
Efficacy and safety of neoadjuvant tislelizumab combined with chemotherapy in locally advanced non-small cell lung cancer—a retrospective cohort study
Highlight box
Key findings
• Neoadjuvant tislelizumab combined with chemotherapy showed good response and tolerability to neoadjuvant therapy. The pathological complete response and objective response rates were 58.33% and 75.00%, respectively, indicating good pathological and clinical remission.
What is known and what is new?
• Neoadjuvant immunotherapy is superior to adjuvant immunotherapy and chemotherapy in prolonging survival, reducing distant recurrence, and inducing anti-tumor immunity.
• Neoadjuvant tislelizumab combined with chemotherapy shows good efficacy and safety in patients with locally advanced non-small cell lung cancer (NSCLC) and has no significant adverse effects on perioperative outcomes.
What is the implication, and what should change now?
• The regimen of neoadjuvant tislelizumab combined with chemotherapy provides a basis for selecting the optimal immunotherapy mode for locally advanced NSCLC patients.
Introduction
According to Global Cancer Statistics 2020, lung cancer is one of the malignant tumors with high morbidity and mortality globally, and has become a major global disease burden. In recent years, the morbidity and mortality of primary lung cancer have been on the rise all over the world (1). According to statistics from the National Cancer Center, in 2016, the incidence and mortality of lung cancer in China ranked first among malignant tumors, with approximately 828,000 new cases and 657,000 deaths (2). At present, radical surgery is still the most effective treatment strategy for patients with resectable lung cancer (3), whereas comprehensive treatment is mainly used for patients with advanced lung cancer. However, the long-term survival rate of these patients is not ideal. According to data from the International Lung Cancer Staging Association (8th edition), the 5-year survival rate of patients with stage III lung cancer is only 12–41% (4).
Compared with adjuvant therapy, neoadjuvant therapy may have more advantages, including better tolerance, shrinking of primary lesions, reduced clinical stage, increased chances of radical surgery, and reduced postoperative recurrence (5,6). However, these advantages will also require more clinical practice to prove in the future. Past studies have confirmed that neoadjuvant chemotherapy can increase the 5-year survival rate of patients by approximately 5% (7), but the 5-year survival rate for patients with advanced lung cancer remains low. Therefore, further optimization of the treatment strategy and prolonging the survival of patients require further research. In recent years, the rise in immunotherapies has greatly changed the treatment prospects of advanced non-small cell lung cancer (NSCLC) and has become part of the standard treatment for advanced lung cancer patients. They have been shown to be associated with improvements in progression-free survival and overall survival (OS) in NSCLC (8,9). Although it is not known whether neoadjuvant or adjuvant immunotherapy is better at prolonging survival clinically. Some studies have reported that neoadjuvant immunotherapy may be superior to adjuvant immunotherapy in prolonging survival, reducing distant recurrence, and inducing anti-tumor immunity (10). The CheckMate 816 study showed that compared with chemotherapy alone, neoadjuvant nivolumab combined with chemotherapy can significantly prolong event-free survival, and the proportion of patients with pathological complete remission is higher without increasing the incidence of adverse events or affecting the feasibility of surgery (11). Therefore, people are also paying more attention to the effectiveness and safety of neoadjuvant immunotherapy in patients with NSCLC.
Tislelizumab is a humanized monoclonal antibody with high affinity and binding specificity to programmed death receptor-1 (PD-1) (12), which is used as 1st line therapy for advanced squamous NSCLC, has shown good safety and antitumor activity (13). However, its role in neoadjuvant therapy for advanced NSCLC still needs to be further explored. Therefore, this study aimed to investigate the efficacy and safety of neoadjuvant tislelizumab combined with chemotherapy in the treatment of patients with locally advanced cell lung cancer. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1103/rc).
Methods
Clinical data
This was a single-arm, non-interventional, retrospective study. From January 1, 2021, to November 30, 2022, 12 patients with locally advanced NSCLC were recruited from the Fujian Medical University Union Hospital. These patients are not expected to achieve the ideal radical resection of lung cancer, or surgery was more difficult, and they were treated with tislelizumab combined with chemotherapy before surgery. Tumor staging was performed according to the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) system (8th edition) (14), and histopathological classification was based on the 2021 World Health Organization diagnostic criteria for lung cancer (15).
