
Effects of occupational exposure to dust, gas, vapor and fumes on chronic bronchitis and lung function
Highlight box
Key findings
• Vapors, gases, dust and fumes (VGDF) exposure is associated with chronic bronchitis, respiratory symptoms and decreased lung function.
What is known and what is new?
• Previous studies documented the inconsistent association between occupational exposure with chronic bronchitis and lung function mainly in western countries.
• We explore the effects of VGDF exposures on lung function and chronic bronchitis in middle-aged adults and the elderly in southern China.
What is the implication, and what should change now?
• VGDF might contribute to the pathogenesis and progression of chronic obstructive pulmonary disease.
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of mortality and morbidity worldwide (1,2), and is characterized by incompletely reversible airflow limitation (3). Chronic bronchitis, defined as cough and sputum production lasting for at least 3 months in 2 consecutive years, is the core manifestation of COPD (4). Chronic bronchitis has been associated with more frequent exacerbations and more rapid lung function decline (5-7). Identification of the modifiable risk factors [such as vapors, gases, dust and fumes (VGDF)] for the early or excessive lung function decline and the development of chronic bronchitis has important significance (8). Several studies have analyzed the association between occupational exposures to VGDF and chronic bronchitis and the relevant respiratory symptoms based on the studies conducted in Europe [European Community Respiratory Health Survey (ECRHS)], Australia and Singapore (8-11). However, these studies have revealed inconsistent results. For instance, Sunyer et al. (9) reported an association between occupational exposures to dust with chronic phlegm but not with chronic bronchitis, while Lytras et al. (10) and LeVan et al. (11) reported a positive association between mineral dust exposure and chronic bronchitis.
As the main source of occupational exposure, the impact of VGDF exposure on lung function has been increasingly recognized (12-14). Findings on the association between VGDF exposure and lung function decline have been conflicting, with some studies reporting a positive association (12-15) but others not (9,16). To date, none of the published studies has been conducted in developing countries such as China. Further studies are warranted to provide the evidence confirming the association between occupational exposure to VGDF and lung function changes.
Previous studies regarding the association between occupational exposure with chronic bronchitis, respiratory symptoms and lung function were mainly conducted in industrial groups with high levels of exposures (17,18). This might render these studies to have suffered from the healthy worker effect and selection bias (the affected workers would have to leave from the highly exposed jobs), which could have collectively resulted in an underestimation of the true risk (19,20). Community-based studies recruiting participants from the general population can help minimize the bias mentioned above. However, nearly all these studies have been conducted in Western countries. In addition, occupational exposure to VGDF may frequently co-exist in real-world scenarios. However, few studies have evaluated the association between dual exposure (dust plus gas/vapor/fumes) and respiratory symptoms and lung function in developing countries, including China (21).
We hypothesized that occupational exposure to VGDF would contribute to decreased lung function and the incidence of chronic bronchitis. Here, we sought to explore the effects of VGDF exposures on lung function and chronic bronchitis in middle-aged adults and the elderly in southern China. Our findings might provide further scientific evidence regarding the adverse effects of the more broadly defined occupational exposure to VGDF in the general population. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-646/rc).
Methods
Study design and participants
Between 2014 and 2019, we adopted a multistage, probability-based sampling strategy for COPD surveillance in six districts or counties of Guangdong province (22). Briefly, residents aged ≥40 years living in the current surveillance point for at least 6 months were eligible for participation. Residents with cognitive defects, language or mental disorders, cancer, paraplegia, or were pregnant or breastfeeding were excluded. Data were collected during a consultation in a healthcare facility by trained staff from the local health stations or community clinics. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the ethics review committee of the National Center for Chronic and Non-Communicable Disease Control and Prevention, China Center for Disease Control and Prevention in 2014 (approval No. 201410) and the ethics review committee of the Guangdong Provincial Center for Disease Control and Prevention in 2019 (approval No. 201901). All participants had provided written informed consent. In our study, a total of 7,418 and 5,249 participants were included for analyzing the effects of occupation exposure to VGDF on chronic bronchitis and lung function, respectively (Figure S1).
Clinical assessments
Trained staffs conducted pre- and post-bronchodilator spirometry among all eligible participants by using commercial spirometers (MasterScreen Pneumo, Jaeger, Germany), in accordance with the international guidelines (3,23). We obtained at least three technically acceptable and repeatable maneuvers for each participant. The highest values of forced expiratory volume in one second (FEV1), forced vital capacity (FVC), and maximal mid-expiratory flow (MMEF) were selected. The absolute values of lung function parameters, measured with post-bronchodilator spirometry, were adopted in subsequent analysis.
