
Fascial structure of the anterior mediastinum: the surgical figure of the sternopericardial ligament and the role of affixing the thymus to the pericardium
Highlight box
Key findings
• The essence of the sternopericardial ligament (SPL) is not a two-corded ligament but a sagittal layer of fibrous tissues between the pericardium and the sternum. A nodular portion that extends from the pericardium in the SPL fixes the thymus to the pericardium.
What is known and what is new?
• The SPL is often depicted as a two-corded structure bridging in the anterior mediastinum. The structure concerning the affixion of the thymus has not been explained in detail.
• The SPL is a sagittal fibrous layer connecting the endothoracic fascia and the fibrous pericardium. The inferior SPL is just adhesion. A specific strip-like portion in the SPL fixes the thymus to the pericardium. The SPL was detectable in computed tomography.
What is the implication, and what should change now?
• Incorrect classical descriptions regarding the SPL should be corrected.
Introduction
A precise understanding of the fascial anatomy supporting the organs plays a pivotal role in surgical procedures. Although numerous studies (1-5) have explored fascial structures in the neck, the retrosternal space, which connects to the neck, has not received adequate research attention until now.
Within the retrosternal space, Terminología Anatómica (TA) defines a solitary fascial structure known as the sternopericardial ligament (SPL) (6). However, SPL descriptions are sparse in contemporary anatomy textbooks (7). Classical textbooks, on the other hand, have portrayed the SPL as two independent tendon-like structures that bridge the sternum and pericardium: the superior SPL and the inferior SPL (8-14). Although these descriptions persisted, we did not encounter such structures during surgical observations. We previously assumed that the sternotomy might have disrupted the retrosternal connective tissues, rendering them invisible.
Presently, advances in medical technology have enabled us to perform thoracoscopy, a non-destructive method that allows for the precise observation of retrosternal structures, offering a magnified, close-up, and recordable view. However, our extensive experience with endoscopic anterior mediastinal surgery has often observed differences between these conventional depictions and our surgical reality. Thus, this study aimed to investigate the precise figure and clinical role of the SPL in the fascial anatomy of the anterior mediastinum during thoracoscopic mediastinal surgery. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1602/rc).
Methods
The participants were patients who underwent video-assisted thoracoscopic surgery for anterior mediastinal lesions at the National Hospital Organization Saitama Hospital between July 2009 and August 2018 consecutively. Reoperations were excluded. We observed fascial structures in the retrosternal space within the range necessary for intraoperative surgery. Individual procedures were to be performed to the extent necessary and sufficient for treatment and not to perform any additional intervention for research purpose such as exposing the entire anterior mediastinum or only certain structures. A high-definition camera (IMAGE1HD; KARL STORZ GmbH & Co. KG, Tuttlingen, Germany) was used to observe tissues during thoracoscopic surgery. The surgeries were recorded as a movie file.
The observations were the distribution of the connective tissues and their relationship to the surrounding organs, and no quantitative measurement of specific structures was performed. The entire fascial structure was analyzed based on the observational results from all consecutively enrolled patients.
Chest radiography and computed tomography (CT) scan were performed for diagnostic purposes in all patients preoperatively and referred to in our analysis. No quantitative observational items were measured in individual patients, and no statistical methods, such as group comparisons, were employed.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee of National Hospital Organization Saitama Hospital (No. R2016-10) and individual consent for this retrospective analysis was waived.
Statistical analysis
Statistical analysis was not used since this study comprises a comprehensive anatomical analysis conducted as a retrospective study based on surgical observations.
Results
Fifty-five thoracoscopic surgical cases of anterior mediastinal disease were investigated in this study (Table 1). These included 11 extended thymectomies for myasthenia gravis with or without thymomas, 37 total or partial resections of the thymus for thymic tumors, and seven simple extirpations of benign tumors such as pericardial cysts or hemangiomas. All patients underwent preoperative radiography and CT scans.