Inclusion criteria: (I) age 18–70 years old, regardless of the gender; (II) squamous NSCLC confirmed by pathology (histology or cytology); (III) patients with locally advanced NSCLC: AJCC Eighth Edition TNM stage IIB–IIIB tumor. The volume is too large or close to the surrounding tissues and organs, it were not expected to achieve the ideal radical resection of lung cancer or patients anticipated with a more difficult surgery; (IV) according to the Eastern Cooperative Oncology Group (ECOG) patients with a score of 0 to 1; (V) patients with epidermal growth factor receptor (EGFR) mutation, anaplastic lymphoma kinase (ALK) gene, and ROS1 translocation negative; (VI) patients who had not received radiotherapy, chemotherapy and targeted therapy before enrollment; and with normal function of main organs; and the lung function meeting the requirements; (VII) patients who joined the study voluntarily and signed the informed consent, with good compliance, and cooperating with follow-ups.
Exclusion criteria: (I) in addition to primary NSCLC, patients with other tumors at the same time or with a history of other tumors in the past 5 years; (II) patients who had previously received chemotherapy, radiotherapy, targeted therapy, or immunotherapy other than tislelizumab; (III) the medication regimen specified in the usage plan was less than three courses of treatment; (IV) the survival period is less than 6 months; (V) the main detection indicators before and after the trial were incomplete or the research-related curative effect data were missed; (VI) the investigator believes that there are any conditions that may harm the subject or cause the subject to be unable to meet or perform the research requirements.
This study was reviewed and approved by the Ethics Committee of the Fujian Medical University Union Hospital (No. 2022KY021), and informed consent was obtained from all patients. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Treatment options
The specific treatment regimen received by the patient was tislelizumab 200 mg (on the first day) combined with chemotherapy, which included but was not limited to the following regimens: platinum combined with vinorelbine/gemcitabine/paclitaxel/etoposide/pemetrexed and others received a total of three cycles of neoadjuvant immunotherapy combined with chemotherapy, with 21 days as a cycle. Surgery was decided 4–6 weeks after neoadjuvant therapy. The surgeon determined the specific surgical method.
Clinical evaluation
Imaging evaluation
The patients underwent clinical evaluation before starting neoadjuvant therapy and after completing three cycles of neoadjuvant therapy. Surgery was considered for patients with tumor shrinkage and R0 resection after multi-disciplinary treatment. Imaging results were evaluated according to the Response Evaluation Criteria in Solid Tumors Version 1.1 (RECIST 1.1) (16).
Pathological evaluation
The pathological evaluation was performed on surgically resected specimens, which were mainly divided into pathological complete response (pCR) and major pathological response (MPR). pCR was defined as no residual viable tumor cells in the original tumor lesion after neoadjuvant therapy, and MPR was defined as 10% or less tumor cells in the original tumor lesion after neoadjuvant therapy (17).
Evaluation of adverse events
Adverse events that occurred during the medication and within three months after the end of the medication were evaluated in accordance with the Common Terminology Criteria for Adverse Events version 5.0 issued by the US National Cancer Institute (18).
Study endpoints
The primary endpoint of this study was pCR, and the secondary endpoints were objective response rate (ORR), R0 resection rate, and safety.
Statistical analysis
Statistical analyses were performed using SPSS 22.0. For continuous variables, the number of cases, mean, standard deviation, median, minimum and maximum values, quartiles, etc., were used for statistical description. For categorical variables, frequency and percentage were used for statistical description. Unless otherwise stated, P<0.05 (two-sided) was considered statistically significant.
Results
Basic characteristics of patients
From January 1, 2021, to November 30, 2022, we enrolled twelve patients with NSCLC, all of whom received three cycles of neoadjuvant tislelizumab combined with chemotherapy. Five patients (5/12, 41.67%) were ≥60 years old, seven patients (7/12, 58.33%) were <60 years old, 10 patients (83.33%) were male, and all had a smoking history; 12 cases (12/12, 100.0%) were squamous cell carcinoma; one case (1/12, 8.33%) was stage IIB, seven cases (7/12, 58.33%) were stage IIIA, and four cases (4/12, 33.33%) were stage IIIB; three patients (3/12, 25.00%) were N1 and nine cases were N2 (9/12, 75.00%) (Table 1).
