
Histopathologic fate of resected pulmonary pure ground glass nodule: a systematic review and meta-analysis
Highlight box
Key findings
• The proportion of invasive adenocarcinoma (IA) among pure ground glass nodules (GGNs) is quite significant (27%) and half of them are non-lepidic predominant adenocarcinoma.
What is known and what is new?
• Pure GGN has been regarded as pre-invasive or lepidic-predominant adenocarcinoma.
• This study suggests different histologic outcomes even among pure GGN.
What is the implication, and what should change now?
• As there are significant portion of IA among pure GGN, the role of surgery for pure GGN still exists and further study aiming to find invasive lesions is necessary.
Introduction
Screening programs with high-resolution computed tomography have been introduced to lower the global burden of lung cancer-related mortality, demonstrating promising results (1-3). The advancement of screening programs has led to assessing lung cancer prognoses according to their radiological characteristics. Specifically, ground glass nodules (GGNs), which are non-specific findings with pulmonary lesions without obscuring vascular marking, represent an important issue among clinicians regarding diagnosis and management. As most GGNs are related to inflammation, respiratory infection, or other benign causes, the differentiation of malignant lesions among GGN is crucial. Several screening trials, including NELSON, revealed that a significant portion of GGN disappeared during follow-up (2). However, in many studies, a persistent pure GGN has been found to be malignant (4-6).
The 8th TNM classification categorizes GGN as indolent or lepidic predominant adenocarcinoma (7). However, there have been contradictory reports about the histopathologic diagnosis of pure GGN. Notably, subtypes other than the lepidic predominant type are frequently observed (5,8,9). As there are no comprehensive pathological reports relating to resected pure GGN, the optimal surgical candidate for pure GGN remains uncertain. If there are significant portion of invasive adenocarcinoma (IA) among pure GGN, it would be difficult to monitor those lesions without treatment. Additionally, minimally invasive surgical technique has also evolved and it has become more feasible to resect relatively early-stage lung cancer preserving lung parenchyme. Therefore, this study aimed to systematically review the pathological features of pure GGN to suggest proper surgical treatment indications. This study would give a generalized review of pathologic results so that clinicians to understand pathologic variability in pure GGN. We present this article in accordance with the PRISMA reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1089/rc).
Methods
This study was registered with PROSPERO (CRD42021273975).
Search strategy and study selection
We searched MEDLINE, PubMed, EMBASE, and Scopus databases until June 14, 2022. The search terms are listed in Table S1. Two authors (W.W. and V.K.) independently reviewed the titles and abstracts, and disagreements were resolved by discussion with a third author (S.L.). The full literature search strategy and selection process are shown in Figure 1. We included studies wherein pathological diagnoses of pure GGN were reported. The exclusion criteria were as follows: (I) studies on mixed GGN and (II) studies without pathologic reports of pure GGN. All studies were limited to those published in the English language and involving humans. Abstracts, case reports, conference presentations, editorials, and reviews were also excluded.
Data extraction
The primary outcomes of interest were the proportion of IA, minimally invasive adenocarcinoma (MIA), atypical adenomatous hyperplasia (AAH), and adenocarcinoma in situ (AIS). The secondary outcomes included histologic patterns among patients with IA and long-term clinical outcomes, such as overall survival and recurrence-free survival. Other extracted data included patient demographics, radiologic characteristics and protocol, number of participants, and extent of surgery.
Statistical analysis
To estimate the proportion of each pathologic stage, we performed a meta-analysis to estimate the summary effects with a proportion of each variable and 95% confidence interval (CI), using random-effect models since there was significant heterogeneity among the included studies, with I2>50%. Publication bias was not assessed because the proportion of meta-analyses was not comparable owing to the lack of a control arm. Statistical significance was defined as a two-sided P value <0.05. Statistical analyses were performed using R version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria) and Review Manager (RevMan) software version 5.2.3 (The Nordic Cochrane Centre, Copenhagen, Denmark).