Table 1
| Characteristic | Values (n=55) |
|---|---|
| Sex | |
| Male | 27 |
| Female | 28 |
| Age (years) | 61.6±15.1 [24–86] |
| Pathological diagnosis† | |
| Thymic tumor | 22 |
| Thymoma | 20 |
| Thymic carcinoma | 2 |
| Thymic cyst | 21 |
| Pericardial cyst | 3 |
| Foregut cyst | 2 |
| Normal thymus | 2 |
| Thymic hyperplasia | 1 |
| Others‡ | 4 |
| Surgical procedure | |
| Extended thymectomy | 11 |
| Total or partial thymectomy | 37 |
| Tumor extirpation | 7 |
| Operation time (min) | 221.3±141.2 [54–625] |
Values are presented as mean ± standard deviation [range] or number. †, pathological diagnosis is based on the surgical specimens and includes eleven myasthenia gravis; three without tumors and eight with thymoma. ‡, including teratoma, hemangioma, sarcoidosis, cyst unknown origin.
Surgical findings
A connective fibrous layer was found running sagittally in the midline of the anterior mediastinum (Figure 1). Hereafter, we refer to this sagittal layer as the sternopericardial fibrous layer (SPFL). The other fibrous layers were around any organ or structure and corresponded to the well-known endothoracic fascia, subpleural fascia, and visceral fascia, including the sheath, adventitia, thymic capsule, and outer layer of the fibrous pericardium (Figure 1B).

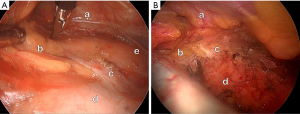
The sagittal layer was bridged between the endothoracic fascia on the back of the sternal body and the frontal face of the pericardium. In front of the thymus near the thymic isthmus, the fibrous tissues of the layer covered the capsule of the thymus and were converted to a lower bifurcation angle made by the thymic glands (hereinafter, the inferior thymic angle). Posteriorly, they gradually concentrated to a small area and formed a sagittal membrane at the narrow thymic gap where the thymic glands were in contact with each other (hereinafter thymic valley). Finally, this layer was fused to the outer fibrous layer of the fibrous pericardium. The layer disappeared near the arterial hilum of the pericardium between the aorta and the pulmonary artery trunk. Near this upper terminal, a small part of the layer was thickened just in front of the pericardium (Figure 1B) and often formed a white nodular strip (Figure 2) or triangular shape (Figure 3). The size was approximately 5–10 mm, based on a thoracoscopic comparison with the size of the endoscopic instrument. The thickened portion was too firm to be bluntly dissected. The portion that migrated to the pericardium without a boundary resembled a pericardial process. Cutting the portion, the pericardial sac was not opened (Figure 3D). Around the nodular portion, the SPFL was dense and firmly fused to the thymic capsule (Figures 2A,2B,3C). Only near this portion, the thymus was firmly fixed on the posterior midline in the mediastinum through the SPFL. The other part of the thymic capsule is loosely fixed to the surrounding tissues and fasciae.

In the caudal area where the thymic glands were apart, the distribution of the fibrous tissues of the SPFL was gradually broader and looser and then ended at the adhesion, where the pericardium contacted the sternum (Figure 4).

The SPFL begins from the endothoracic fascia on the entire sternal body and ends on the fibrous pericardium. The SPFL divided the retrosternal space into two compartments below the inferior thymic angle, and the thymic glands with adipose tissue along the internal mammary artery were placed in each compartment (Figure 1A-1C).
Radiologic findings of SPFL
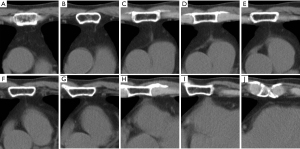
A sagittal line between the sternum and pericardium was observed on the CT images (Figure 5). The line was not a shadow of the pleura or vessels. It began at the back of the sternal body, ran in the posterior direction between the thymic glands, and ended at the pericardium, where it was in contact with the sternum. The line was generally thin, but became thicker near the pericardium (Figure 5C,5D). The pericardium showed a triangular thickness, where the line contacted the pericardium (Figure 5H,5I). The line was almost midline, but tilted slightly to the left, possibly along the cardiac axis (Figure 6).

