
Prognostic implication of Vasoactive Inotropic Score in adult patients with cardiogenic shock on veno-arterial extracorporeal membrane oxygenation
Highlight box
Key findings
• Firstly, higher maximum level of Vasoactive Inotropic Score (VIS) within the first 6 hours before extracorporeal membrane oxygenation (ECMO) initiation independently predicted poorer clinical outcomes in patients supported with ECMO for cardiogenic shock (CS). Secondly, VIS exceeding 20 was significantly associated with increased risks of in-hospital mortality and 30-day mortality. Thirdly, when categorized by the cause of CS, a high VIS exhibited good predictive ability in patients with acute myocardial infarction, heart failure, and acute myocarditis.
What is known and what is new?
• The adverse outcomes were associated with excessive use of vasoactive drugs and the vasoactive drug scoring system has emerged as a valuable prognostic tool.
• This study examines the correlation between VIS and patient prognosis in ECMO-supported patients with different etiologies of cardiogenic shock. When categorized by the cause of CS, a high VIS exhibited good predictive ability in patients with acute myocardial infarction, heart failure, and acute myocarditis.
What is the implication, and what should change now?
• This study fills the gap in the current study of the correlation between the VIS score and the prognosis of patients supported by ECMO and additional research is required in the future to explore and improve the practicality of VIS scoring in clinical settings.
Introduction
Cardiogenic shock (CS) is a common terminal stage in the disease progression of critically ill patients with cardiovascular diseases (1). Vasopressors and inotropes are typically employed to enhance tissue perfusion in patients who are unable to maintain hemodynamic stability after receiving fluids (2-4). However, the extensive use of these agents may give rise to significant adverse events, such as arrhythmias and myocardial ischemia, which can cause multiorgan dysfunction and even death (5-7). Hence, vasoactive medications are often included in mortality prediction scores like Sequential Organ Failure Assessment (SOFA) and Vasoactive Inotropic Score (VIS) (8,9). Recently, several studies have demonstrated that higher VIS values are predictive of unfavorable outcomes, including mortality and other complications, in pediatric patients (10,11). Furthermore, higher VIS scores have also been associated with poorer outcomes in adult populations undergoing cardiac surgery (12,13).
However, these studies have several limitations. The causes of shock, treatment strategies, and patient responses to therapeutic interventions can vary significantly among individuals. In cases where patients received treatment with an intra-aortic balloon pump (IABP) or extracorporeal membrane oxygenation (ECMO), the need for vasopressors and inotropes may be reduced. Thus, the objective of this study was to investigate the prognostic implications of VIS in adult patients with CS who underwent veno-arterial ECMO (VA-ECMO) and determine whether the predictive ability of VIS differs among patients with different causes of CS. We present this article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-823/rc).
Methods
Study design
This study is a multicenter retrospective study. The data were collected from the national data platform for ECMO patients established by Beijing Anzhen Hospital, Capital Medical University. Patients who received VA-ECMO support for shock between 2015 and 2021 were selected from this platform. The exclusion criteria for the study were as follows: (I) under 18 years old; (II) non-CS; (III) out-of-hospital cardiac arrest; (IV) without essential clinical data. We collected information on population characteristics, in-hospital management, laboratory data, procedural data, ECMO management and clinical outcomes via web-based case report forms. Finally, patients presenting with CS treated with VA-ECMO were divided into two groups based on the maximum VIS (VISmax) during the first 6 hours before the initiation of ECMO, using the cut-off values established in a previous study (14): 0–20 and >20. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the institutional review board of the Beijing Anzhen Hospital (No. 2021021X). Informed consent for demographic, physiological and hospital-outcome data analyses was waived because this observational study did not modify existing diagnostic or therapeutic strategies.