Results
Following a systematic search, 24 studies were included in this analysis. The selection process is described in Figure 1 (4-6,8-28). The study design and eligibility criteria of each article are demonstrated in Table S2. Fifteen articles applied the size criteria (4-6,11-13,18-25,27), while four studies (11,18,21,25) mentioned the persistence of pure GGN. Patient characteristics, including radiologic findings, surgical strategies, and clinical outcomes, are shown in Table 1. Male patients accounted for 27.5–63% of the patients, and 10 studies with clinical outcomes reported nearly no recurrence or death during the follow-up other than one study.
Table 1
| Author | Year | Age, years | Demographics | Radiologic variables | Surgical extent | Prognosis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Never smoker | Size, mm | Hounsfield unit | Lobectomy | Sublobar resection | Follow up periods, months | Clinical outcome | ||||||
| Zhu (20) | 2022 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |||
| Sun (13) | 2022 | Mean 61.2 (SD 6.9) |
35/69 | 55/69 | Mean 34 (SD 4) | Mean −550.6 (SD 77.0) |
66/69 | 3/69 | Median 70.3 (range, 60.1–137.4) |
No recurrence or metastasis | |||
| Fu (26) | 2021 | N/A | 119/432 | 364/432 | N/A | N/A | 120/432 | 312/432 | Median 51.6 | 5-year RFS 100%, 5-year 99.5% | |||
| Wang (28) | 2021 | Median 59 (IQR, 52–64) |
103/273 | 229/273 | Median 19 (IQR, 15–24) |
Mean −511 (SD 104.5) |
185/273 | 88/273 | Median 68 (IQR, 60–84) |
5-year RFS 100% | |||
| Sun (10) | 2020 | Mean 56.38 (SD 10.69) |
28/102 | 95/102 | N/A | N/A | N/A | N/A | Median 30.8 | No recurrence or metastasis | |||
| Li (15) | 2020 | Mean 55 (SD 9.99; range, 26–83) | 35/90 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |||
| Chen (27) | 2019 | Median 49 (range, 28–72) |
21/59 | N/A | Mean 7.8 (range, 4–18) |
N/A | 3/59 | 56/59 | N/A | N/A | |||
| Lee (4) | 2019 | Median 59.5 (range, 34–77) |
11/36 | 32/36 | 8.5 (range, 4–19) |
Median −614 (range, −770 to 442) |
7/44 | 37/44 | N/A | N/A | |||
| Mao (9) | 2019 | Median 58 (range, 39–78) |
46/109 | N/A | N/A | N/A | 109/109 | N/A | N/A | N/A | |||
| Wang (17) | 2019 | Mean 36.52 (SD 5.07) |
30/91 | 64/91 | Mean 8.65 (SD 2.34) |
N/A | 3/91 | 72/91 | N/A | N/A | |||
| Ye (24) | 2018 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |||
| Moon (16) | 2018 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |||
| Li (14) | 2018 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |||
| Sawada (19) | 2009 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Median 45.0 (range 1.6–95) |
No recurrence or death | |||
| Yamaguchi (22) | 2015 | N/A | N/A | N/A | N/A | N/A | 17/33 | 16/33 | Median 30.4 (range 4.9-102.5) |
No recurrence or metastasis | |||
| Ichinose (6) | 2014 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |||
| Lim (11) | 2013 | Median 59 (range, 43–71) |
20/46 | 32/46 | Mean 16.6 (SD 5.5; range, 10.1–30) |
N/A | 27/46 | 19/46 | Median 51.5 (range 36-98) |
No recurrence or metastasis | |||
| Cho (23) | 2013 | Mean 57.9 (range, 31–80) |
29/46 | 22/46 | Mean 9.0 (range, 6–18) |
N/A | 23/46 | 23/46 | N/A | N/A | |||
| Eguchi (8) | 2014 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | No recurrence or metastasis | |||
| Liang (25) | 2015 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |||
| Kakinuma (18) | 2016 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |||
| Fournel (12) | 2017 | N/A | N/A | N/A | N/A | N/A | 6/27 | 21/27 | N/A | N/A | |||
| Zha (5) | 2016 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | No recurrence | |||
| Kitami (21) | 2016 | Median 64 (range, 39–83) |
32/72 | N/A | Mean 12.9 (SD 6.1) | Mean −569 (SD 126) |
31/78 | 47/78 | Median 46 | Three cases with solid predominant had recurrence | |||
Sublobar resection includes wedge resection and segmentectomy. N/A, not available; SD, standard deviation; RFS, recurrence-free survival; IQR, interquartile range.