Table 1
| Patient No. | Sex | Age (years) | Histologic type | cTNM | Surgical approach | Tumor shrinkage | Response per RECIST1.1 | Tumor regression grade | ypTNM |
|---|---|---|---|---|---|---|---|---|---|
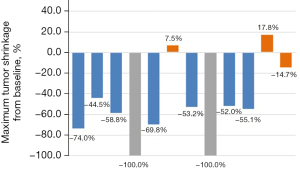
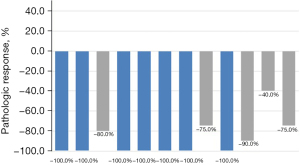
| 1 | M | 57 | LUSC | cT4N2M0 (IIIB) | Lobe | −74.0% | PR | III | ypT0N0M0 (pCR) |
| 2 | M | 48 | LUSC | cT2N2M0 (IIIA) | Lobe | −44.5% | PR | III | ypT0N0M0 (pCR) |
| 3 | M | 62 | LUSC | cT4N2M0 (IIIB) | Pneu | −58.8% | PR | IIa | ypT1cN0M0 (IA3) |
| 4 | M | 55 | LUSC | cT3N2M0 (IIIB) | Lobe | −100% | CR | III | ypT0N0M0 (pCR) |
| 5 | M | 55 | LUSC | cT3N1M0 (IIIA) | Lobe | −69.8% | PR | III | ypT0N0M0 (pCR) |
| 6 | M | 56 | LUSC | cT2N2M0 (IIIA) | Lobe | +7.5% | SD | III | ypT0N0M0 (pCR) |
| 7 | M | 62 | LUSC | cT3N2M0 (IIIB) | Lobe | −53.2% | PR | III | ypT0N0M0 (pCR) |
| 8 | M | 55 | LUSC | cT1N1M0 (IIB) | Lobe | −100% | CR | IIa | ypT1aN0M0 (IA1) |
| 9 | W | 59 | LUSC | cT2N2M0 (IIIA) | Lobe | −52.0% | PR | III | ypT0N0M0 (pCR) |
| 10 | M | 60 | LUSC | cT3N1M0 (IIIA) | Lobe | −55.1% | PR | IIb | ypT2bN1M0 (IIB) |
| 11 | M | 71 | LUSC | cT2N2M0 (IIIA) | Lobe | +17.8% | SD | IIa | ypT1cN1M0 (IIB) |
| 12 | W | 68 | LUSC | cT2N2M0 (IIIA) | Lobe | −14.7% | SD | IIa | ypT2aN0M0 (IB) |
cTNM, clinical TNM stage; TNM, tumor-node-metastasis; RECIST1.1, Response Evaluation Criteria in Solid Tumors version 1.1; ypTNM, pathological TNM stage after induction treatment; M, man; LUSC, lung squamous cell carcinoma; Lobe, lobectomy; PR, partial response; pCR, pathological complete response; Pneu, pneumonectomy; CR, complete response; SD, stable disease; W, woman.
Imaging manifestations
Among the 12 patients enrolled, the efficacy was evaluated according to RECIST 1.1. Two patients (2/12, 16.67%) achieved complete response (CR), seven patients (7/12, 58.33%) achieved partial response (PR), three patients (3/12, 25.00%) achieved stable disease (SD), and the overall ORR was 75.00%. There was no statistically significant correlation between imaging findings and pathological responses (P=0.346) (Table 1, Figure 1).