Definitions and outcomes
We defined shock as follows: (I) systolic blood pressure <90 mmHg for 30 minutes or systolic blood pressure ≥90 mmHg with vasopressors or inotropes support; (II) the presence of tissue hypoperfusion (serum lactate levels ≥2.0 mmol/L) (7). Maximal VIS was connected with the maximal dosing rates of vasoactive and inotropic agents, including dopamine, dobutamine, epinephrine, norepinephrine, vasopressin, and milrinone during the first 6 hours before the initiation of ECMO. VISmax was calculated by the following formula: VIS = dopamine dose (µg/kg/min) + dobutamine dose (µg/kg/min) + 100 × epinephrine dose (µg/kg/min) + 100 × norepinephrine dose (µg/kg/min) +10 × milrinone dose (µg/kg/min) + 10,000 × vasopressin dose (U/kg/min) (9). We define experienced medical units as those that have conducted a total of 50 ECMO cases and continue to perform more than 30 cases annually. The definition of kidney injury is based on the clinical guidelines provided by Kidney Disease: Improving Global Outcomes. The primary outcome in this study was in-hospital mortality. And secondary endpoints were 30-day mortality, length of stay in the intensive care unit (ICU) and hospital, complications related with ECMO and other complications including renal injury, gastrointestinal bleeding, infections, and neurological complications, among others.
Statistical analysis
All the analyses were performed with SPSS 24.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were compared between groups as mean standard deviation or median with interquartile ranges (IQRs). Categorical variables were presented as numbers and relative frequencies. Categorical variables were compared with chi-squared or Fisher’s exact tests, and continuous variables were compared with Student’s t-test or the Mann-Whitney U test. When the missing values do not exceed 10% of the total data, we consider them as minor missing and do not take any additional actions. If there are more missing values but not exceeding 20%, we fill these gaps with the mean value. Cumulative survival after ECMO initiation was analyzed with the Kaplan-Meier method, and the significance level was assessed with a log rank test. Univariable and multivariable regression analyses were performed to estimate the contribution of VIS to in-hospital mortality, and to identify risk factors for prediction of in-hospital mortality. In multivariable models, covariates relevant in univariate analysis based on a P value of less than 0.1. The odds ratio (OR) of each variable is presented with the 95% confidence interval (95% CI). Receiver operating characteristic (ROC) curve analysis was used to assess the predictive accuracy of the VIS. P values less than 0.05 were considered statistically significant.
Results
Populations
A total of 1,742 patients were included in this study (Figure 1). The baseline clinical characteristics of these patients, categorized according to VIS, are presented in Table 1. The mean age of the patients was 53.5±15.3 years, and 1,185 (68%) patients were male. The median maximal VIS values were 8.25 (IQR, 3, 16). There were no significant differences in age, sex, and comorbidities between the two VIS groups. However, a higher proportion of patients in the high VIS group had a history of previous cardiac surgery (6.8% versus 10.1% for VIS ≤20 and >20, P=0.017). Cardiac surgery and acute myocardial infarction were the leading causes of CS, accounting for 33.6% and 31.7% of all cases, respectively. In the high VIS group, patients were more likely to experience shock secondary to cardiac surgery, acute myocardial infarction, and acute myocarditis. Notably, patients in the high VIS group exhibited more severe clinical manifestations, as evidenced by significantly lower systolic blood pressure (71.8 versus 57.9 for VIS ≤20 and >20, P<0.001) and higher blood lactic acid levels (7.6 versus 11.9 for VIS ≤20 and >20, P<0.001). Moreover, patients in the higher VIS group had a higher SOFA score (11.2 versus 15.0 for VIS ≤20 and >20, P<0.001). Some experienced medical units observed a higher prevalence of lower doses of vasoactive agents among patients with CS. Additionally, patients with higher VIS scores more frequently underwent continuous renal replacement therapy (CRRT) (45.5% versus 59.1% for VIS ≤20 and >20, P<0.001), whereas the use of IABP did not show a significant difference.