The records of respiratory symptoms, including chronic cough and phlegm, were derived from the standardized questionnaire based on the Epidemiologic Standardization Project Questionnaire of the American Thoracic Society (ATS-DLD-78-A) (24). Persistent cough denoted coughing on most days (≥4 days per week) for at least three months each year in the absence or presence of cold (participants responding ‘yes’ to several items on coughing). Persistent phlegm denoted sputum production on most days (≥4 days per week) for at least three months each year in the absence or presence of cold (participants responding ‘yes’ to this question item). Respondents also reported the frequency of respiratory symptoms. Chronic bronchitis was defined as coughing up phlegm for at least three months in two consecutive years (25).
Exposure assessment
A standardized questionnaire was used to ascertain occupational exposure to VGDF. Participants were requested to answer to the occupational information including the job title, industry, and the duration of occupation which were associated with occupational exposure to VGDF. Participants with exposure to any item of VGDF for more than 1 year over their lifetime were considered as occupationally exposed. All participants were divided into four groups: exposure to dust only, exposure to gas/vapor/fume only, dual exposure to dust and, gas/vapor/fume, and non-occupational exposure.
Covariates
We captured the following covariates from the questionnaire survey: age, sex (male, female), education level (none + primary school education/middle school education or higher), marriage status (married/unmarried), region of residence (urban/rural), body mass index (BMI) (underweight/normal/overweight/obese), smoking status (no/yes) and biomass fuel (no/yes). The type and definition of the exposure and covariates are shown in Table S1.
Statistical analysis
We analyzed the fundamental characteristics of participants in the exposure groups of dust, gas/vapor/fumes and VGDF. Continuous variables were demonstrated with the mean and standard deviation, and categorical variables with counts and frequencies. The t-tests were employed to analyze the association of continuous variables, and when indicated, transformation was applied. Contingency tables and chi-squared tests were applied for categorical variables. The odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated for the association between occupational exposure to VGDF and chronic bronchitis and individual respiratory symptoms (cough and phlegm) by performing univariate and multivariate logistic regression. Multivariate linear regression model was adopted to calculate the changes of FEV1, FVC, FEV1/FVC and MMEF in two groups with different occupational exposure status. The magnitude of collinearity was assessed based on the variance inflation factor (VIF). The VIF of 5 or greater indicated collinearity among the variables. Variables with the evidence of a significant collinearity were excluded from the model. We performed three regression models by adjusting for different variables as follow:
- Model 1: occupational exposure to dust, gas/vapor/fume or dust/gas/vapor/fume;
- Model 2: Model 1 further adjusted with the age, sex, education level, marriage status, region of residence;
- Model 3: Model 2 further adjusted with the BMI, height, smoking status and biomass fuel.
Results were deemed materially changed when there was a transition (e.g., from significant to no significance, or the vice versa) in the direction of associations of Model 1 to Model 3.
Because of the notable differences in occupational category and intensities between males and females in the same occupationally exposed industry, we performed an analysis stratified by sex to examine the differential effect of occupational exposure of VGDF on lung function. When statistically significant effect was observed, a likelihood test was further performed.
We further conducted sensitivity analyses based on two methods of propensity score (PS) methods to evaluate the robustness of our results: (I) inverse probability weighting; (II) including the PS as an additional covariate. Age, sex, height, education level, marriage status, region of residence, BMI, height, smoking status and biomass fuel were adopted to calculate the PS. In all analyses, non-occupationally exposure groups were treated as the reference.
All statistical analyses were performed with SAS software 9.4 (SAS Institute, Inc., Cary, NC, USA). The threshold for statistical significance was set to P<0.05.
Results
Baseline characteristics of the study participants
The baseline characteristics of the study population are summarized in Table 1. The proportion of males, younger participants, smokers and participants with normal BMI status was consistently higher among those working with exposure to dust. In addition, participants exposed to dust had a higher frequency of cough, phlegm, and had slightly higher FEV1, FVC and MMEF (P<0.05). Participants with occupational exposure to gas/vapor/fume were mainly young people living in rural areas and who have mostly received primary school education or lower. Exposure to gas vapor and fumes was associated with cough, and lower FEV1/FVC and MMEF (P<0.05). Participants with VGDF exposures were more likely to be males, living in rural areas and smoking. Apart from having a lower level of education, these participants had a normal BMI status and a higher frequency of cough and phlegm, with higher FVC and lower FEV1/FVC and MMEF (all P<0.05).