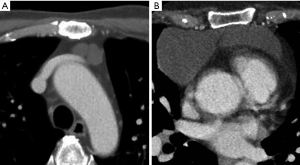
In clinical cases with giant thymic cysts in the lower thymic body, CT showed two cysts and a low-density boundary band (Figure 7). However, it was surgically found that the SPFL divided the large cyst into two parts (Figure 3).
Discussion
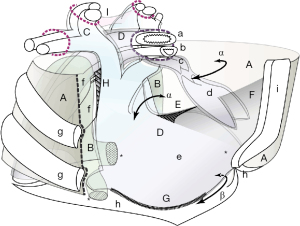
Unlike other fibrous fasciae in the mediastinum, the SPFL is structurally different in that a simple fibrous layer bridges the two organs. The SPFL should correspond to the SPL defined in the TA (6). However, the form differs from the description of the two cord-like tendons in classical textbooks (8,9,14). This study indicated that the SPFL comprised only a fibrous layer but showed a complex appearance between the sternum and pericardium (Figures 1,8). The SPFL broadly arises from the connective tissues beneath the sternal body and should be an extension of the endo-thoracic fascia.
Role of SPFL in thymic fixation
From a surgical viewpoint, the most significant aspect of the SPFL is fixing the thymus to the pericardium at the midline rather than the pericardium. If the thymus rods only on the pericardium, it can freely move laterally in the rich adipose tissue in the anterior mediastinum. Although thymic tumors are heavier than normal fatty thymic glands in many clinical cases, the contralateral thymic gland rarely moves toward the tumor. In addition, large thymomas on the midline do not deviate laterally. In the present case, with a large cyst near the inferior thymic angle, a notch-like lobular sign (Figure 7) was formed on the cyst by an SPFL composed of stiff connective tissue (Figure 3). We suppose that a ‘curvilinear strand extending from the displaced left thymic lobe to the anterosuperior aspect of the pericardial sac’ that Low et al. reported in a neonatal case of spontaneous multiloculated multiseptate pneumomediastinum (15) must correspond to the SPFL in this study. These clinical findings indicate a role for the SPFL in thymic fixation.
Dense portion of SPFL fixing the thymus to the pericardium
Thymus fixation was primarily attributed to the dense portion of the SPFL located posterior to the inferior thymic angle rather than the membranous region of the SPFL lodged between the thymic glands (Figure 3C). Henceforth, we refer to this dense portion as the thymopericardial node (TPN). Surgical removal of the TPN allowed easy movement of the thymus within the mediastinum (Figure 3D). TPN serves a crucial role as an anchor to secure the thymic capsule to the pericardium. The TPN that fixes the thymus to the pericardium can be regarded as a ligament and may be called the thymopericardial ligament.
Characteristics of TPN
Based on our observations of adult surgical cases, the TPN appeared white and strip-like (Figure 2A,2B), sometimes exhibiting a cone-like or triangular shape (Figure 3C) located anteriorly to the arterial hilum of the pericardium (16). These characteristics differed from those of the surrounding SPL tissues. It continued smoothly into the fibrous pericardium; therefore, we assumed that it was part of the fibrous pericardium.
The shape of the TPN is similar to that of the SPL described in older anatomical textbooks. However, the observed TPN is often relatively short (14) extends from the thymus to the pericardium, not the sternum. As the pericardium near the TPN was not resected during our surgical procedures, microscopic findings were unavailable. In our observations, the TPN was a part of the fibrous pericardium or extension (17,18). We contend that depicting the entire SPL as a tendon directly connecting the sternum and pericardium would be inaccurate.