Table 1
| Parameter | Overall (n=1,742) | VIS ≤20 (N=1,146) | VIS >20 (N=596) | P value |
|---|---|---|---|---|
| Male | 1,185 (68.0) | 792 (69.1) | 393 (65.9) | 0.178 |
| Age, years | 53.5±15.3 | 53.5±15.5 | 54.0±16.1 | 0.991 |
| BMI, kg/m2 | 24.8±3.6 | 25.1±3.6 | 24.6±3.5 | 0.558 |
| Previous cardiac surgery | 138 (7.9) | 78 (6.8) | 60 (10.1) | 0.017 |
| Previous PCI | 247 (14.2) | 160 (14.0) | 87 (14.6) | 0.709 |
| Previous myocardial Infarction | 228 (13.1) | 156 (13.6) | 72 (12.1) | 0.360 |
| Previous heart failure | 265 (15.2) | 162 (14.1) | 103 (17.3) | 0.085 |
| Hypertension | 677 (38.9) | 456 (39.8) | 221 (37.1) | 0.256 |
| Dyslipidemia | 268 (15.4) | 181 (15.8) | 87 (14.6) | 0.518 |
| Diabetes mellitus | 327 (18.8) | 221 (19.3) | 106 (17.8) | 0.444 |
| Cause of cardiogenic shock | ||||
| Cardiac surgery | 586 (33.6) | 349 (30.5) | 237 (39.8) | <0.001 |
| Acute myocardial infarction | 552 (31.7) | 394 (34.4) | 158 (26.5) | 0.001 |
| Heart failure | 106 (6.1) | 62 (5.4) | 44 (7.4) | 0.102 |
| Acute myocarditis | 259 (14.9) | 194 (16.9) | 65 (10.9) | 0.001 |
| Cardiomyopathy | 140 (8.0) | 82 (7.2) | 58 (9.7) | 0.061 |
| Others | 99 (5.7) | 65 (5.7) | 34 (5.7) | 0.978 |
| Systolic blood pressure, mmHg | 67.8±25.8 | 71.8±27.4 | 57.9±23.5 | <0.001 |
| Diastolic blood pressure, mmHg | 41.3±17.1 | 43.8±17.5 | 34.8±15.5 | <0.001 |
| Heart rate, beats/min | 109.0±48.8 | 110.0±43.8 | 104.7±54.5 | 0.220 |
| pH | 7.3±0.2 | 7.3±0.2 | 7.2±0.2 | <0.001 |
| HCO3−, mmol/L | 19.3±7.5 | 19.8±5.2 | 17.4±6.5 | <0.001 |
| PO2, mmHg | 111.6±91.4 | 110.9±79.9 | 113.2±97.9 | 0.667 |
| PCO2, mmHg | 40.9±21.5 | 40.1±25.2 | 43.2±27.0 | 0.054 |
| Lactic acid, mmol/L | 8.9±5.3 | 7.6±3.8 | 11.9±5.5 | <0.001 |
| SOFA | 12.3±3.5 | 11.2±3.0 | 15±3.3 | <0.001 |
| Experienced medical units | 1,262 (72.4) | 864 (75.4) | 398 (66.8) | <0.001 |
| Transfer from other hospital | 248 (14.2) | 169 (14.7) | 79 (13.3) | 0.398 |
| IABP | 641 (36.8) | 413 (36.0) | 228 (38.3) | 0.363 |
| CRRT | 874 (50.2) | 522 (45.5) | 352 (59.1) | <0.001 |
Values are means ± standard deviations or n (%). ECMO, extracorporeal membrane oxygenation; VIS, Vasoactive Inotropic Score; BMI, body mass index; PCI, percutaneous coronary intervention; SOFA, sequential organ failure assessment; IABP, intra-aortic balloon pump; CRRT, continuous renal replacement therapy.