Table 1
| Characteristics | Dust | Gas, vapor and fumes | Dust, gas, vapor and fumes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No (N=4,219) | Yes (N=912) | P | No (N=4,219) | Yes (N=638) | P | No (N=4,219) | Yes (N=1,649) | P | |||
| SDI | |||||||||||
| Sex, n (%) | <0.001 | 0.80 | <0.001 | ||||||||
| Male | 2,019 (47.85) | 533 (58.44) | 2,019 (47.85) | 309 (48.43) | 2,019 (47.85) | 939 (56.94) | |||||
| Female | 2,200 (52.15) | 379 (41.56) | 2,200 (52.15) | 329 (51.57) | 2,200 (52.15) | 710 (43.06) | |||||
| Age (years), mean (SD) | 57.83 (9.74) | 55.23 (8.38) | <0.001 | 57.83 (9.74) | 56.84 (8.24) | 0.01 | 57.83 (9.74) | 57.32 (8.81) | 0.05 | ||
| Educational level, n (%) | 0.20 | <0.001 | <0.001 | ||||||||
| None + primary school education | 2,332 (55.27) | 482 (52.85) | 2,332 (55.27) | 406 (63.64) | 2,332 (55.27) | 1,046 (63.43) | |||||
| Middle school education or higher | 1,887 (44.73) | 430 (47.15) | 1,887 (44.73) | 232 (36.36) | 1,887 (44.73) | 603 (36.57) | |||||
| Marriage status, n (%) | <0.001 | 0.06 | <0.001 | ||||||||
| Married | 3,796 (89.97) | 853 (93.53) | 3,796 (89.97) | 589 (92.32) | 3,796 (89.97) | 1,526 (92.54) | |||||
| Unmarried | 423 (10.03) | 59 (6.47) | 423 (10.03) | 49 (7.68) | 423 (10.03) | 123 (7.46) | |||||
| Region of residence, n (%) | 0.30 | <0.001 | <0.001 | ||||||||
| Urban | 1,729 (40.98) | 392 (42.98) | 1,729 (40.98) | 143 (22.41) | 1,729 (40.98) | 490 (29.71) | |||||
| Rural | 2,490 (59.02) | 520 (57.02) | 2,490 (59.02) | 495 (77.59) | 2,490 (59.02) | 1,159 (70.29) | |||||
| Biomass fuel, n (%)* | 0.30 | <0.001 | <0.001 | ||||||||
| No | 2,678 (66.75) | 569 (65.03) | 2,678 (66.75) | 307 (49.44) | 2,678 (66.75) | 697 (43.24) | |||||
| Yes | 1,334 (33.25) | 306 (34.97) | 1,334 (33.25) | 314 (50.56) | 1,334 (33.25) | 915 (56.76) | |||||
| Smoking, n (%)* | <0.001 | >0.9 | <0.001 | ||||||||
| No | 2,545 (60.49) | 451 (49.45) | 2,545 (60.49) | 384 (60.28) | 2,545 (60.49) | 819 (49.73) | |||||
| Yes | 1,662 (39.51) | 461 (50.55) | 1,662 (39.51) | 253 (39.72) | 1,662 (39.51) | 828 (50.27) | |||||
| Symptoms, n (%) | |||||||||||
| Chronic bronchitis | 0.40 | 0.90 | <0.001 | ||||||||
| No | 4,143 (98.20) | 892 (97.81) | 4,143 (98.20) | 626 (98.12) | 4,143 (98.20) | 1,591 (96.48) | |||||
| Yes | 76 (1.80) | 20 (2.19) | 76 (1.80) | 12 (1.88) | 76 (1.80) | 58 (3.52) | |||||
| Cough | <0.001 | 0.005 | <0.001 | ||||||||
| No | 3,950 (93.62) | 822 (90.13) | 3,950 (93.62) | 578 (90.60) | 3,950 (93.62) | 1,483 (89.93) | |||||
| Yes | 269 (6.38) | 90 (9.87) | 269 (6.38) | 60 (9.40) | 269 (6.38) | 166 (10.07) | |||||
| Phlegm | <0.001 | 0.20 | <0.001 | ||||||||
| No | 3,792 (89.88) | 774 (84.87) | 3,792 (89.88) | 562 (88.09) | 3,792 (89.88) | 1,396 (84.66) | |||||
| Yes | 427 (10.12) | 138 (15.13) | 427 (10.12) | 76 (11.91) | 427 (10.12) | 253 (15.34) | |||||
| Anthropometry | |||||||||||
| BMI (kg/m2), mean (SD) | 24.04 (3.92) | 23.73 (3.37) | 0.001 | 24.04 (3.92) | 23.80 (3.42) | 0.11 | 24.04 (3.92) | 23.60 (3.38) | <0.001 | ||
| BMI category, n (%) | 0.003 | 0.40 | <0.001 | ||||||||