Surgical implications of the TPN
Thymic surgeons should be mindful of the presence of a rigid TPN situated between the thymus and pericardium near the aortic trunk. Even in a normal thymus, the pericardium is drawn toward the thymus owing to the strong traction exerted by TPN, resulting in a deformation that might resemble a malignant indentation. This portion is so firm that surgeons need caution to avoid misinterpreting it as part of a malignant tumor or direct pericardial invasion of the malignancy during surgery. Conversely, it is worth noting that thymomas originating near the thymic isthmus could potentially infiltrate the pericardium more readily, given the close proximity of the thymus to the pericardium in this region.
Previous studies of SPFL and TPN
A few studies have described SPL in infant with pneumomediastinum (15,17,19). Quattromani et al. (17), in neonatal autopsy cases, reported fibrous connective tissues that extend over the thymus and heart tenting outward from the inferior pole of the thymus and extend laterally to parietal pleural reflection and described it as a ‘distinct fascial band’. In this case, the fibrous pericardium merged histologically with the thymic capsule. They also presented a ‘small triangular density’ where the band was attached to the heart in the chest radiograph of infants with pneumomediastinum. Although our result is limited to adult surgical observation, it can be said that the former ‘band’ corresponds to our SPFL and the latter ‘triangle’ to our TPN.
Inferior SPL
If the area contacting the pericardium and lower sternum is considered the inferior SPL, it can consist of two ligaments. Although the contact portion drawn in the figure appears to be an independent ligamentous structure (Figure 1B), in our observation, the contact was due to adhesion, and no ligamentous structures such as the phrenicopericardial ligament between the pericardium and diaphragm were detected here, nor were any dense fibrous structures such as the SPL running between the thymic lobes (Figure 4). As well-known to surgeons, this adhesion is so loose that it could be dissected with a finger before a median sternotomy (20).
SPLF as ligaments in the thorax
The role of the SPFL in fixing the pericardium to the thoracic cage can be regarded as a ligament and should be referred to as the SPL. The pericardium was anteriorly fixed to the thorax by the SPL and posteriorly to the tracheobronchi by the tracheobronchopericardial ligament (13,21). The tracheobronchi connect to the esophagus fascially, and the esophagus connects to the vertebra fascially. The SPL is a component of the fascial train from the sternum to the vertebrae on the midline of the thorax.
SPFL dividing the retrosternal space
The SPFL is a part of a wall that forms a thymic lodge and can be regarded as the caudal floor of Grodinsky’s Space 3 (Figure 8). Grodinsky reported that space 3 is isolated by dense adhesions between the fibrous pericardium and the sternum (1). Our observations align with his description. The caudal floor in the anterior mediastinum of Space 3 could consist of the pericardium, pleurae, and SPFL.
Therefore, the mediastinal compartment above the SPFL can be regarded as a fascial space called the previsceral space (20) or the thymic compartment (12). This compartment lies between the visceral and endothoracic fasciae and continues into the space between the visceral and middle layers of the deep cervical fascia. In other words, the compartment is the thoracic part of space 3, and only the thymus is the primary organ. At the four lateral corners of the compartment, abundant adipose tissues are found along the internal mammary arteries and pericardiacophrenic arteries craniocaudally, and the corners of the caudal floor of the compartment, which are called the cardiophrenic angles, are filled with adipose tissues. The retrosternal space, which is the anterior mediastinum for surgeons, is filled with the thymic gland and corner fat tissues.
Because the SPFL below the thymus extends vertically across the retrosternal space, it functions as a watershed for the lower anterior mediastinum. For example, descending mediastinitis in the anterior mediastinum usually extends posterolaterally below the thymic isthmus (2,22-26). We frequently observed mediastinitis extending laterally along the edges of the SPFL.
SPFL in radiologic images
Although SPFL can often be detected on radiologic images, radiologic findings have rarely been described (27-30). The finding of the SPFL on coronal CT images is compatible with radiological findings known as the anterior junction line (27,31,32) on chest radiographs (Figure 6). We propose that the anterior junction line should consist not only of the pleura and fat tissue between the mediastinal pleura, and that the SPFL should be an essential component of the anterior junction line.