The histopathological outcomes of the included studies are shown in Table 2. Among these, 18 studies specifically described the proportion of IA among resected cases of pure GGN. The pooled proportion of IA was 29% in the overall estimation and 27% (95% CI: 18–37%, I2=95%) in the meta-analysis (Figure 2). The pooled proportion of non-lepidic predominant IA was 37% by overall estimation and 50% (95% CI: 35–65%, I2=91%) in the meta-analysis (Figure 3). The proportions of other types (MIA, 24%; AIS, 36%; AAH, 11%) are described in Table 3 (Figures S1-S3).
Table 2
| Author | Year | Pathologic criteria | AAH | AIS | MIA | IA | Lepidic predominant IA | Acinar predominant IA | Papillary predominant IA | Micropapillary or solid predominant IA |
|---|---|---|---|---|---|---|---|---|---|---|
| Zhu (20) | 2022 | WHO 2021 | AAH or AIS 116/653 | MIA or IA 537/653 | N/A | N/A | N/A | N/A | ||
| Sun (13) | 2022 | IASLC/ATS/ERS 2011 | N/A | 8/69 | 5/69 | 56/69 | 35/56 | 10/56 | 11/56 | N/A |
| Fu (26) | 2021 | IASLC/ATS/ERS 2011 | N/A | 118/432 | 213/432 | 101/432 | 64/101 | 31/101 | 0/101 | 6/101 |
| Wang (28) | 2021 | IASLC/ATS/ERS 2011 | N/A | N/A | N/A | 273/273 | 239/273 | 13/273 | 21/273 | 0/273 |
| Sun (10) | 2020 | IASLC/ATS/ERS 2011 | N/A | N/A | N/A | 102/102 | 28/102 | Acinar or papillary 74/102 | 0/102 | |
| Li (15) | 2020 | IASLC/ATS/ERS 2011 | N/A | 20/90 | 22/90 | 48/90 | N/A | N/A | N/A | N/A |
| Chen (27) | 2019 | WHO 2015 | 25/59 | 32/59 | 2/59 | 0/59 | N/A | N/A | N/A | N/A |
| Lee (4) | 2019 | WHO 2015 | 1/44 | 18/44 | 15/44 | 10/44 | 2.0/10 | 7.0/10 | 1.0/10 | 0/10 |
| Mao (9) | 2019 | IASLC/ATS/ERS 2011 | N/A | N/A | N/A | 109 | 63/109 | 28/109 | 18/109 | 0/109 |
| Wang (17) | 2019 | IASLC/ATS/ERS 2011 | 8/91 | 16/91 | 42/91 | 13/91 | N/A | N/A | N/A | N/A |
| Ye (24) | 2018 | IASLC/ATS/ERS 2011 | N/A | AIS or MIA 475/534 | 59/534 | N/A | N/A | N/A | N/A | |
| Moon (16) | 2018 | WHO 2015 | N/A | 37/106 | 60/106 | 36/106 | N/A | N/A | N/A | N/A |
| Li (14) | 2018 | IASLC/ATS/ERS 2011 | N/A | AIS or MIA 90/167 | 77/167 | N/A | N/A | N/A | N/A | |
| Sawada (19) | 2009 | WHO 2004 | N/A | AIS or MIA 53/63 | 10/63 | 0/10 | 1.0/10 | 9.0/10 | N/A | |
| Yamaguchi (22) | 2015 | IASLC/ATS/ERS 2011 | 3/47 | 29/47 | 4/47 | 8/47 | N/A | N/A | N/A | N/A |
| Ichinose (6) | 2014 | IASLC/ATS/ERS 2011 | 6/114 | 70/114 | 16/114 | 13/114 | 1.0/13 | 2.0/13 | 10.0/13 | N/A |
| Lim (11) | 2013 | IASLC/ATS/ERS 2011 | N/A | 19/46 | 9/46 | 18/46 | 8.0/18 | 8.0/18 | 2.0/18 | N/A |
| Cho (23) | 2013 | IASLC/ATS/ERS 2011 | 3/46 | 23/46 | 2/46 | 3/46 | N/A | N/A | N/A | N/A |