| Under weight | 276 (6.54) | 34 (3.73) | 276 (6.54) | 35 (5.49) | 276 (6.54) | 83 (5.03) | |||||
| Normal | 1,975 (46.81) | 462 (50.66) | 1,975 (46.81) | 318 (49.84) | 1,975 (46.81) | 888 (53.85) | |||||
| Over weight | 1,476 (34.98) | 300 (32.89) | 1,476 (34.98) | 219 (34.33) | 1,476 (34.98) | 517 (31.35) | |||||
| Obesity | 492 (11.66) | 116 (12.72) | 492 (11.66) | 66 (10.34) | 492 (11.66) | 161 (9.76) | |||||
| Before bronchodilator use, mean (SD) | |||||||||||
| FEV1, L | 2.27 (0.60) | 2.43 (0.57) | <0.001 | 2.27 (0.60) | 2.25 (0.54) | 0.50 | 2.27 (0.60) | 2.29 (0.59) | 0.30 | ||
| FVC, L | 2.92 (0.74) | 3.13 (0.68) | <0.001 | 2.92 (0.74) | 2.96 (0.67) | 0.30 | 2.92 (0.74) | 3.01 (0.72) | <0.001 | ||
| FEV1/FVC | 77.73 (8.63) | 77.66 (7.94) | 0.80 | 77.73 (8.63) | 76.27 (8.36) | <0.001 | 77.73 (8.63) | 76.12 (8.67) | <0.001 | ||
| MMEF, L/min | 2.04 (0.90) | 2.16 (0.89) | 0.001 | 2.04 (0.90) | 1.90 (0.83) | 0.002 | 2.04 (0.90) | 1.95 (0.87) | 0.005 | ||
| After bronchodilator use, mean (SD) | |||||||||||
| FEV1, L | 2.32 (0.60) | 2.48 (0.56) | <0.001 | 2.32 (0.60) | 2.30 (0.53) | 0.40 | 2.32 (0.60) | 2.35 (0.59) | 0.20 | ||
| FVC, L | 2.93 (0.73) | 3.13 (0.67) | <0.001 | 2.93 (0.73) | 2.96 (0.66) | 0.40 | 2.93 (0.73) | 3.02 (0.72) | <0.001 | ||
| FEV1/FVC | 79.53 (8.74) | 79.46 (7.93) | 0.80 | 79.53 (8.74) | 78.17 (8.51) | 0.002 | 79.53 (8.74) | 78.01 (8.78) | <0.001 | ||
| MMEF, L/min | 2.26 (0.96) | 2.38 (0.93) | 0.002 | 2.26 (0.96) | 2.10 (0.89) | <0.001 | 2.26 (0.96) | 2.17 (0.92) | 0.005 | ||
*, the variable had missing values. SDI, social demographic indices; SD, standard deviation; BMI, body mass index; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; FEV1/FVC, forced expiratory volume in one second/forced vital capacity; MMEF, maximal mid-expiratory flow.
Association between VGDF and respiratory symptoms
Occupational exposure to dust was associated with an increased risk of cough (OR: 1.60, 95% CI: 1.22 to 2.08, P=0.001 in Model 3) and phlegm (OR: 1.49, 95% CI: 1.19 to 1.85, P<0.001 in Model 3). We only noted significantly increased risk of cough (OR: 1.53, 95% CI: 1.11 to 2.07, P=0.008 in Model 3) but no other symptoms for occupational exposure to gas/vapor/fume group. Notably, dual occupational exposure to dust and gas/vapor/fume was associated with significantly increased risk of chronic bronchitis. Results were shown in Figure 1 and Table S2.
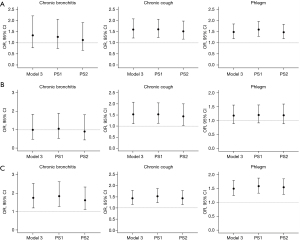
Similar associations between VGDF exposure and chronic bronchitis and respiratory symptoms have been observed in males. However, the impact of VGDF exposures on chronic bronchitis and respiratory syndromes was not significant in females. After adjusting for important covariates, exposure to dust was associated only with phlegm, while VGDF exposure was associated with both cough and phlegm in females. Detailed results are shown in Table S3. Likelihood test showed that the gender differences in the effects of dust exposure on phlegm, VGDF exposure on both cough and phlegm were not statistically significant (both P>0.05).