Differences between normal physiological conditions and surgeries
Under normal physiological conditions, in which the chest cavities are airtight and negative pressure is applied, the SPFL is compressed bilaterally by the expanded lungs and accumulates centrally. Therefore, the SPFL exists as a thin and dense sagittal layer even below the thymic valley. However, once the airtightness is broken, the SPFL cannot maintain its membrane shape. The mediastinal pleurae move apart and the density of the SPFL among the pleurae is looser. Therefore, during surgery, the SPFL was observed as a broad and loose fibrous area when the lungs collapsed under normal atmospheric pressure.
Surgical technique and retrosternal fascial anatomy
The fascial structure of the anterior mediastinum is simple. It is comprised of a fatty thymus and massive adipose connective tissue. However, the monotony of this structure can pose challenges for surgeons performing thoracoscopic procedures within the anterior mediastinum, making it challenging to maintain precise orientation during surgery. To avoid disorientation, surgeons must develop a thorough understanding of the fascial anatomy and meticulously follow the continuity of the fibrous connective tissues. The atlas of fibrous tissues presented in this study will help navigate anterior mediastinal surgery. This will be valuable, particularly for thoracoscopic surgeries performed under limited and magnified views.
Conclusions
In this study, we showed the detailed structure of the SPL and the fascial anatomy in the anterior mediastinum, revealing differences in some previous descriptions of the SPL as two ligaments or elongated tendon-like structures.
The structure described as the superior SPL in previous textbooks was the essence of the SPL, which is a sagittal layer of simple fibrous tissues between the pericardium and sternum. By contrast, the inferior SPL showed adhesions with minimal connective tissue. We confirmed that a specific strip-like portion of the SPL serves as an anchor to fix the thymus to the pericardium. We also showed that the SPL formed a segment of the caudal floor of Grodinsky’s space 3, effectively dividing the anterior mediastinum into the right and left compartments. This fibrous layer was detectable on radiological images and suggested to be a potential component of the anterior junctional line.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1602/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1602/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1602/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1602/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee of National Hospital Organization Saitama Hospital (No. R2016-10) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Grodinsky M, Holyoke EA. The fasciae and fascial spaces of the head, neck and adjacent regions. Am J Anat 1938;63:367-408.
- Lindner HH. The anatomy of the fasciae of the face and neck with particular reference to the spread and treatment of intraoral infections (Ludwig's) that have progressed into adjacent fascial spaces. Ann Surg 1986;204:705-14. [Crossref] [PubMed]
- Guidera AK, Dawes PJ, Stringer MD. Cervical fascia: a terminological pain in the neck. ANZ J Surg 2012;82:786-91. [Crossref] [PubMed]
- Gavid M, Dumollard JM, Habougit C, et al. Anatomical and histological study of the deep neck fasciae: does the alar fascia exist? Surg Radiol Anat 2018;40:917-22. [Crossref] [PubMed]
- Feigl G, Hammer GP, Litz R, et al. The intercarotid or alar fascia, other cervical fascias, and their adjacent spaces - a plea for clarification of cervical fascia and spaces terminology. J Anat 2020;237:197-207. [Crossref] [PubMed]
- Federative Committee on Anatomical Terminology. Terminologia anatomica. Stuttgart: Thieme;1998.
- Gray H, Standring S, Berkovitz BKB. Gray’s anatomy: The Anatomical Basis of Clinical Practice. 39th ed. Philadelphia: Elsevier Churchill Livingstone;2005.
- Toldt C, Dalla RA, Paul E. An atlas of human anatomy for students and physicians vol. 5 Anatomischer atlas für Studierende und Ärtze. New York: Rebman Company; 1919.
- Byce TH, Walmsley T. Quain’s Elements of Anatomy (eleventh). London: Longmans Green &Co; 1929.
- Perlemuter L, Waligora J. Cahiers d’anatomie: thorax. Paris: Masson; 1987.