| Eguchi (8) | 2014 | IASLC/ATS/ERS 2011 | N/A | 5/33 | 15/33 | 12/33 | 5.0/12 | 4.0/12 | 3.0/12 | N/A |
| Liang (25) | 2015 | IASLC/ATS/ERS 2011 | 26/74 | 30/74 | MIA or IA 18/74 | N/A | N/A | N/A | N/A | |
| Kakinuma (18) | 2016 | IASLC/ATS/ERS 2011 | 5/35 | 21/35 | 9/35 | N/A | N/A | N/A | N/A | N/A |
| Fournel (12) | 2017 | IASLC/ATS/ERS 2011 | 0/27 | 8.0/27 | 8.0/27 | 10/27 | 3.0/10 | 2.0/10 | 4.0/10 | N/A |
| Zha (5) | 2016 | IASLC/ATS/ERS 2011 | N/A | 137/553 | 146/553 | 270/553 | 156/270 | 41/270 | 48/270 | 15/270 |
| Kitami (21) | 2016 | IASLC/ATS/ERS 2011 | 10/78 | 30/78 | 19/78 | 18/78 | 14/18 | N/A | N/A | N/A |
AAH, atypical adenomatous hyperplasia; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; IA, invasive adenocarcinoma; WHO, World Health Organization; N/A, not available; IASLC, International Association for the Study of Lung Cancer; ATS, American Thoracic Society; ERS, European Respiratory Society.
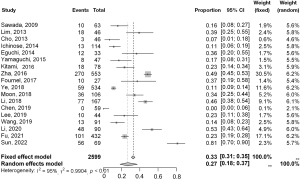

Table 3
| Pathology variables | Number of studies | Total number of patients | Number of events | Proportion (overall), % | Proportion by meta-analysis [95% CI], % | I2 (P value) | τ2 | |
|---|---|---|---|---|---|---|---|---|
| Random effect | Fixed effect | |||||||
| IA | 18 | 2,599 | 762 | 29 | 27 [18–37] | 33 [31–35] | 95% (<0.01) | 0.990 |
| Non-lepidic predominant IA | 13 | 1,002 | 373 | 37 | 50 [35–65] | 38 [35–42] | 91% (<0.01) | 1.012 |
| MIA | 16 | 1,870 | 587 | 31 | 24 [16–34] | 34 [32–37] | 91% (<0.01) | 0.837 |
| AIS | 17 | 1,944 | 621 | 32 | 36 [28–44] | 33 [31–35] | 89% (<0.01) | 0.473 |
| AAH | 10 | 615 | 87 | 14 | 11 [6–21] | 19 [16–23] | 87% (<0.01) | 0.981 |
| Sublobar resection | 11 | 1,198 | 694 | 58 | 60 [38–78] | 58 [55–62] | 95% (<0.01) | 1.969 |
CI, confidence interval; IA, invasive adenocarcinoma; MIA, minimally invasive adenocarcinoma; AIS, adenocarcinoma in situ; AAH, atypical adenomatous hyperplasia.
In terms of the surgical strategy, 12 studies described the extent of surgery, such as lobectomy or sublobar resection (Table 1). The proportion of sublobar resection was 58% by overall estimation and 60% (95% CI: 38–78%, I2=95%) by meta-analysis (Figure 4). Regarding lymph node dissection, only two studies reported the absence of lymph node metastasis (4,26).

Table 4 summarizes the predictive factors related to IA among resected cases of pure GGN. Among nine studies, five suggested the size of pure GGN as a predictive factor for IA (4,11,12,26,27). Hounsfield unit (HU) was found to be significant in two studies (13,15) and other radiologic characteristics (25), including maximal standard uptake value (SUVmax) (6), were also suggested.