Association between VGDF and lung function
We did not identify significant associations between occupational dust exposure and decreased lung function (Figure 2, Table S4). The association was not present in both males and females (Table S5). Compared with the reference group, the mean FEV1/FVC was −1.05 (95% CI: −1.85, −0.26, P=0.01 in Model 3), MMEF was −0.15 L/min (95% CI: −0.23, −0.07, P<0.001 in Model 3) in participants with occupational exposure to gas/vapor/fume (Figure 2, Table S4). The mean MMEF was 0.13 L/min lower in males (Table S5), while the corresponding mean FEV1/FVC and MMEF was 1.34 and 0.17 L/min lower in females with occupational exposure to gas/vapor/fume (Table S5). The mean FVC was 0.04 L higher (95% CI: 0.01, 0.07, P=0.004 in Model 3), the FEV1/FVC was −0.74 (95% CI: −1.28, −0.20, P=0.01 in Model 3) and the mean MMEF was −0.06 L/min (95% CI: −0.12, −0.01, P=0.03 in Model 3) in participants with dual occupational exposure when compared with those without exposure to VGDF (Figure 2, Table S4). The mean FVC was 0.06 L higher in males (Table S5), while the corresponding mean FEV1/FVC and MMEF was 0.95 and 0.09 L/min lower in females with dual occupational exposure (Table S5). Likelihood test showed that the gender differences in the effects of gas/vapor/fume exposure on MMEF were not statistically significant.

Sensitivity analysis
Sensitivity analysis showed that the results were not substantially changed. In the analyses which had included the PS score as an additional covariate, and in the analysis that had included the inverse probability weighting, participants exposed to dust were consistently more likely to suffer from cough and phlegm than those who did not have VGDF exposures. Occupational exposure to gas/vapor/fume was also associated with phlegm based on the sensitivity analysis. Participants with dual occupational exposure were more likely to develop chronic bronchitis and respiratory symptoms than those exposed to neither dust nor gas/vapor/fume. Results were shown in Figure 1 and Table S6. The mean FEV1/FVC and MMEF were lower in participants with occupational exposure to gas/vapor/fume and VGDF compared with those without occupational exposure (Figure 2, Table S7).
Discussion
This cross-sectional study has provided solid population-based evidence supporting the fact that simultaneous occupational exposure to VGDF was associated with increased risk of chronic bronchitis. We found an increased risk of the salient respiratory symptoms, including cough and/or phlegm among participants with three occupational exposure groups of dust only, gas/vapor/fume only and VGDF. Furthermore, occupational exposure to gas/vapor/fume or VGDF was associated with lower lung function.
A number of population-based studies have reported the association between dust exposure and symptoms related to chronic bronchitis (26). However, few studies have evaluated the association between chronic bronchitis and gas, vapor and fume, particularly dual exposure of dust and gas/vapor/fume. Our study has added substantially to the of evidence pertaining to the association between occupational exposure to VGDF and chronic bronchitis. Similar to the finding of an earlier analysis in the cohort of ECRHS, there was no statistically significant association between occupational exposures to mineral dust and chronic bronchitis in young adults (9). However, the latest cohort study of ECRHS showed an increased risk of chronic bronchitis associated with mineral dust exposure (10). A positive association between the exposure to gas/vapor/fume and chronic bronchitis was not demonstrated in the latest cohort study of ECRHS (10), which was similar to the findings of our study. These indicated that social economic status including age (27), life exposure, residential area and education level might have collectively explained for the inconsistent findings across the studies. After adjusting for the age, sex, height, education level, marriage status, region of residence, BMI, smoking status and biomass fuel by using the PS algorithm, results of the occupational effects of dust or gas/vapor/fume did not change materially, rendering our results robust. A key research question of our study was whether the adverse effects of dual occupational exposure to dust and gas/vapor/fume would be synergistic. Indeed, based on the cross-sectional survey in Guangdong province, we have noted a higher risk of chronic bronchitis in participants with dual occupational exposure to VGDF when compared with those exposed to dust or gas/vapor/fume alone. The mechanisms pertaining to the effect of occupational exposure to VGDF on chronic bronchitis are less clear. VGDF are a heterogeneous category of exposures, which have been linked to various forms of pulmonary toxicity (28-30), for instance, the significant association between vanadium exposure and chronic bronchitis have been reported (28,30).