Surgery and complications
Twelve patients (12/12, 100.0%) received surgical treatment, and the median interval between the last neoadjuvant treatment and surgery was 62.17±47.56 days [interquartile range (IQR), 34.00–74.75]. Eleven patients (11/12, 91.67%) underwent lobectomy (one patient underwent right middle and right lower lobe resection, and others underwent single lobectomy), one patient (1/12, 8.33%) underwent left pneumonectomy, one patient was converted to thoracotomy, and the remaining eleven patients successfully underwent radical surgery under video-assisted thoracoscopic surgery (VATS). Pleural adhesions were found in two patients (2/12, 16.67%) during surgery, and pulmonary infection occurred in one patient (1/12, 8.33%) after surgery. The average operation time was 155.33±36.50 minutes (IQR, 121.00–187.50), and the average blood loss was 45.83±28.43 mL (IQR, 30.00–50.00). The average hospitalization time was 9.17±3.95 days (IQR, 4.75–13.75). Twelve patients (12/12, 100.0%) who underwent surgery successfully achieved R0 resection, and the mortality rate within 90 days after surgery was 0%. No recurrence or distant metastasis occurred in the follow-ups to date (Table 2).
Table 2
| Characteristics | Values |
|---|---|
| R0 resection, n (%) | 12 (100.00) |
| Interval between the neoadjuvant treatment and surgery (days), mean ± SD [IQR] | 62.17±47.56 [34.00–74.75] |
| Surgical approach, n (%) | |
| Video-assisted thoracoscopic surgery | 11 (91.67) |
| Thoracotomy | 1 (8.33) |
| Resection type, n (%) | |
| Lobectomy | 11 (91.67) |
| Pneumonectomy | 1 (8.33) |
| Nodal downstaging in patients (n=12), n (%) | |
| N1 to N0 | 3 (25.00) |
| N2 to N0 | 7 (58.33) |
| N2 to N1 | 1 (8.33) |
| N1 to N1 | 1 (8.33) |
| No. of lymph nodes harvested, mean ± SD [IQR] | 18.42±10.42 [12.00–21.75] |
| Intraoperative adhesion, n (%) | 2 (16.67) |
| Estimated blood loss (mL), mean ± SD [IQR] | 45.83±28.43 [30.00–50.00] |
| Length of postoperative hospital stay (days), mean ± SD [IQR] | 9.17±3.95 [4.75–13.75] |
| Postoperative pulmonary infection, n (%) | 1 (8.33) |
| Operation time (min), mean ± SD [IQR] | 155.33±36.50 [121.00–187.50] |
| Treatment-related adverse events during neoadjuvant treatment, n (%) | |
| Abnormal thyroid function | 3 (25.00) |
| Abnormal liver function | 1 (8.33) |
| Rash | 1 (8.33) |
SD, standard deviation; IQR, interquartile range.
Pathological manifestations
Of the 12 patients who underwent surgery, seven patients (7/12, 58.33%) achieved pCR. There was no significant difference in pathological response between stage IIB, IIIA, and IIIB subgroups [pCR: 0/1 (0.0%) vs. 4/7 (57.1%) vs. 3/4 (75.0%), P=0.394]. All 12 patients (12/12, 100.0%) who received treatment achieved downstaging. According to the tumor regression grading criteria determined by Junker et al. (19,20), seven patients (7/12, 58.33%) were grade III, four patients (4/12, 33.33%) were IIa, and one patient (1/12, 8.33%) were classified as class IIb (Table 1, Figure 2).
Adverse events
In this study, five patients (5/12, 41.67%) had treatment-related adverse reactions, all of which were grade I–II adverse reactions according to the Common Terminology Criteria for Adverse Events (CTCAE) classification, including three patients (3/12, 25.00%) with abnormal thyroid function and one case (1/12, 8.33%) with abnormal liver function, one case (1/12, 8.33%) with rash. No adverse reactions of grade III or above were found, and there were no treatment terminations or treatment-related deaths caused by related adverse reactions (Table 2).
Discussion
It is reported that the research of neoadjuvant tislelizumab combined with chemotherapy in patients with locally advanced NSCLC is still in progress. This study aimed to explore the treatment of neoadjuvant tislelizumab combined with chemotherapy in patients with locally advanced lung cancer. Currently, we enrolled twelve patients, all of whom successfully received three cycles of neoadjuvant immunotherapy combined with chemotherapy, and finally successfully underwent surgery. The results showed that 58.33% of the patients achieved pCR, and no serious adverse reactions of grade III or above were found, showing good safety and efficacy. Certainly, this is a study with a small sample size, excellent pCR rate may be derived from selection bias.