Table 4
| Author | Year | Factors | P value | Size effect |
|---|---|---|---|---|
| Sun (13) | 2022 | Mean HU attenuation | 0.0087 | |
| Fu (26) | 2021 | Radiologic size | <0.001 | OR 47.165 (95% CI: 19.279–115.390) |
| Li (15) | 2020 | Mean HU attenuation | 0.019 | N/A |
| Chen (27) | 2019 | Radiologic characteristics: irrelevant margin; bubble lucency; air bronchogram; size | N/A | N/A |
| Lee (4) | 2019 | Radiologic size (10 mm cut off) | 0.005 | OR 24.05 (95% CI: 2.607–221.908) |
| Ichinose (6) | 2014 | Positive on PET (SUVmax >0.8) | <0.001 | OR 16.0 |
| Lim (11) | 2013 | Radiologic size | 0.010 | OR 1.236 |
| Liang (25) | 2015 | Amount of blood vessels | 0.050 | OR 3.13 |
| Fournel (12) | 2017 | Radiologic size (13 mm cut off) | N/A | N/A |
HU, Hounsfield unit; OR, odds ratio; CI, confidence interval; N/A, not available; PET, positron emission tomography; SUVmax, maximal standard uptake value.
Discussion
With the increasing number of GGNs in patients with lung cancer, persistent pure GGN has also received attention from thoracic surgeons. However, management strategies, such as surgery and close monitoring, vary among institutions. Clinicians has regarded pure GGN as indolent lesions, and their pathologic diagnosis has been empirically considered as lepidic predominant lesions (29). However, this study integrated histopathologic outcomes of resected pure GGN and suggested more evidence-based results of pure GGN. Notably, the proportion of IA was relatively high at 27%, and half of the IA in cases of pure GGN was not lepidic predominant. This analysis could guide surgeons to have a more comprehensive understanding and identify a suitable surgical candidate among pure GGN.
The current guidelines classify pure GGN as a lepidic predominant lesion, and the clinical stage of lesions with pure GGN is classified as clinical stage 0 (29). However, based on the analysis in this article, this approach should be reconsidered. From a histopathological perspective, there was a significant proportion of IA in cases of pure GGN, and even acinar- or papillary-dominant lesions were found at a higher frequency than expected. Since lung cancers that presented as pure GGN demonstrated excellent prognosis, it may be appropriate to have other classifications for this relatively indolent radiologic type of lung cancer.
The surgical strategy for pure GGN is not standardized, although an increasing number of studies have been favoring sublobar resection in this group (30,31). The JCOG 0804 trial demonstrated excellent outcomes of sublobar resection among tumors with a maximum diameter of ≤20 mm and consolidation-to-tumor ratio (CTR) ≤0.25 (32). Moreover, the clinical benefit of sublobar resection was achieved in radiologically invasive pulmonary lesions with a CTR >0.5 in the JCOG 0802 trial (33). Therefore, the standard extent of surgery in cases of pure GGN should be sublobar resection. In this study, the proportion of sublobar resection was approximately 60%. Further, since most of the articles included in this meta-analysis were published between 2009 and 2022, our findings represent the current preference for sublobar resection over lobectomy.
In terms of proper lymphadenectomy, there is insufficient evidence for comparing different types of mediastinal lymph node dissection (MLND) procedures. Zhang et al. reported lymph node metastasis among 151 tumors with CTR ≤0.5, and no lymph node involvement was observed regardless of tumor size (34). Moreover, a study comparing hilar lymph node dissection and MLND among part-solid adenocarcinoma described no significant difference in clinical outcomes after propensity score matching (35). Although surgeons should consider patient risk factors and tumor characteristics to choose the appropriate MLND, extensive dissection may be inappropriate for pure GGN. Recently, several ablative treatments that only control the main tumor lesions have exhibited superior clinical outcomes among ground glass opacity-dominant lesions. Indeed, Mikami et al. reported no local or regional recurrence in 126 patients after SBRT (36), and radiofrequency ablation also showed promising results, with a 5-year cancer-specific survival rate of 100% (37). A multidisciplinary discussion would be appropriate to determine the optimal lymphadenectomy or treatment modality for pure GGN.