In this study, we have evaluated the association between occupational exposures and chronic bronchitis symptoms of cough and phlegm separately, which were less specific to chronic bronchitis. Although some studies have documented that the VGDF inhalation exposure did not lead to the development of chronic cough (26,31) or chronic phlegm (31), we have now identified the associations between VGDF and multiple respiratory symptoms including cough and phlegm, which mirrored the previous findings. A cohort study has demonstrated that occupational exposure to VGDF resulted in the respiratory symptoms related to bronchitis (32,33). Another population-based study has demonstrated an association between occupational exposure to VGDF and chronic cough and phlegm (34). Moreover, the effects of VGDF exposures on cough, phlegm and bronchitis varied considerably between males and females. Further study is warranted to confirm the differential association between males and females.
Previous studies have provided some evidence for an association between VGDF exposure and lower levels of lung function, but the findings remained contradictory. Several cross-sectional studies (35-37) and a longitudinal study (38) did not suggest an association between exposure to VGDF and accelerated lung function decline. A five-year follow-up study reported that fume exposure was associated with significantly decreased FEV1 among individuals with early-stage COPD (12). These studies were conducted solely based on the young adults, patients with a known diagnosis of COPD, a single industry or occupational category, or lung function parameters reflecting large airway disorders (decreased FEV1). By contrast, our study sought to address different questions. For instance, we were more concerned whether the occupational exposure to VGDF would affect the lung function in the general population. Second, we probed into the hypothesis whether the adverse effects of dual occupational exposure to dust and gas/vapor/fume on lung function would be synergistic. Third, we added MMEF as one of the important indices of lung function, which has been adopted as the key parameter to indicate small airway obstruction in the large population-based studies (39,40). From the standpoint of the study design, our study has included the older general population from the community. These findings highlighted the role of VGDF exposure in small airway obstruction (partly evidenced by the decreased MMEF), resulting in the progression of chronic airway obstructive diseases such as COPD (41). Our study was not designed to specifically address the plausible mechanisms how VGDF exposure could dampen the lung function. We speculated different mechanisms leading to airflow limitation related to different kinds of VGDF, depending on the biochemical pathways as well as the vapor and aerosol droplet size.
This study has certain limitations. First, the prevalence of chronic bronchitis in females (0.77%) was relatively lower than in males (3.63%), therefore the CIs of effect estimates for VGDF and chronic bronchitis were wide in females. Future study which recruits more women and more comprehensively assess the effect modification by gender is needed. Second, the magnitude of association might have been biased by the self-reported exposure, respiratory symptoms and symptoms without frequencies. Third, owing to the small number of participants reporting chronic bronchitis and the relevant respiratory symptoms, we did not conduct further subgroup analyses based on the duration of exposure. Although MMEF is not an optimal small airway parameter, it remains the most widely used parameter derived from spirometry and could indicate small airway obstruction in most large-scale population-based studies (39,40). However, the objective assessment of occupational exposure and the development of job exposure matrix are neither practical nor feasible in such a larger scale study like ours. Although we have observed associations between occupational exposure to gas/vapor/fume or VGDF and the decreased FEV1/FVC, the clinical significance should also be considered. Despite the aforementioned limitations, our study findings remained mostly robust because the estimated odds of chronic bronchitis have taken into account five stepwise models and has been subject to sensitivity analysis that included two methods of PS to determine the validity. The association of all models were not modified materially compared with the overall analysis.
Conclusions
We have now provided the evidence regarding the association of occupational exposure to VGDF and chronic bronchitis and the individual respiratory symptoms. Occupational exposure to VGDF is associated with a trend of decreased lung function in the general population. These findings highlight the role of VGDF in driving the pathogenesis and clinical presentation of COPD. Avoidance of these exposures is highly relevant for occupational prevention or control among the general population.
Acknowledgments
We thank Prof. Li-Wen Fang and Dr. He-Ling Bao (National Center for Chronic and Non-Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention) for their organization and instruction of the program. We thank the working staff from the local centers for disease control and prevention, health stations and community clinics for the program organization and implementation, epidemiological survey and lung function test.