Clinical outcomes
Overall, a total of 882 (50.6%) patients died in the hospital, and the in-hospital mortality rates were significantly higher in those with higher VIS scores (41.4% versus 68.3% for VIS ≤20 and >20, P<0.001), as indicated in Table 2. Similar trends were observed in the 30-day mortality among these patients (40.7% versus 64.9% for VIS ≤20 and >20, P<0.001). The lower VIS group had a higher success rate in weaning (63.1% versus 44.5% for VIS ≤20 and >20, P<0.001), while patients in the higher VIS group were discharged earlier from the ICU (16.7 versus 9.1 days for VIS ≤20 and >20, P<0.001). Concurrently, the high VIS group had a higher likelihood of experiencing kidney injury (22.3% versus 30.2% for VIS ≤20 and >20, P<0.001), as well as bleeding complications (23.4% versus 31.0%, P=0.001). Among the patients, 461 (26.5%) had infectious complications, and the incidence was lower in the high VIS group (28.6% versus 22.3% for VIS ≤20 and >20, P=0.005). Additionally, 96 (5.5%) patients experienced neurological complications, and 117 (6.7%) had limb complications, with no significant difference between the two groups.
Table 2
| Outcomes | Overall (n=1,742) | VIS ≤20 (N=1,146) | VIS >20 (N=596) | P value |
|---|---|---|---|---|
| In-hospital mortality | 882 (50.6) | 475 (41.4) | 407 (68.3) | <0.001 |
| 30-days mortality | 853 (49.0) | 466 (40.7) | 387 (64.9) | <0.001 |
| Successful weaning | 988 (56.7) | 723 (63.1) | 265 (44.5) | <0.001 |
| Length of ECMO, h | 127 [49, 161] | 127 [56, 150] | 126 [63, 166] | 0.951 |
| Length of hospital stay, days | 21 [8, 28] | 22 [10, 27] | 19 [10, 29] | 0.013 |
| Length of ICU stay, days | 17.2 [4, 17.2] | 16.7 [6, 17] | 9.1 [6, 17] | <0.001 |
| Kidney injury | 436 (25.0) | 256 (22.3) | 180 (30.2) | <0.001 |
| Bleeding complications | 453 (26.0) | 268 (23.4) | 185 (31.0) | 0.001 |
| Gastrointestinal tract | 95 (5.5) | 48 (4.2) | 47 (7.9) | 0.001 |
| Surgical site | 99 (5.7) | 48 (4.2) | 51 (8.6) | <0.001 |
| Peripheral cannulation site | 240 (13.8) | 140 (12.2) | 100 (16.8) | 0.009 |
| Hemolysis | 19 (1.1) | 6 (0.5) | 13 (2.2) | 0.002 |
| Cerebral hemorrhage | 34 (2.0) | 21 (1.8) | 13 (2.2) | 0.618 |
| Other sites | 56 (3.2) | 38 (3.3) | 18 (3.0) | 0.740 |
| Neurologic complications | 96 (5.5) | 64 (5.6) | 32 (5.4) | 0.852 |
| Infectious complications | 461 (26.5) | 328 (28.6) | 133 (22.3) | 0.005 |
| Limb complications | 117 (6.7) | 74 (6.5) | 43 (7.2) | 0.287 |
Values are medians [interquartile ranges] or n (%). VIS, Vasoactive Inotropic Score; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit.
Prognostic implication of VIS according to the cause of CS
Univariate regression analysis presented in Table 3 demonstrated that a VIS >20 was a significant risk factor for in-hospital mortality (OR 3.042, 95% CI: 2.47–3.75, P<0.001). In the subsequent multivariable regression analysis, where numerous predictors of in-hospital mortality were considered, VIS >20 emerged as an independent predictor (OR 2.64; 95% CI: 2.10–3.33, P<0.001), after excluding the influence of confounding factors. Additionally, an elevated lactate acid level >8 mmol/L (OR 1.48; 95% CI: 1.19–1.84, P=0.001) and the use of CRRT (OR 1.83; 95% CI: 1.48–2.25, P<0.001) were identified as independent predictors.
