et al. Endobronchial ultrasound with a guide sheath for small malignant pulmonary nodules: a retrospective comparison between central and peripheral locations. J Thorac Dis 2015;7:596-602. [Crossref] [PubMed]
- Steinfort DP, Bonney A, See K, et al. Sequential multimodality bronchoscopic investigation of peripheral pulmonary lesions. Eur Respir J 2016;47:607-14.
- Ali MS, Trick W, Mba BI, et al. Radial endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions: A systematic review and meta-analysis. Respirology 2017;22:443-53. [Crossref] [PubMed]
- Shinagawa N, Yamazaki K, Onodera Y, et al. Factors related to diagnostic sensitivity using an ultrathin bronchoscope under CT guidance. Chest 2007;131:549-53. [Crossref] [PubMed]
- Asahina H, Yamazaki K, Onodera Y, et al. Transbronchial biopsy using endobronchial ultrasonography with a guide sheath and virtual bronchoscopic navigation. Chest 2005;128:1761-5.
- Matsumoto Y, Izumo T, Sasada S, et al. Diagnostic utility of endobronchial ultrasound with a guide sheath under the computed tomography workstation (ziostation) for small peripheral pulmonary lesions. Clin Respir J 2017;11:185-92.
- Naidich DP, Sussman R, Kutcher WL, et al. Solitary pulmonary nodules. CT-bronchoscopic correlation. Chest 1988;93:595-8.
- Minezawa T, Okamura T, Yatsuya H, et al. Bronchus sign on thin-section computed tomography is a powerful predictive factor for successful transbronchial biopsy using endobronchial ultrasound with a guide sheath for small peripheral lung lesions: a retrospective observational study. BMC Med Imaging 2015;15:21.
- Zhan P, Zhu QQ, Miu YY, et al. Comparison between endobronchial ultrasound-guided transbronchial biopsy and CT-guided transthoracic lung biopsy for the diagnosis of peripheral lung cancer: a systematic review and meta-analysis. Transl Lung Cancer Res 2017;6:23-34.
- Yamada N, Yamazaki K, Kurimoto N, et al. Factors related to diagnostic yield of transbronchial biopsy using endobronchial ultrasonography with a guide sheath in small peripheral pulmonary lesions. Chest 2007;132:603-8. [Crossref] [PubMed]
- Tsuboi E, Ikeda S, Tajima M, et al. Transbronchial biopsy smear for diagnosis of peripheral pulmonary carcinomas. Cancer 1967;20:687-98. [Crossref] [PubMed]
- Tachihara M, Tamura D, Kiriu T, et al. Bronchoscopy Using Virtual Navigation and Endobronchial Ultrasonography with a Guide Sheath (EBUS-GS) with or without Fluoroscopy for Peripheral Pulmonary Lesions. Kobe J Med Sci 2018;63:E99-E104.
- Wagner U, Walthers EM, Gelmetti W, et al. Computer-tomographically guided fiberbronchoscopic transbronchial biopsy of small pulmonary lesions: a feasibility study. Respiration 1996;63:181-6. [Crossref] [PubMed]
- Heyer CM, Kagel T, Lemburg SP, et al. Transbronchial biopsy guided by low-dose MDCT: a new approach for assessment of solitary pulmonary nodules. AJR Am J Roentgenol 2006;187:933-9.