Funding: The study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-646/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-646/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-646/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-646/coif). X.Y.Z., Y.J.X., Y.W., N.X., C.L., Z.X.H., X.M.T., R.L.M., W.J.G., L.F.L. report funding from the Guangdong Provincial Medical Science and Technology Research Funding (No. C2021083); and the Science and Technology Foundation of Guangdong Province (No. 2023A1515012328). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the ethics review committee of the National Center for Chronic and Non-Communicable Disease Control and Prevention, China Center for Disease Control and Prevention in 2014 (approval No. 201410) and the ethics review committee of the Guangdong Provincial Center for Disease Control and Prevention in 2019 (approval No. 201901). All participants had provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1545-602. [Crossref] [PubMed]
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1459-544. [Crossref] [PubMed]
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med 2017;195:557-82. [Crossref] [PubMed]
- Izquierdo-Alonso JL, Rodriguez-Gonzálezmoro JM, de Lucas-Ramos P, et al. Prevalence and characteristics of three clinical phenotypes of chronic obstructive pulmonary disease (COPD). Respir Med 2013;107:724-31. [Crossref] [PubMed]
- Kim V, Han MK, Vance GB, et al. The chronic bronchitic phenotype of COPD: an analysis of the COPDGene Study. Chest 2011;140:626-33. [Crossref] [PubMed]
- Woodruff PG, Barr RG, Bleecker E, et al. Clinical Significance of Symptoms in Smokers with Preserved Pulmonary Function. N Engl J Med 2016;374:1811-21. [Crossref] [PubMed]
- de Marco R, Accordini S, Cerveri I, et al. Incidence of chronic obstructive pulmonary disease in a cohort of young adults according to the presence of chronic cough and phlegm. Am J Respir Crit Care Med 2007;175:32-9. [Crossref] [PubMed]
- Holm M, Kim JL, Lillienberg L, et al. Incidence and prevalence of chronic bronchitis: impact of smoking and welding. The RHINE study. Int J Tuberc Lung Dis 2012;16:553-7. [Crossref] [PubMed]
- Sunyer J, Zock JP, Kromhout H, et al. Lung function decline, chronic bronchitis, and occupational exposures in young adults. Am J Respir Crit Care Med 2005;172:1139-45. [Crossref] [PubMed]
- Lytras T, Kogevinas M, Kromhout H, et al. Occupational exposures and incidence of chronic bronchitis and related symptoms over two decades: the European Community Respiratory Health Survey. Occup Environ Med 2019;76:222-9. [Crossref] [PubMed]
- LeVan TD, Koh WP, Lee HP, et al. Vapor, dust, and smoke exposure in relation to adult-onset asthma and chronic respiratory symptoms: the Singapore Chinese Health Study. Am J Epidemiol 2006;163:1118-28. [Crossref] [PubMed]
- Harber P, Tashkin DP, Simmons M, et al. Effect of occupational exposures on decline of lung function in early chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;176:994-1000. [Crossref] [PubMed]
- Krzyzanowski M, Jedrychowski W, Wysocki M. Factors associated with the change in ventilatory function and the development of chronic obstructive pulmonary disease in a 13-year follow-up of the Cracow Study. Risk of chronic obstructive pulmonary disease. Am Rev Respir Dis 1986;134:1011-9. [Crossref] [PubMed]
- Lytras T, Kogevinas M, Kromhout H, et al. Occupational exposures and 20-year incidence of COPD: the European Community Respiratory Health Survey. Thorax 2018;73:1008-15. [Crossref] [PubMed]
- Alif SM, Dharmage SC, Benke G, et al. Occupational exposure to pesticides are associated with fixed airflow obstruction in middle-age. Thorax 2017;72:990-7. [Crossref] [PubMed]
- Lindberg A, Jonsson AC, Rönmark E, et al. Ten-year cumulative incidence of COPD and risk factors for incident disease in a symptomatic cohort. Chest 2005;127:1544-52. [Crossref] [PubMed]
- Jaakkola JJ, Piipari R, Jaakkola MS. Occupation and asthma: a population-based incident case-control study. Am J Epidemiol 2003;158:981-7. [Crossref] [PubMed]
- Zock JP, Cavallé N, Kromhout H, et al. Evaluation of specific occupational asthma risks in a community-based study with special reference to single and multiple exposures. J Expo Anal Environ Epidemiol 2004;14:397-403. [Crossref] [PubMed]
- Petsonk EL, Daniloff EM, Mannino DM, et al. Airway responsiveness and job selection: a study in coal miners and non-mining controls. Occup Environ Med 1995;52:745-9. [Crossref] [PubMed]
- Eisen EA, Holcroft CA, Greaves IA, et al. A strategy to reduce healthy worker effect in a cross-sectional study of asthma and metalworking fluids. Am J Ind Med 1997;31:671-7. [Crossref] [PubMed]
- Caillaud D, Lemoigne F, Carré P, et al. Association between occupational exposure and the clinical characteristics of COPD. BMC Public Health 2012;12:302. [Crossref] [PubMed]
- Zheng XY, Li ZL, Li C, et al. Effects of cigarette smoking and biomass fuel on lung function and respiratory symptoms in middle-aged adults and the elderly in Guangdong province, China: A cross-sectional study. Indoor Air 2020;30:860-71. [Crossref] [PubMed]
- Standardization of Spirometry. 1994 Update. American Thoracic Society. Am J Respir Crit Care Med 1995;152:1107-36. [Crossref] [PubMed]
- Ferris BG. Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis 1978;118:1-120.