Table 3
| Variables | Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Age (per 1 increase) | 1.023 | 1.017–1.030 | <0.001 | 1.025 | 1.017–1.033 | <0.001 | |
| Hypertension | 1.412 | 1.163–1.715 | <0.001 | ||||
| Dyslipidemia | 1.310 | 1.008–1.703 | 0.044 | ||||
| Diabetes mellitus | 1.284 | 1.008–1.636 | 0.043 | ||||
| HCO3− | 0.981 | 0.966–0.996 | 0.014 | ||||
| PO2 | 1.001 | 1.000–1.002 | 0.030 | 1.002 | 1.000–1.003 | 0.008 | |
| PCO2 | 1.009 | 1.003–1.015 | 0.003 | ||||
| Lactate acid >8 mmol/L | 2.260 | 1.865–2.739 | <0.001 | 1.477 | 1.185–1.841 | 0.001 | |
| Experienced medical units | 1.274 | 1.032–1.573 | 0.024 | ||||
| IABP | 1.200 | 0.987–1.459 | 0.067 | ||||
| CRRT | 2.153 | 1.778–2.607 | <0.001 | 1.827 | 1.483–2.250 | <0.001 | |
| VIS >20 | 3.042 | 2.469–3.748 | <0.001 | 2.644 | 2.102–3.327 | <0.001 | |
OR, odds ratio; CI, confidence interval; IABP, intra-aortic balloon pump; CRRT, continuous renal replacement therapy; VIS, Vasoactive Inotropic Score.
According to the causes of CS, there were variations in the predictive ability of VIS, as shown in Table 4. While patients with high VIS in the overall group had a significantly elevated risk of in-hospital mortality, this trend was not observed in patients with cardiomyopathy-related shock and high VIS values. Conversely, high VIS demonstrated good predictive ability in patients with acute myocardial infarction (OR 3.78, 95% CI: 2.37–6.02, P<0.001), heart failure (OR 3.75, 95% CI: 1.39–10.17, P=0.009), and acute myocarditis (OR 3.73, 95% CI: 1.84–7.56, P<0.001). The ROC analysis revealed that VIS, as a predictor of in-hospital mortality, exhibited an area under the curve (AUC) of 0.65 (95% CI: 0.63–0.68), with an optimal cutoff value of 20.1 (Figure 2). Notably, when the ROC curve was assessed based on the causes of CS (Figure 3), VIS exhibited the highest predictive ability for in-hospital mortality in patients with acute myocarditis (AUC 0.70, 95% CI: 0.63–0.78, P<0.001).
Table 4
| Outcomes | In-hospital mortality, n (%) | Unadjusted | Adjusted* | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |||
| Total population (N=1,742) | ||||||
| 0< VIS ≤20 (N=1,146) | 475 (41.4) | Reference | Reference | Reference | Reference | |
| VIS >20 (N=596) | 407 (68.3) | 3.042 (2.469–3.748) | <0.001 | 2.644 (2.102–3.327) | <0.001 | |
| Cardiac surgery (N=586) | ||||||
| 0< VIS ≤20 (N=349) | 174 (49.9) | Reference | Reference | Reference | Reference | |
| VIS >20 (N=237) | 160 (67.5) | 2.090 (1.482–2.946) | <0.001 | 1.787 (1.216–2.626) | 0.003 | |
| Acute myocardial infarction (N=552) | ||||||
| 0< VIS ≤20 (N=394) | 155 (39.3) | Reference | Reference | Reference | Reference | |
| VIS >20 (N=158) | 120 (75.9) | 4.869 (3.209–7.389) | <0.001 | 3.775 (2.368–6.018) | <0.001 | |
| Heart failure (N=106) | ||||||
| 0< VIS ≤20 (N=62) | 23(37.1) | Reference | Reference | Reference | Reference | |
| VIS >20 (N=44) | 29 (65.9) | 3.278 (1.460–7.360) | 0.004 | 3.752 (1.385–10.167) | 0.009 | |
| Acute myocarditis (N=259) | ||||||
| 0< VIS ≤20 (N=194) | 36 (18.6) | Reference | Reference | Reference | Reference | |
| VIS >20 (N=65) | 32 (49.2) | 4.256 (2.321–7.803) | <0.001 | 3.733 (1.844–7.556) | <0.001 | |
| Cardiomyopathy (N=140) | ||||||
| 0< VIS ≤20 (N=82) | 52 (63.4) | Reference | Reference | Reference | Reference | |
| VIS >20 (N=58) | 41 (70.7) | 1.391 (0.676–2.865) | 0.060 | 1.757 (0.717–4.305) | 0.218 | |
*, variables adjusted for included age, hypertension, dyslipidemia, diabetes mellitus, HCO3−, PO2, PCO2, lactic acid >8 mmol/L, experienced medical units, IABP, CRRT, and VIS >20. VIS, Vasoactive Inotropic Score; OR, odds ratio; CI, confidence interval; IABP, intra-aortic balloon pump; CRRT, continuous renal replacement therapy.