Overdiagnosis and treatment are important issues in the management of pure GGN (3). Although most cases of pure GGN are considered slow- or non-growing lesions, specific indications for surgery should be discussed based on the pathologic diagnosis. As properly resected AIS/MIA has a favorable prognosis after a 10-year follow-up (38), detecting IA among pure GGN would be a suitable strategy to identify surgical candidates. For this purpose, radiologic characteristics and size were considered relevant factors. Although the optimum size cut-off value was not determined, it is reportedly in the range of 10 to 15 mm (4,12,26). Several radiologic characteristics, such as irregular margin, bubble lucency, air bronchogram (27), and proportion of blood vessels (25) have been suggested; however, their standardization would be necessary to obtain reproducible results in other institutions. With the development of radiomics studies in this field, we expect a further detailed analysis of radiological variables to provide more reliable criteria for IA among pure GGN.
This study had several limitations. First, there has been a shift in determining pathologic diagnosis of early-stage lung cancer. Bronchoalveolar carcinoma, which was defined in the WHO 2004 classification, was later further differentiated into AIS, MIA, and IA based on the 2011 International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) guideline. Though most studies other than one introduced the concepts of new classification, there could be some variations. Another factor is interobserver variability in the pathologic diagnosis of GGN. Depending on patients’ population and the number of experienced pathologists, final diagnosis could vary from institutions (39-41). Though several studies evaluated good correlation between pulmonary pathologists, the discrepancy exists due to complicated lung pathology such as emphysema, fibrosis, or inflammatory tissue. Third, there was a significant bias in patient selection. As the persistence of pure GGN is important to predict its malignancy potential, a period of observation was necessary; however, only four studies mentioned the persistence of lesions. If some studies performed surgical resection without a sufficient observation period, benign or less-invasive malignant lesions would have also been more included. Additionally, different size criteria for inclusion could significantly impact pathological outcomes. Fourth, patient demographics and other surgical variables may be confounding factors. Owing to limited accessibility to patient data, we could not describe the impact of age, sex, smoking history, and surgical strategies. Especially, the smoking status was found as a contributing factor for the growth of GGN (42). These factors should be matched to interpret the fate of pure GGNs. Lastly, there was substantial heterogeneity among the outcomes, which may be related to the study design, number of participants, surgical strategies, or other unidentifiable factors. Therefore, result interpretation should be applied cautiously and further prospective studies with the collaboration from multiple institutions are necessary.
Conclusions
This is the first systematic review and meta-analysis of the histopathological outcomes of pure GGN. The proportion of IA was higher than expected, with different subtypes of IA observed rather than the lepidic predominant type alone. Considering the possible radiologic factors that predict IA among pure GGN, the criteria for resection or follow-up of patients with pure GGN should be investigated.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1089/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1089/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1089/coif). The authors have no conflicts of interest to declare.
Ethical Statements: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yang ZG, Sone S, Takashima S, et al. High-resolution CT analysis of small peripheral lung adenocarcinomas revealed on screening helical CT. AJR Am J Roentgenol 2001;176:1399-407. [Crossref] [PubMed]
- Horeweg N, van der Aalst CM, Thunnissen E, et al. Characteristics of lung cancers detected by computer tomography screening in the randomized NELSON trial. Am J Respir Crit Care Med 2013;187:848-54. [Crossref] [PubMed]
- Silva M, Prokop M, Jacobs C, et al. Long-Term Active Surveillance of Screening Detected Subsolid Nodules is a Safe Strategy to Reduce Overtreatment. J Thorac Oncol 2018;13:1454-63. [Crossref] [PubMed]
- Lee GD, Park CH, Park HS, et al. Lung Adenocarcinoma Invasiveness Risk in Pure Ground-Glass Opacity Lung Nodules Smaller than 2 cm. Thorac Cardiovasc Surg 2019;67:321-8. [Crossref] [PubMed]
- Zha J, Xie D, Xie H, et al. Recognition of "aggressive" behavior in "indolent" ground glass opacity and mixed density lesions. J Thorac Dis 2016;8:1460-8. [Crossref] [PubMed]
- Ichinose J, Kohno T, Fujimori S, et al. Invasiveness and malignant potential of pulmonary lesions presenting as pure ground-glass opacities. Ann Thorac Cardiovasc Surg 2014;20:347-52. [Crossref] [PubMed]
- Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204-23.
