
A comparison of GOLD 2019 and 2023 recommendations to contemporaneous real-world inhaler treatment patterns for chronic obstructive pulmonary disease management in Singapore
Highlight box
Key findings
• At a single tertiary center in Singapore, a significantly higher proportion of chronic obstructive pulmonary disease (COPD) patients would have escalated treatment under the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2019/2023 eosinophil-directed algorithms compared with contemporaneous prescribing patterns.
What is known and what is new?
• Since 2019, GOLD has suggested using blood eosinophil count to guide treatment adjustments in COPD patients at high exacerbation risk. Previously, treatment escalation was commonly managed without relying on blood eosinophil count.
• Of 268 patients, 84% would have been eligible for treatment escalation under the GOLD 2019/2023 strategies, indicating that implementing updated strategies would impact the majority of COPD patients.
What is the implication, and what should change?
• In our institution, a gap exists between real-world practice and treatment recommendations, highlighting a need for systematic, coordinated interventions to better align clinical practice with treatment guidelines. This may encourage more individualized treatment based on specific treatable traits.
Introduction
Prior to 2019, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommended that patients with chronic obstructive pulmonary disease (COPD) should be assessed using the “ABCD assessment tool” and categorized into one of four groups for the purpose of selecting an initial maintenance therapy (1). For patients who experience exacerbations despite initial pharmacotherapy, an escalation from long-acting muscarinic antagonist (LAMA) monotherapy to LAMA plus long-acting beta2-agonist (LABA), or LAMA + LABA plus inhaled corticosteroid (ICS) was recommended.
Several studies have raised concerns about the associations between ICS plus a long-acting bronchodilator regimens and an increased risk of pneumonia in patients with COPD, with the increased relative risk found to be as high as 39% (2). Consequently, researchers have turned their attention to blood eosinophil levels as a potential predictor for both frequent exacerbations and effective COPD management. Previous studies have reported that high blood eosinophil levels may predict more frequent exacerbations as well as a good therapeutic response to ICS-containing regimens for the prevention of exacerbations in patients with COPD (3-5). The risk of pneumonia has been found to be lower in patients with high blood eosinophils (≥2%) (6). However, caution must be exercised when considering ICS therapy, particularly when blood eosinophil counts are low (7).
Based on these studies, the GOLD 2019 strategy provided updated guidance regarding follow-up treatment for patients with COPD who continue to experience exacerbations despite initial pharmacological treatment, recommending that blood eosinophil count levels be considered to help decide on whether to add ICS treatment (Figure 1) (7,8). For patients initially receiving LAMA or LABA therapy, escalation to ICS + LABA was considered if the blood eosinophil count was ≥300 cells/µL, or ≥100 cells/µL with ≥1 hospitalization or ≥2 moderate exacerbations. If blood eosinophil levels were not elevated, an escalation to LAMA + LABA was recommended. For patients already receiving LAMA + LABA, an escalation to triple therapy (LAMA + LABA + ICS) was considered if the blood eosinophil count was ≥100 cells/µL. For patients already receiving ICS + LABA, an escalation to triple therapy was recommended, but a switch to LAMA + LABA was considered if there was pneumonia, if ICS had originally been inappropriately prescribed, or if there was a previous lack of response to ICS.

More recently, GOLD 2023 provided further updates, including the discouragement of LABA + ICS as a COPD treatment regimen. Instead, patients on LAMA or LABA monotherapy with persistent exacerbations should now be escalated directly to triple therapy (LABA + LAMA + ICS) if blood eosinophil count is ≥300 cells/µL, or to LABA + LAMA if blood eosinophil count is ≤300 cells/µL (9). In line with the 2019 GOLD recommendations, patients already receiving LAMA + LABA are considered for an escalation to triple therapy (LAMA + LABA + ICS) if the blood eosinophil count is ≥100 cells/µL. For patients already receiving ICS + LABA, GOLD 2023 recommends an escalation to triple therapy if there are further exacerbations. A switch to LAMA + LABA is considered if the patient has major symptoms without exacerbations.