Tislelizumab is a humanized monoclonal antibody (IgG4 variant) against PD-1 of which Fc region has been engineered to minimize the interaction between the antibody Fc region and macrophages. The combination of cells eliminates the phagocytosis of T cells by macrophages [antibody dependent cellular phagocytosis (ADCP) effect], avoids the consumption of T cells by macrophages, and mediates potential resistance to anti-PD-1 therapy (21,22). It has high affinity and binding specificity for PD-1, and the dissociation rate of tislelizumab from PD-1 is slower than that of pembrolizumab and nivolumab (23). The RATIONALE 307 study showed that in the first-line treatment of patients with advanced squamous NSCLC, tislelizumab combined with chemotherapy significantly prolonged the primary endpoint progression free survival (PFS) compared with chemotherapy alone (median period 7.6 vs. 5.5 months, P<0.001; the median period was 7.6 vs. 5.5 months, P<0.001), and showed manageable safety and tolerability characteristics (13). Tislelizumab combined with carboplatin and paclitaxel (or nab-paclitaxel) has been approved as the first-line treatment for advanced squamous cell carcinoma of the lung.
With the great success of immunotherapy in the exploration and treatment of advanced lung cancer, some studies on neoadjuvant immunotherapy have also reported preliminary results, all of which have achieved very good pathological response data. Particularly for neoadjuvant immunotherapy combined with chemotherapy, it is reported that the MPR and pCR rates for immunotherapy are only 14–45% and 0–16.2%, respectively; whereas the reported MPR and pCR rates for neoadjuvant immunotherapy combined with chemotherapy are 15–86% and 9–63%, respectively (24). In an open, multicenter phase III trial (CheckMate 816), the efficacy and safety of nivolumab combined with chemotherapy as neoadjuvant therapy compared with neoadjuvant chemotherapy alone in patients with resectable NSCLC showed that nivolumab had a pCR rate of 24.0% [95% confidence interval (CI): 18.0–31.0%] in the nivolumab combined with chemotherapy group and 2.2% (95% CI: 0.6–5.6%) in the neoadjuvant chemotherapy group alone (25). At the same time, the immune neoadjuvant therapy of nivolumab combined with chemotherapy can significantly prolong the patient’s event free survival (EFS) (31.6 vs. 20.8 months) and reduce the risk of disease progression, recurrence or death compared with chemotherapy alone (11). These results further confirmed that neoadjuvant immunotherapy has shown a high pathological response rate and manageable treatment-related adverse reactions and may significantly improve the long-term prognosis of patients, which is expected to eventually translate into OS benefits. The newly published results of the KEYNOTE-671 study also reported that in patients with resectable stage II, IIIA or IIIB (N2) NSCLC, neoadjuvant pembrolizumab plus chemotherapy followed by resection and adjuvant pembrolizumab significantly improved event-free survival at 24 months, MPR (30.2% vs. 11.0%), and pCR (18.1% vs. 4.0%) as compared with neoadjuvant chemotherapy alone followed by surgery (26). And immunotherapy combined with chemotherapy represented by NADIMII, perioperative treatment with nivolumab plus chemotherapy resulted in a higher percentage of patients with a pCR (37% vs. 7%) and longer survival than chemotherapy alone (25). RATIONALE 315 is a randomized, double-blind, placebo-controlled phase III clinical study (NCT04379635), designed to study neoadjuvant tislelizumab combined with platinum-containing double-drug chemotherapy versus platinum-containing double-drug chemotherapy as neoadjuvant therapy in patients with resectable stage II–IIIA NSCLC, comparing the efficacy and safety between the two groups of patients. The results of this study have not yet been released publicly, and it is believed that the future RATIONALE 315 study can achieve satisfactory positive results.
Although OS is currently a recognized standard endpoint, pCR, MPR, ORR, etc., are still the main surrogate endpoints in most trials due to the need for long-term follow-up data. Related studies have shown that pCR or MPR may be important predictors of long-term OS and disease-free survival (DFS) in NSCLC patients treated with neoadjuvant therapy (27,28). In our study, the pCR and ORR of neoadjuvant chemotherapy combined with immunotherapy were 58.33% and 75.00%, respectively, showing a good pathological and clinical remission rate. This also shows that the regimen of neoadjuvant tislelizumab combined with chemotherapy may be beneficial to patients with locally advanced NSCLC. The number of patients currently enrolled in this trial is small, and there is a lack of long-term follow-up data. The results of this study need to be confirmed by large-scale or prospective studies.