- Eguchi T, Kondo R, Kawakami S, et al. Computed tomography attenuation predicts the growth of pure ground-glass nodules. Lung Cancer 2014;84:242-7. [Crossref] [PubMed]
- Mao R, She Y, Zhu E, et al. A Proposal for Restaging of Invasive Lung Adenocarcinoma Manifesting as Pure Ground Glass Opacity. Ann Thorac Surg 2019;107:1523-31. [Crossref] [PubMed]
- Sun F, Huang Y, Yang X, et al. Solid component ratio influences prognosis of GGO-featured IA stage invasive lung adenocarcinoma. Cancer Imaging 2020;20:87. [Crossref] [PubMed]
- Lim HJ, Ahn S, Lee KS, et al. Persistent pure ground-glass opacity lung nodules ≥ 10 mm in diameter at CT scan: histopathologic comparisons and prognostic implications. Chest 2013;144:1291-9. [Crossref] [PubMed]
- Fournel L, Etienne H, Mansuet Lupo A, et al. Correlation between radiological and pathological features of operated ground glass nodules. Eur J Cardiothorac Surg 2017;51:248-54. [Crossref] [PubMed]
- Sun K, Xie H, Zhao J, et al. A clinicopathological study of lung adenocarcinomas with pure ground-glass opacity > 3 cm on high-resolution computed tomography. Eur Radiol 2022;32:174-83. [Crossref] [PubMed]
- Li M, Wang Y, Chen Y, et al. Identification of preoperative prediction factors of tumor subtypes for patients with solitary ground-glass opacity pulmonary nodules. J Cardiothorac Surg 2018;13:9. [Crossref] [PubMed]
- Li S, Yu J, Meng X, et al. The feasibility of non-contrast enhanced plus contrast-enhanced computed tomography in discriminating invasive pure ground-glass opacity from pre-invasive pure ground-glass opacity. J Cardiothorac Surg 2020;15:162. [Crossref] [PubMed]
- Moon Y, Park JK, Lee KY, et al. Predictive factors for invasive adenocarcinoma in patients with clinical non-invasive or minimally invasive lung cancer. J Thorac Dis 2018;10:6010-9. [Crossref] [PubMed]
- Wang J, Ma H, Ni CJ, et al. Clinical characteristics and prognosis of ground-glass opacity nodules in young patients. J Thorac Dis 2019;11:557-63. [Crossref] [PubMed]
- Kakinuma R, Noguchi M, Ashizawa K, et al. Natural History of Pulmonary Subsolid Nodules: A Prospective Multicenter Study. J Thorac Oncol 2016;11:1012-28. [Crossref] [PubMed]
- Sawada S, Komori E, Nogami N, et al. Evaluation of lesions corresponding to ground-glass opacities that were resected after computed tomography follow-up examination. Lung Cancer 2009;65:176-9. [Crossref] [PubMed]
- Zhu M, Yang Z, Wang M, et al. A computerized tomography-based radiomic model for assessing the invasiveness of lung adenocarcinoma manifesting as ground-glass opacity nodules. Respir Res 2022;23:96. [Crossref] [PubMed]
- Kitami A, Sano F, Hayashi S, et al. Correlation between histological invasiveness and the computed tomography value in pure ground-glass nodules. Surg Today 2016;46:593-8. [Crossref] [PubMed]
- Yamaguchi M, Furuya A, Edagawa M, et al. How should we manage small focal pure ground-glass opacity nodules on high-resolution computed tomography? A single institute experience. Surg Oncol 2015;24:258-63. [Crossref] [PubMed]
- Cho S, Yang H, Kim K, et al. Pathology and prognosis of persistent stable pure ground-glass opacity nodules after surgical resection. Ann Thorac Surg 2013;96:1190-5. [Crossref] [PubMed]
- Ye T, Deng L, Xiang J, et al. Predictors of Pathologic Tumor Invasion and Prognosis for Ground Glass Opacity Featured Lung Adenocarcinoma. Ann Thorac Surg 2018;106:1682-90. [Crossref] [PubMed]