The use of blood eosinophil count as a predictor of high exacerbation risk and response to ICS has remained in the GOLD recommendations since its inclusion in 2019. However, the potential impact of these updated GOLD strategies and how significantly they would differ from existing real-world practice is currently not known. This study aimed to evaluate the potential impact of updated recommendations outlined in both the GOLD 2019 and GOLD 2023 on current clinical practice. More specifically, this study aimed to assess the proportion of patients who would have required treatment escalations if they had been managed based on these updated guidelines. This will provide valuable insights into current prescribing behaviors and patterns, helping to identify potential barriers and challenges that need to be addressed as we transition towards a more individualized approach to COPD treatment. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1769/rc).
Methods
Study design and data source
This retrospective cohort analysis utilized prospectively collected data from the Changi General Hospital (CGH) COPD data warehouse. The data warehouse was developed for the purpose of routine clinical management, service evaluation and clinical audit. It includes data such as hospitalization demographics, laboratory and radiology tests, medication management, lung function tests, and case management particulars, collated from multiple information systems. To be included in the data warehouse, patients are identified using primary or secondary International Classification of Diseases, Tenth Revision, Australian Modification (ICD-10-AM) codes specifically related to COPD diagnoses (J440, J441, J448, J449) for either an inpatient or emergency department (ED) visit to CGH. The accuracy of these diagnostic codes was validated during development of the data warehouse and demonstrated a positive predictive value of 77.6% for COPD confirmed either by spirometry [post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity ratio <0.7] or computed tomography imaging evidence of emphysema. The CGH COPD data warehouse contains records starting from October 2017 and is regularly updated with new data. At the time of analysis, data were available up to October 2020.
Study population
Patients were eligible for inclusion if they experienced a hospitalization for a COPD exacerbation on or after October 1, 2018 (primary diagnosis of J44). This allowed for a 1-year period (baseline period) of data for all patients prior to hospitalization. Patients were also required to be aged ≥40 years and receiving LAMA, LAMA + LABA, or ICS + LABA maintenance therapy at the time of hospital admission. LABA monotherapy is not routinely prescribed for patients with COPD in Singapore, and no patients in the data warehouse were receiving LABA monotherapy at the time of hospital admission. Patients receiving triple therapy (i.e., LAMA + LABA + ICS) at the time of hospital admission were excluded from the analysis because the key focus of the study was treatment escalation. If a patient fulfilled the eligibility criteria at several time points (i.e., multiple exacerbations resulting in hospitalization), data for the most recent hospitalization episode were collected and analyzed.
Outcome measures
The primary objective of the study was to describe the proportion and characteristics of hospitalized patients who would have been eligible for escalation to other COPD maintenance treatment according to the GOLD 2019 and 2023 strategies. It should be noted that the study period was contemporaneous with the publication of the GOLD 2019 strategy and pre-dated the GOLD 2023 report, and hence this represents the potential impact of new recommendations on existing practice and not an evaluation of quality of care, as recommendations cannot be retrospectively applied, and new recommendations may take time to be implemented in real-world practice. The secondary objective was to compare the characteristics of patients whose treatment escalation would have been concordant with GOLD 2019 or 2023 and those whose treatment escalation would not have been concordant with GOLD 2019 or 2023. This was stratified for each of the three maintenance groups (i.e., LAMA, LAMA + LABA, or ICS + LABA). In Singapore, medications on admission are prescribed based on most recent maintenance therapy, and if a decision is made to change long-term maintenance therapy during the admission, the new therapy is prescribed at discharge. Based upon comparison between maintenance therapies prescribed at hospital admission and hospital discharge, patients were classified as “concordant” or “discordant” to the GOLD 2019 and 2023 strategies.
GOLD 2019-concordant treatment escalations were defined as: an escalation from LAMA to ICS + LABA if blood eosinophil count is ≥100 cells/µL, an escalation from LAMA to LAMA + LABA if blood eosinophil count is <100 cells/µL, an escalation from LAMA + LABA to triple therapy if blood eosinophil count is ≥100 cells/µL, or an escalation from ICS + LABA to triple therapy (Figure 1).
GOLD 2023-concordant treatment escalations were defined as: an escalation from LAMA to triple therapy if blood eosinophil count is ≥300 cells/µL, an escalation from LAMA to LAMA + LABA if blood eosinophil count is <300 cells/µL, an escalation from LAMA + LABA to triple therapy if blood eosinophil count is ≥100 cells/µL, or an escalation from ICS + LABA to triple therapy (Figure 2).