In terms of safety, our study also showed that tislelizumab was well tolerated and controlled by all patients. Although five patients (5/12, 41.67%) had immune-related adverse reactions, according to CTCAE were all grades I–II, no adverse reactions of grade III and above were found, and no treatment termination and treatment-related death caused by related adverse reactions occurred. Related studies have also shown that immunotherapy combined with chemotherapy did not increase the incidence of grade III or higher adverse reactions (29). The common adverse reactions in this study were mainly abnormal thyroid function and liver function, which is also consistent with current research at home and abroad (30).
At present, the impact of neoadjuvant immunotherapy on the feasibility and safety of surgery is being explored. Related studies have shown that neoadjuvant immunotherapy may lead to mediastinal and hilar fibrosis (31), which may undoubtedly increase the difficulty of surgery. However, the results of the NEOSTAR study showed that immunotherapy had little effect on the surgical resection rate and surgical complexity; it also had no significant adverse effect on perioperative outcomes (32). In our study, all patients were successfully operated, among which one patient was converted to thoracotomy, and the remaining 11 patients successfully underwent radical surgery under VATS. Pleural adhesions were found in two patients during operation, and only one patient was observed lung infection. The average operation time was 155.33±36.50 minutes (IQR, 121.00–187.50), and the average blood loss was 45.83±28.43 mL (IQR, 30.00–50.00). The operative time, blood loss, and postoperative complications of the patients included in this study were not significantly affected, and the R0 resection rate was 100%. In conclusion, based on the available data, there is no conclusive evidence that neoadjuvant immunotherapy has major adverse effects on the feasibility and safety of surgery.
Our study has several limitations. First the study was conducted in a single center, and the number of patients currently enrolled was small; therefore, it should be further verified in a larger phase III trial in the future. Second, despite the high pathological remission rate in this study, long-term follow-up data are still lacking. Further attention should be paid to the prognosis of patients, including the 3-year or 5-year survival rate and DFS.
Conclusions
In conclusion, we reported 12 cases of tislelizumab combined with chemotherapy in the neoadjuvant treatment of locally advanced NSCLC. All 12 patients showed a favourable response and tolerability to neoadjuvant therapy. The pCR and ORR rates were 58.33% and 75.00%, respectively, indicating good pathological and clinical remission. However, this is a study with a small sample size, its indications require further clinical trials to verify and further large-scale prospective studies are also needed in the future to provide a basis for the selection of the best immunotherapy model for patients.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1103/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1103/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1103/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1103/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by approved by the Ethics Committee of the Fujian Medical University Union Hospital (No. 2022KY021) and informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Zheng R, Zhang S, Zeng H, et al. Cancer incidence and mortality in China, 2016. J Natl Cancer Cent 2022;2:1-9.
- Wyld L, Audisio RA, Poston GJ. The evolution of cancer surgery and future perspectives. Nat Rev Clin Oncol 2015;12:115-24. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Scagliotti GV, Pastorino U, Vansteenkiste JF, et al. Randomized phase III study of surgery alone or surgery plus preoperative cisplatin and gemcitabine in stages IB to IIIA non-small-cell lung cancer. J Clin Oncol 2012;30:172-8. [Crossref] [PubMed]
- Pisters KM, Vallières E, Crowley JJ, et al. Surgery with or without preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer: Southwest Oncology Group Trial S9900, an intergroup, randomized, phase III trial. J Clin Oncol 2010;28:1843-9. [Crossref] [PubMed]
- Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 2014;383:1561-71. [Crossref] [PubMed]
- Duma N, Santana-Davila R, Molina JR. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin Proc 2019;94:1623-40. [Crossref] [PubMed]
- Pan C, Liu H, Robins E, et al. Next-generation immuno-oncology agents: current momentum shifts in cancer immunotherapy. J Hematol Oncol 2020;13:29. [Crossref] [PubMed]
- Cascone T, Hamdi H, Zhang F, et al. Superior efficacy of neoadjuvant compared to adjuvant immune checkpoint blockade in non-small cell lung cancer. Cancer Res 2018;78:abstr 1719.
