
Sternal cleft: new options for reconstruction
Introduction
Sternal cleft (SC), also called sternal fissure or bifid sternum (1), is a rare congenital malformation caused by the failure of the mesenchymal plate fusion process during the eighth week of gestation (1). It is present in 2 out of every 100,000 newborns (2), showing a preference in females. Some associated malformations are facial hemangioma, ectopia cordis, cardiac defects, and pectus excavatum (3).
Diagnosis is generally made after birth due to the paradoxical midline movement, although it can be done prenatally by ultrasonography (4). A computerized tomography scan (CT scan) can not only classify SC, but also be a useful tool for surgical planning.
SC can be classified as complete or incomplete, and can be subdivided into superior or inferior. Surgery is mainly indicated to protect intrathoracic structures from potential injuries, and also for aesthetical concerns. Lannelongue described the first attempt, a limited repair, in 1888, followed by Burton in 1949 (5), and since then numerous techniques have been described.
We first reported our approach in 1998 (5), and also published one of the biggest series in the English literature in 2009 (6). Since then, however, technical difficulties have been seen frequently. Therefore, we are proposing modifications to the technique, as well as suggesting new materials, in response to some of this technical adversity.
Material and methods
This study describes the first cases that demonstrate how to manage the difficulties in building the posterior sternal wall (PSW) when the periosteum of the two hafts of the sternal bars is insufficient.
We describe a total of three female cases, all of whom underwent SC repair between 2018–2019.
Changes in the new approach
- We use a double layer of bovine pericardial patch where the periosteum flaps cannot be primarily sutured, which becomes part of the PSW. Interrupted PDS (polydioxanone) sutures are needed for prosthesis fixation (Figure 1).
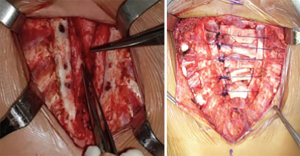 Figure 1 Intra-operative aspect, preparing the posterior sternal wall bilaterally. The anterior periosteum of the sternal bars was vertically incised and medially rotated. Intra-operative aspect, where the chondral grafts have already been subperichondrially resected. They were kept in place by PDS (polydioxanone) sutures in the sternal bars.
Figure 1 Intra-operative aspect, preparing the posterior sternal wall bilaterally. The anterior periosteum of the sternal bars was vertically incised and medially rotated. Intra-operative aspect, where the chondral grafts have already been subperichondrially resected. They were kept in place by PDS (polydioxanone) sutures in the sternal bars. - Three or four PDS number 1 interrupted stitches are then performed to bring the sternal bars closer and maintain the chondral grafts middle line in position (Figure 1).
- A single layer of bovine pericardial patch should be considered when the pectoralis major cannot be brought together. It must be positioned between the edges to improve the tension-free closure and to buttress the suture line (Figure 2).
- The suction drains are placed above and below the muscle plane to avoid seromas and local complications (Figure 2).
Results
The mean age was 4.6 years, range of 4–6 years (Table 1), and the mean duration of hospital stay was 6 days, range of 5–7 days. We did not face any intraoperative complications, nor any other complications during the postoperative period in the hospital. Two of the three cases stayed in intensive care unit (ICU) during the first post-operative day for pain management and monitoring. Suction drains were removed before hospital discharge.
Table 1
| Sex | Age, years | SC classification | Comorbidities |
|---|---|---|---|
| Female | Six | Incomplete superior | None |
| Female | Four | Complete | Interatrial communication, closed spontaneously |
| Female | Four | Complete | Facial hemangioma |
SC, sternal cleft.
The follow-up period varied from 12 to 21 months at an outpatient clinic. The only complication observed during this period was a seroma in the first case, requiring aspiration twice (10 and 5 cc) in the first 15 postoperative days.
All patients were examined in the outpatient clinic less than 15 days after the surgery, 45 days after the surgery, and at least every 3 months up to the present time. The anterior chest wall was considered stable, without the paradoxical movement seen previously. We highlight that the superior portion of the new sternum has the appearance of the manubrium.
During the last physical exam, it was possible to feel the beginning of the formation of a new sternum body, as well as cartilage growth in the places where the chondral grafts had been removed. The pectoralis major was also in place and working properly.
New image findings
The CT scan findings showed a good aspect of PSW, reaffirming the successful outcomes using biocompatible materials.
Discussion
Most papers advocate that surgery for SC is performed in the newborn because of the maximal flexibility of the thoracic cage (4) and minimal compression of underlying mediastinal structures. This is the ideal scenario, even though it is not accomplished in all cases. In developing countries in particular, as is the case in our series, most patients are referred to us as a child or adolescent. We prefer to close the defect utilizing mainly autologous or absorbable tissues and materials. From this point of view, we provide an easy, feasible and safe solution to challenges that a surgeon performing SC closure may face during the procedure. Good outcomes were observed, with a low complication rate. The CT scans showed an acceptable post-operative status (neosternum aspect) (Figure 3), and it was cosmetically effective. At the present time, all the patients are doing well, and no paradoxical movement has been observed, due to the rigidity and strength of the technique.

Several techniques have been described for SC treatment, also including cardiac repair (7,8). Zamfir et al. (3) described one of the primary closures in newborns after testing increased intra-thoracic (9) pressure for 10 min. Jabbad et al. (10) published maneuvers that saw the left pleura opened wide, slowly bringing the sternal bars together, making it possible to use titanium plates and screws. Torre et al. (2) cited other alternatives to primary closure, such as partial or total thymectomy (11) and clavicle dislocation. De Campos et al. (6) reported the use of polypropylene mesh in two cases, as the primary method of bringing muscles together was not possible. Due to the age of the patients, we could not count on having a flexible chest. On the other hand, a bovine pericardium patch makes the closure possible, thus avoiding more invasive procedures and the use of non-absorbable prostheses.