- Lahousse L, Seys LJM, Joos GF, et al. Epidemiology and impact of chronic bronchitis in chronic obstructive pulmonary disease. Eur Respir J 2017;50:1602470. [Crossref] [PubMed]
- Blanc PD, Torén K. Occupation in chronic obstructive pulmonary disease and chronic bronchitis: an update. Int J Tuberc Lung Dis 2007;11:251-7.
- James AL, Palmer LJ, Kicic E, et al. Decline in lung function in the Busselton Health Study: the effects of asthma and cigarette smoking. Am J Respir Crit Care Med 2005;171:109-14. [Crossref] [PubMed]
- Irsigler GB, Visser PJ, Spangenberg PA. Asthma and chemical bronchitis in vanadium plant workers. Am J Ind Med 1999;35:366-74. [Crossref] [PubMed]
- Nemery B. Metal toxicity and the respiratory tract. Eur Respir J 1990;3:202-19.
- Yu D, Walters DM, Zhu L, et al. Vanadium pentoxide (V(2)O(5)) induced mucin production by airway epithelium. Am J Physiol Lung Cell Mol Physiol 2011;301:L31-9. [Crossref] [PubMed]
- Anyfantis ID, Rachiotis G, Hadjichristodoulou C, et al. Respiratory Symptoms and Lung Function among Greek Cotton Industry Workers: A Cross-Sectional Study. Int J Occup Environ Med 2017;8:32-8. [Crossref] [PubMed]
- Senthilselvan A, Chénard L, Ulmer K, et al. Excess respiratory symptoms in full-time male and female workers in large-scale swine operations. Chest 2007;131:1197-204. [Crossref] [PubMed]
- Christiani DC, Wang XR, Pan LD, et al. Longitudinal changes in pulmonary function and respiratory symptoms in cotton textile workers. A 15-yr follow-up study. Am J Respir Crit Care Med 2001;163:847-53. [Crossref] [PubMed]
- Matheson MC, Benke G, Raven J, et al. Biological dust exposure in the workplace is a risk factor for chronic obstructive pulmonary disease. Thorax 2005;60:645-51. [Crossref] [PubMed]
- Rodríguez E, Ferrer J, Zock JP, et al. Lifetime occupational exposure to dusts, gases and fumes is associated with bronchitis symptoms and higher diffusion capacity in COPD patients. PLoS One 2014;9:e88426. [Crossref] [PubMed]
- Zock JP, Sunyer J, Kogevinas M, et al. Occupation, chronic bronchitis, and lung function in young adults. An international study. Am J Respir Crit Care Med 2001;163:1572-7. [Crossref] [PubMed]
- Torén K, Vikgren J, Olin AC, et al. Occupational exposure to vapor, gas, dust, or fumes and chronic airflow limitation, COPD, and emphysema: the Swedish CArdioPulmonary BioImage Study (SCAPIS pilot). Int J Chron Obstruct Pulmon Dis 2017;12:3407-13. [Crossref] [PubMed]
- de Jong K, Boezen HM, Kromhout H, et al. Association of occupational pesticide exposure with accelerated longitudinal decline in lung function. Am J Epidemiol 2014;179:1323-30. [Crossref] [PubMed]
- Niu Y, Yang T, Gu X, et al. Long-Term Ozone Exposure and Small Airway Dysfunction: The China Pulmonary Health (CPH) Study. Am J Respir Crit Care Med 2022;205:450-8. [Crossref] [PubMed]
- Xiao D, Chen Z, Wu S, et al. Prevalence and risk factors of small airway dysfunction, and association with smoking, in China: findings from a national cross-sectional study. Lancet Respir Med 2020;8:1081-93. [Crossref] [PubMed]
- Oppenheimer BW, Goldring RM, Herberg ME, et al. Distal airway function in symptomatic subjects with normal spirometry following World Trade Center dust exposure. Chest 2007;132:1275-82. [Crossref] [PubMed]