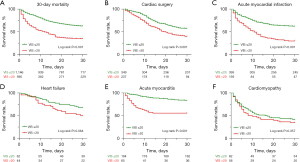
A total of 853 patients (49.0%) died within 30 days after initiation of ECMO. The 30-day mortality rate was significantly higher in the group with high VIS compared to the group with low VIS (40.7% versus 64.9% for VIS ≤20 and >20, P<0.001). The Kaplan-Meier survival curve illustrated in Figure 4A demonstrates a statistically significant difference in the 30-day survival rates between the two groups (Log rank P<0.001). Furthermore, significant disparities in the 30-day survival curves were observed in the high VIS group of patients with cardiac surgery, acute myocardial ischemia, and acute myocarditis (Figure 4B-4D), while no notable differences were observed in patients with heart failure or cardiomyopathy (refer to Figure 4E,4F).
Discussion
In this study, we have the following findings. First, we find higher maximum level of VIS during the first 6 hours before the initiation of ECMO was shown to be an independent predictor for poorer clinical outcomes in patients with CS supported with ECMO. Second, VIS greater than 20 was connected with a significantly higher risk of in-hospital and 30-day mortality regardless of the causes of CS. Third, according to the cause of CS, high VIS demonstrated good predictive ability in patients with acute myocardial infarction, heart failure, and acute myocarditis.
Currently, numerous studies have been conducted on the topic of VIS; however, limited research has been specifically focused on ECMO-supported patients. This study aims to explore this particular aspect. Firstly, by leveraging the benefits of multicenter big data, this study investigates the relationship between VIS and the prognosis of ECMO-supported patients with CS. The inclusion of data from nearly 2,000 patients enhances the alignment of the research results with real-world scenarios and increases the reliability of the conclusions. Secondly, this study examines the correlation between VIS and patient prognosis in ECMO-supported patients with different etiologies of CS. Additionally, the study analyzes the predictive ability of VIS in different patients to determine its suitability for specific patient populations.
This study examined the prognostic differences between high VIS and low VIS patients by using the maximum value of VIS within the first 6 hours of ECMO support as the study object. The primary endpoint was in-hospital mortality. The findings revealed that patients in the high VIS group had higher in-hospital mortality rates and 30-day mortality rates compared to the low VIS group. This can be attributed to the fact that a higher VIS indicates more severe shock prior to ECMO installation, resulting in prolonged periods of inadequate end-organ perfusion and multiple organ dysfunction (15,16). Even with ECMO support, the chances of organ function recovery are lower, leading to relatively higher mortality rates. Moreover, there was a higher incidence of kidney injury and neurological complications, providing further evidence for this relationship. However, it was observed that patients in the high VIS group had shorter ICU and hospital stays compared to those in the low VIS group. This may be because patients in the high VIS group had more severe conditions, making it challenging to save their lives even with ECMO support, resulting in some patients experiencing rapid mortality, consequently reducing the overall treatment duration for the high VIS group. Furthermore, as the duration of patient support increased, there was an elevated incidence of infection complications (17), which explains the relatively lower infection rate among patients in the high VIS group.