Alternative synthetic materials biologic mesh grafts (12), such as VitaGraft™ (DMC Equipamentos, São Carlos, Brazil), can be considered. VitaGraft™ is an osteoinductor and osteoconductor used for bone regeneration. It is a biocompatible, absorbable, non-cytotoxic, non-immunogenic, and non-pyrogenic synthetic material. It consists of a nanometric ceramic of tricalcium phosphate in the β phase (β-TCP) and copolymer polylactic glycolic acid. We have already performed a repair of SC on a 5-year-old patient by using VitaGraft™. The surgical procedure was performed to treat an incomplete SC that was 5 cm × 2 cm in size. All the steps, such as a vertical incision in the periosteum of sternal bars, medial rotation, PSW construction, and chondrectomy were the same. The only difference was the VitaGraft™ placement between the sternal bars (Figure 4). The patient had an uneventful recovery and follow-up. The CT scan showed good radiological results (Figure 5).


Conclusions
The rigidity of the chest wall, the patient’s age and the lack of new space to accommodate the intrathoracic organs must be considered, particularly in large defects, where it is almost impossible to bring the sternal bars together (13). The heart is the organ that is least tolerant to reduced space inside the thoracic cage. Therefore, the enlargement of the thoracic cage diameter is the main reason for our decision to use PSW. We build the PSW with periosteal flaps raised from the sternal bars, together with the chondral grafts, adding a small patch of bovine pericardium when necessary. In other words, we prepare a natural bed for new bone formation. Even though the chondral grafts will be absorbed, it will provide a new sternum to protect the mediastinal structures. Similar to de Campos et al. (6), we did not encounter acquired Jeune’s syndrome as a consequence of chondral graft removal during follow-up.
One of the biggest limitations of our study, which is shared by much of the chest wall literature, is that it is based on case series studies, in response to low prevalence. SC has a reported incidence of <1% (14,15), so in this referred period, only three cases were eligible for surgery in our thoracic surgery reference center. Forwarding the patients to reference centers in all cases might be the correct strategy to deal with this rare disease. It allows the standardization of the procedure and improves outcomes, and makes it easier to publish the results. There should be a long follow-up of patients while they are growing, with a greater number of patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Erik de Loos, José Ribas de Campos and Jean Daemen) for the series “Chest Wall Resections and Reconstructions” published in Journal of Thoracic Disease. The article has undergone external peer review.
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-645/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-645/coif). The special series “Chest Wall Resections and Reconstructions” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients or their legal guardians for the publication of this article and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bostanci K, Stamenovic D. Repair of Sternal Cleft in Children. Curr Surg Rep 2018;6:1-5.
- Torre M, Rapuzzi G, Carlucci M, et al. Phenotypic spectrum and management of sternal cleft: literature review and presentation of a new series. Eur J Cardiothorac Surg 2012;41:4-9. [Crossref] [PubMed]
- Zamfir C, Zamfirescu A, Tanase C, et al. Sternal cleft – A rare congenital malformation. J Pediatr Surg Case Rep 2014;2:97-100.
- Yuksel M, Kuru P, Ermerak NO, et al. Intrauterine diagnosed sternal cleft patient and her management. J Vis Surg 2016;2:48. [Crossref] [PubMed]
- de Campos JR, Filomeno LT, Fernandez A, et al. Repair of congenital sternal cleft in infants and adolescents. Ann Thorac Surg 1998;66:1151-4. [Crossref] [PubMed]
- de Campos JR, Das-Neves-Pereira JC, Velhote MC, et al. Twenty seven-year experience with sternal cleft repair. Eur J Cardiothorac Surg 2009;35:539-41. [Crossref] [PubMed]
- Ates MS, Duvan I, Onuk BE, et al. Isolated Sternal Cleft in a Patient With Coronary Artery Disease. World J Pediatr Congenit Heart Surg 2016;7:238-40. [Crossref] [PubMed]
- Kojima A, Okamura T, Shikata F, et al. Staged Repair of Complete Sternal Cleft and Interrupted Aortic Arch Associated With PHACES Syndrome in a Very Low Birth Weight Infant. Int Surg 2016;101:313-7.
- Singh S, Lahoti BK, Garge S, et al. Sternal cleft repair: a report of two cases and review of literature. Afr J Paediatr Surg 2010;7:211-3. [Crossref] [PubMed]
- Jabbad H, Shehata R, Al-Ebrahim K. Successful surgical repair of complete sternal cleft in an adult. Asian Cardiovasc Thorac Ann 2010;18:376-8. [Crossref] [PubMed]
- Saha AK, Sardar SK, Sur A. Congenital Sternal Cleft along with Persistent Left-Sided Superior Vena Cava: A Rare Presentation. Case Rep Pediatr 2013;2013:192478. [Crossref] [PubMed]
- Alshomer F, Aldaghri F, Alohaideb N, et al. Reconstruction of Congenital Sternal Clefts: Surgical Experience and Literature Review. Plast Reconstr Surg Glob Open 2017;5:e1567. [Crossref] [PubMed]
- Kabiri el H. Traibi A, Boulahya A. Complete sternal cleft in an adult: case report. Gen Thorac Cardiovasc Surg 2011;59:587-9. [Crossref] [PubMed]
- Klein T, Kellner M, Boemers TM, et al. Surgical Repair of a Superior Sternal Cleft in an Infant. European J Pediatr Surg Rep 2015;3:64-7. [Crossref] [PubMed]
- Smith AEP, Mani A, Jones A, et al. Surgical Repair of Complete Congenital Sternal Cleft Associated With Pectus Excavatum. Ann Thorac Surg 2020;109:e51-3. [Crossref] [PubMed]