- Mellins HZ, Kottmeier P, Kiely B. Radiologic signs of pericardial effusion; an experimental study. Radiology 1959;73:9-16. [Crossref] [PubMed]
- Milhiet H, Jager P. Anatomie et chirurgie du péricarde: Applications à la chirurgie bronchopulmonaire. Paris: Masson; 1951.
- MARCHAND P. The anatomy and applied anatomy of the mediastinal fascia. Thorax 1951;6:359-68. [Crossref] [PubMed]
- Popa GT, Lucinescu E. The Mechanostructure of the Pericardium. J Anat 1932;67:78-107.
- Low AS, Tan-Kendrick AP, Loh M, et al. Spontaneous multiloculated multiseptated pneumomediastinum in a newborn baby: the spinnaker sail is rigged-CT features with pathologic correlation. Pediatr Radiol 2003;33:712-5. [Crossref] [PubMed]
- Pauza DH, Pauziene N, Tamasauskas KA, et al. Hilum of the heart. Anat Rec 1997;248:322-4. [Crossref] [PubMed]
- Quattromani FL, Foley LC, Bowen A, et al. Fascial relationship of the thymus: Radiologic-pathologic correlation in neonatal pneumomediastinum. AJR Am J Roentgenol 1981;137:1209-11. [Crossref] [PubMed]
- Shil SK, Ferdows S, Sutradhar BC, et al. Topographic Anatomy of Visceral Organs of a Spotted Deer (Axis axis). Res J Vet Pract 2014;2:55-7.
- Hodler J, Vock P. Computerized tomography imaging of the anterior cardiophrenic angle. Rofo 1987;146:654-7. [Crossref] [PubMed]
- Pearson FG, Cooper JD, Deslauriers J, et al. Thoracic surgery. 2nd ed. Philadelphia: Elsevier Churchill Livingstone; 2002.
- Nakanishi K, Goto H, Ito T. Fascial reinforcement fixing the bronchi to the heart: its anatomy and clinical significance. Surg Radiol Anat 2017;39:1301-8. [Crossref] [PubMed]
- Wheatley MJ, Stirling MC, Kirsh MM, et al. Descending necrotizing mediastinitis: transcervical drainage is not enough. Ann Thorac Surg 1990;49:780-4. [Crossref] [PubMed]
- Jessup PM. Mediastinal emphysema. Arch Surg 1931;23:760-82.
- Fields JM, Schwartz DS, Gosche J, et al. Idiopathic bilateral anterior mediastinal abscesses. Pediatr Radiol 1997;27:596-7. [Crossref] [PubMed]
- Roberts JR, Smythe WR, Weber RW, et al. Thoracoscopic management of descending necrotizing mediastinitis. Chest 1997;112:850-4. [Crossref] [PubMed]
- Makeieff M, Gresillon N, Berthet JP, et al. Management of descending necrotizing mediastinitis. Laryngoscope 2004;114:772-5. [Crossref] [PubMed]
- Cimmino CV. The anterior mediastinal line on chest roentgenograms. Radiology 1964;82:459-60. [Crossref] [PubMed]
- Sussmann AR, Ko JP. Understanding chest radiographic anatomy with MDCT reformations. Clin Radiol 2010;65:155-66. [Crossref] [PubMed]
- Gibbs JM, Chandrasekhar C, Ferguson EE, et al. Lines and stripes: where did they go? —From conventional radiography to CT. Radiographic 2007;27:33-48. [Crossref] [PubMed]
- Marano R, Liguori C, Savino G, et al. Cardiac silhouette findings and mediastinal lines and stripes: radiograph and CT scan correlation. Chest 2011;139:1186-96. [Crossref] [PubMed]
- Proto AV, Simmons JD, Zylak CJ. The anterior junction anatomy. Crit Rev Diagn Imaging 1983;19:111-73.
- Heitzman ER. The anterior mediastinum. In: The Mediastinum pp77-115. Berlin: Springer; 1988.