The study discovered that a VIS value greater than 20 is an independent risk factor for in-hospital mortality (OR =2.6), and this difference was also statistically significant in the 30-day survival analysis. When categorizing patients based on the underlying causes of shock, it was found that VIS >20 was an independent risk factor for in-hospital mortality in patients with shock due to cardiac surgery, acute myocardial infarction, heart failure, and acute myocarditis. However, in the 30-day survival analysis, only patients with cardiac surgery, acute myocardial infarction, and acute myocarditis exhibited significant differences. This could be attributed to the weaker regulatory effect of vasoactive drugs in patients with chronic heart failure and cardiomyopathy compared to other categories, resulting in less pronounced differentiation between high and low VIS scores. On the other hand, acute myocarditis demonstrated the opposite pattern, indicating its strong predictive ability for in-hospital mortality in ECMO-supported patients.
Similar findings were obtained in Shukla et al.’s (18) study on children’s prognosis, which also selected the VISmax score before ECMO installation. The results revealed that a high VIS score was associated with higher in-hospital mortality and worse brain function, consistent with our own results. Another study by Choi et al. (19) indicated a strong correlation between a high VIS score and adverse outcomes in patients with CS undergoing different treatment methods. Among these methods, the VIS score exhibited the strongest predictive ability in the drug-only treatment group, followed by the IABP group, while the ECMO group had a relatively weaker predictive ability. In that study, the cut-off value for VIS in the ECMO group was 84, significantly higher than the 20 used in our study. This disparity may arise from the fact that patients with lower VIS values tended to receive less invasive drug treatment or IABP, with ECMO only considered when the effectiveness of the first two treatments was unsatisfactory, indicating a more severe condition in patients and higher VIS values
The present study has several limitations. Despite being a multicenter study, it still cannot evade the inherent limitations associated with a retrospective design. As a result, the findings of this study can only offer partial reference value and lack comprehensive guidance for clinical practice. Furthermore, the variability in clinical experiences and treatment strategies among different centers poses a drawback to multicenter studies, potentially introducing biases in the patients’ treatment process influenced by various factors. Moreover, the presence of missing data in this study hampers the analysis of laboratory data. Additionally, this study inevitably involves other potential confounding factors, prompting us to conduct adjusted analyses when evaluating patient outcomes to mitigate these confounders as much as possible.
The present study investigates the prognosis of patients who receive support from VIS and ECMO. However, the limited nature of the retrospective study design and research focus leads to a minimal overall contribution to VIS research. Various unresolved issues persist in VIS research, such as the optimal timing for calculating VIS values, whether before or after ECMO installation. Furthermore, there exist different methods for calculating VIS, with some studies opting for the maximum value within a specific time frame, while others utilize the average value. Addressing these matters necessitates further high-quality research. Moreover, VIS itself represents a dynamic concept. Since its initial introduction by Gaies et al. (9) in 2010, numerous researchers have endeavored to enhance the VIS scoring system, including the Remember (20) score and the Kaddoura et al. (21) study in 2022. Consequently, additional research is required in the future to explore and improve the practicality of VIS scoring in clinical settings.