- Liang J, Xu XQ, Xu H, et al. Using the CT features to differentiate invasive pulmonary adenocarcinoma from pre-invasive lesion appearing as pure or mixed ground-glass nodules. Br J Radiol 2015;88:20140811. [Crossref] [PubMed]
- Fu F, Zhang Y, Wang S, et al. Computed tomography density is not associated with pathological tumor invasion for pure ground-glass nodules. J Thorac Cardiovasc Surg 2021;162:451-459.e3. [Crossref] [PubMed]
- Chen PH, Chang KM, Tseng WC, et al. Invasiveness and surgical timing evaluation by clinical features of ground-glass opacity nodules in lung cancers. Thorac Cancer 2019;10:2133-41. [Crossref] [PubMed]
- Wang Z, Zhu W, Lu Z, et al. Invasive adenocarcinoma manifesting as pure ground glass nodule with different size: radiological characteristics differ while prognosis remains the same. Transl Cancer Res 2021;10:2755-66. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Fu F, Zhang Y, Wen Z, et al. Distinct Prognostic Factors in Patients with Stage I Non-Small Cell Lung Cancer with Radiologic Part-Solid or Solid Lesions. J Thorac Oncol 2019;14:2133-42. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Prognostic impact of a ground glass opacity component in the clinical T classification of non-small cell lung cancer. J Thorac Cardiovasc Surg 2017;154:2102-2110.e1. [Crossref] [PubMed]
- Mimae T, Tsutani Y, Miyata Y, et al. Solid Tumor Size of 2 cm Divides Outcomes of Patients With Mixed Ground Glass Opacity Lung Tumors. Ann Thorac Surg 2020;109:1530-6. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Zhang Y, Fu F, Wen Z, et al. Segment Location and Ground Glass Opacity Ratio Reliably Predict Node-Negative Status in Lung Cancer. Ann Thorac Surg 2020;109:1061-8. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Significance of Lymphadenectomy in Part-Solid Lung Adenocarcinoma: Propensity Score Matched Analysis. Ann Thorac Surg 2018;106:989-97. [Crossref] [PubMed]
- Mikami N, Takeda A, Hashimoto A, et al. CT Findings and Treatment Outcomes of Ground-Glass Opacity Predominant Lung Cancer After Stereotactic Body Radiotherapy. Clin Lung Cancer 2022;23:428-37. [Crossref] [PubMed]
- Kodama H, Yamakado K, Hasegawa T, et al. Radiofrequency ablation for ground-glass opacity-dominant lung adenocarcinoma. J Vasc Interv Radiol 2014;25:333-9. [Crossref] [PubMed]
- Yotsukura M, Asamura H, Motoi N, et al. Long-Term Prognosis of Patients With Resected Adenocarcinoma In Situ and Minimally Invasive Adenocarcinoma of the Lung. J Thorac Oncol 2021;16:1312-20. [Crossref] [PubMed]
- Thunnissen E, Beasley MB, Borczuk AC, et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma. An international interobserver study. Mod Pathol 2012;25:1574-83. [Crossref] [PubMed]
- Butnor KJ. Controversies and challenges in the histologic subtyping of lung adenocarcinoma. Transl Lung Cancer Res 2020;9:839-46. [Crossref] [PubMed]
- Boland JM, Froemming AT, Wampfler JA, et al. Adenocarcinoma in situ, minimally invasive adenocarcinoma, and invasive pulmonary adenocarcinoma--analysis of interobserver agreement, survival, radiographic characteristics, and gross pathology in 296 nodules. Hum Pathol 2016;51:41-50. [Crossref] [PubMed]
- Kobayashi Y, Sakao Y, Deshpande GA, et al. The association between baseline clinical-radiological characteristics and growth of pulmonary nodules with ground-glass opacity. Lung Cancer 2014;83:61-6. [Crossref] [PubMed]