- Forde PM, Spicer J, Lu S, et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med 2022;386:1973-85. [Crossref] [PubMed]
- Shen L, Guo J, Zhang Q, et al. Tislelizumab in Chinese patients with advanced solid tumors: an open-label, non-comparative, phase 1/2 study. J Immunother Cancer 2020;8:e000437. [Crossref] [PubMed]
- Wang J, Lu S, Yu X, et al. Tislelizumab Plus Chemotherapy vs Chemotherapy Alone as First-line Treatment for Advanced Squamous Non-Small-Cell Lung Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol 2021;7:709-17. [Crossref] [PubMed]
- Kay FU, Kandathil A, Batra K, et al. Revisions to the Tumor, Node, Metastasis staging of lung cancer (8(th) edition): Rationale, radiologic findings and clinical implications. World J Radiol 2017;9:269-79. [Crossref] [PubMed]
- Nicholson AG, Tsao MS, Beasley MB, et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J Thorac Oncol 2022;17:362-87. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Hellmann MD, Chaft JE, William WN Jr, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 2014;15:e42-50. [Crossref] [PubMed]
- Freites-Martinez A, Santana N, Arias-Santiago S, et al. Using the Common Terminology Criteria for Adverse Events (CTCAE - Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr (Engl Ed) 2021;112:90-2. [Crossref] [PubMed]
- Junker K, Thomas M, Schulmann K, et al. Tumour regression in non-small-cell lung cancer following neoadjuvant therapy. Histological assessment. J Cancer Res Clin Oncol 1997;123:469-77. [Crossref] [PubMed]
- Junker K, Langner K, Klinke F, et al. Grading of tumor regression in non-small cell lung cancer: morphology and prognosis. Chest 2001;120:1584-91. [Crossref] [PubMed]
- Zhang T, Song X, Xu L, et al. The binding of an anti-PD-1 antibody to FcγRI has a profound impact on its biological functions. Cancer Immunol Immunother 2018;67:1079-90. [Crossref] [PubMed]
- Dahan R, Sega E, Engelhardt J, et al. FcγRs Modulate the Anti-tumor Activity of Antibodies Targeting the PD-1/PD-L1 Axis. Cancer Cell 2015;28:285-95. [Crossref] [PubMed]
- Hong Y, Feng Y, Sun H, et al. Tislelizumab uniquely binds to the CC' loop of PD-1 with slow-dissociated rate and complete PD-L1 blockage. FEBS Open Bio 2021;11:782-92. [Crossref] [PubMed]
- Shao L, Lou G. Neoadjuvant immunotherapy in non-small cell lung cancer: a narrative review on mechanisms, efficacy and safety. J Thorac Dis 2022;14:3565-74. [Crossref] [PubMed]
- Provencio M, Nadal E, González-Larriba JL, et al. Perioperative Nivolumab and Chemotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2023;389:504-13. [Crossref] [PubMed]
- Wakelee H, Liberman M, Kato T, et al. Perioperative Pembrolizumab for Early-Stage Non-Small-Cell Lung Cancer. N Engl J Med 2023;389:491-503. [Crossref] [PubMed]
- Weissferdt A, Pataer A, Vaporciyan AA, et al. Agreement on Major Pathological Response in NSCLC Patients Receiving Neoadjuvant Chemotherapy. Clin Lung Cancer 2020;21:341-8. [Crossref] [PubMed]
- Pataer A, Kalhor N, Correa AM, et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 2012;7:825-32. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Brahmer JR, Lacchetti C, Thompson JA. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline Summary. J Oncol Pract 2018;14:247-9. [Crossref] [PubMed]
- Chaft JE, Hellmann MD, Velez MJ, et al. Initial Experience With Lung Cancer Resection After Treatment With T-Cell Checkpoint Inhibitors. Ann Thorac Surg 2017;104:e217-8. [Crossref] [PubMed]
- Cascone T, William WN Jr, Weissferdt A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med 2021;27:504-14. [Crossref] [PubMed]