Conclusions
Firstly, higher maximum level of VIS within the first 6 hours before ECMO initiation independently predicted poorer clinical outcomes in patients supported with ECMO for CS. Secondly, VIS exceeding 20 was significantly associated with increased risks of in-hospital mortality and 30-day mortality. Thirdly, when categorized by the cause of CS, a high VIS exhibited good predictive ability in patients with acute myocardial infarction, heart failure, and acute myocarditis.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-823/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-823/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-823/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-823/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the institutional review board of the Beijing Anzhen Hospital (No. 2021021X). Informed consent for demographic, physiological and hospital-outcome data analyses was waived because this observational study did not modify existing diagnostic or therapeutic strategies.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Samsky MD, Morrow DA, Proudfoot AG, et al. Cardiogenic Shock After Acute Myocardial Infarction: A Review. JAMA 2021;326:1840-50. [Crossref] [PubMed]
- Tehrani BN, Truesdell AG, Psotka MA, et al. A Standardized and Comprehensive Approach to the Management of Cardiogenic Shock. JACC Heart Fail 2020;8:879-91. [Crossref] [PubMed]
- Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med 2021;47:1181-247. [Crossref] [PubMed]
- Russell JA. Vasopressor therapy in critically ill patients with shock. Intensive Care Med 2019;45:1503-17. [Crossref] [PubMed]
- Ammar MA, Ammar AA, Wieruszewski PM, et al. Timing of vasoactive agents and corticosteroid initiation in septic shock. Ann Intensive Care 2022;12:47. [Crossref] [PubMed]
- Annane D, Ouanes-Besbes L, de Backer D, et al. A global perspective on vasoactive agents in shock. Intensive Care Med 2018;44:833-46. [Crossref] [PubMed]
- Burgunder L, Heyrend C, Olson J, et al. Medication and Fluid Management of Pediatric Sepsis and Septic Shock. Paediatr Drugs 2022;24:193-205. [Crossref] [PubMed]
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707-10. [Crossref] [PubMed]
- Gaies MG, Gurney JG, Yen AH, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med 2010;11:234-8. [Crossref] [PubMed]
- McIntosh AM, Tong S, Deakyne SJ, et al. Validation of the Vasoactive-Inotropic Score in Pediatric Sepsis. Pediatr Crit Care Med 2017;18:750-7. [Crossref] [PubMed]
- Kallekkattu D, Rameshkumar R, Chidambaram M, et al. Threshold of Inotropic Score and Vasoactive-Inotropic Score for Predicting Mortality in Pediatric Septic Shock. Indian J Pediatr 2022;89:432-7. [Crossref] [PubMed]
- Koponen T, Karttunen J, Musialowicz T, et al. Vasoactive-inotropic score and the prediction of morbidity and mortality after cardiac surgery. Br J Anaesth 2019;122:428-36. [Crossref] [PubMed]
- Yamazaki Y, Oba K, Matsui Y, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in adults after cardiac surgery with cardiopulmonary bypass. J Anesth 2018;32:167-73. [Crossref] [PubMed]
- Han J, Pinsino A, Sanchez J, et al. Prognostic value of vasoactive-inotropic score following continuous flow left ventricular assist device implantation. J Heart Lung Transplant 2019;38:930-8. [Crossref] [PubMed]
- Davidson J, Tong S, Hancock H, et al. Prospective validation of the vasoactive-inotropic score and correlation to short-term outcomes in neonates and infants after cardiothoracic surgery. Intensive Care Med 2012;38:1184-90. [Crossref] [PubMed]
- Kwon JH, Yoo SY, Kim S, et al. Vasoactive inotropic score as a predictor of long-term mortality in patients after off-pump coronary artery bypass grafting. Sci Rep 2022;12:12863. [Crossref] [PubMed]
- Kühn D, Metz C, Seiler F, et al. Antibiotic therapeutic drug monitoring in intensive care patients treated with different modalities of extracorporeal membrane oxygenation (ECMO) and renal replacement therapy: a prospective, observational single-center study. Crit Care 2020;24:664. [Crossref] [PubMed]
- Shukla I, Hanson SJ, Yan K, et al. Vasoactive-Inotropic Score and Vasoactive-Ventilation-Renal Score as Outcome Predictors for Children on Extracorporeal Membrane Oxygenation. Front Pediatr 2021;9:769932. [Crossref] [PubMed]
- Choi KH, Yang JH, Park TK, et al. Differential Prognostic Implications of Vasoactive Inotropic Score for Patients With Acute Myocardial Infarction Complicated by Cardiogenic Shock According to Use of Mechanical Circulatory Support. Crit Care Med 2021;49:770-80. [Crossref] [PubMed]
- Wang L, Yang F, Wang X, et al. Predicting mortality in patients undergoing VA-ECMO after coronary artery bypass grafting: the REMEMBER score. Crit Care 2019;23:11. [Crossref] [PubMed]
- Kaddoura R, Mohamed Ibrahim MI, Omar A. Levosimendan for VA-ECMO weaning: the silver lining. ESC Heart Fail 2022;9:236-40. [Crossref] [PubMed]