Data analysis
All variables collected and included in the data warehouse are shown in Table S1. Baseline variables analyzed included: patient demographics (age, sex, ethnicity); smoking history (ex-smoker, current smoker, never-smoker); body mass index (BMI); Elixhauser comorbidities; asthma diagnosis recorded during the baseline period (J45. Asthma); COPD Assessment Test (CAT) score (the median score was taken as a point estimate if repeated measures were available); and lung function (FEV1% predicted and FEV1 absolute value). Variables recorded for each hospitalized COPD exacerbation included: COPD medication on admission, first blood eosinophil count during admission, and COPD medications at the time of discharge. COPD medications were recorded as: LAMA (tiotropium, glycopyrronium, umeclidinium), LAMA + LABA (tiotropium + olodaterol, indacaterol + glycopyrronium, umeclidinium + vilanterol), ICS + LABA (budesonide + formoterol, fluticasone propionate + salmeterol, fluticasone furoate + vilanterol, or fluticasone propionate + formoterol fumarate), or triple therapy [any LAMA with ICS + LABA, or any LAMA + LABA with ICS (budesonide, fluticasone propionate, or beclomethasone)].
Descriptive analyses are reported using number of observations and percentages for categorical variables, mean and standard deviation (SD) for parametric numeric variables, and median and interquartile range (IQR) for non-parametric variables. The proportion of patients who would have had GOLD 2019- or GOLD 2023-concordant treatment escalations was calculated among all patients receiving maintenance treatment (overall), as well as stratified by prior treatment (LAMA, LAMA + LABA, or ICS + LABA).
Statistical analysis
There were insufficient numbers to conduct analyses on escalation vs. non-escalation according to initial therapy. Therefore, a pooled analysis was conducted for all patients who would have been eligible for treatment escalation according to the GOLD 2019 and 2023 strategies. Baseline characteristics for all patients were described. Patients were further stratified according to blood eosinophil count (≥100 and <100 cells/µL). The characteristics of patients who would have had GOLD 2019- or GOLD 2023-concordant treatment escalations were compared with those who would have had discordant escalation. The Kolmogorov–Smirnov test was used to assess the normality of the distribution of described parameters. Data with a normal distribution were expressed as mean ± SD and data that were not normally distributed were expressed as median (IQR). A two-tailed t-test was used to test differences in means and the Fisher’s Exact test was used to compare medians, with P values <0.05 considered statistically significant. Statistical analyses were carried out using International Business Machines Corporation Statistical Package for the Social Sciences statistical software for Macintosh, Version 25.0.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was granted an exemption by the Singhealth Centralised Institutional Review Board (2018/2698). No direct subject contact or primary collection of individual human subject data occurred; therefore, individual consent for this retrospective cohort analysis was waived. In accordance with Singhealth personal data protection policies, the data were submitted to a trusted third party for deidentification and/or anonymization prior to analysis.
Results
Study population and baseline characteristics
Between October 2018 and April 2020, there were a total of 454 patients with ≥1 hospital admission for an exacerbation of COPD (Figure 3). Of these, 273 patients were receiving either LAMA, LAMA + LABA, or ICS + LABA at admission. Five patients died during admission and were excluded. The remaining 268 patients who had available data relating to maintenance treatment at hospital discharge were included in this analysis. For the included patients, mean ± SD age was 73±10 years and the majority (91.0%) were male (Table 1). The predominant ethnicity was Chinese (56.7%), followed by Malay (28.4%), and Indian (6.0%). A total of 103 patients (n=252; 40.9%) were current smokers. The median (IQR) BMI (n=182) was 21.5 (18.6–25.5) kg/m2 and median (IQR) CAT score (n=200) was 12 (7–17). In total, 176 patients (65.7%) had a recorded lung function test and the median FEV1% predicted was 63%. The most common comorbidities were hypertension (45.1%), diabetes mellitus (31.0%), and ischemic heart disease (35.8%). A total of 63 patients (23.5%) had a diagnosis of pneumonia at admission, or during the previous year. Comorbid asthma was recorded in 47 patients (17.5%). Median (IQR) blood eosinophil count (n=268) was 200 (0–400) cells/µL. The demographics of patients in the three treatment groups were broadly similar at the time of hospital admission; however, there was a higher percentage of females in patients receiving ICS + LABA. In patients receiving LAMA + LABA, there was a lower average FEV1% predicted, and a higher number of ED visits/hospital admissions in the past year.
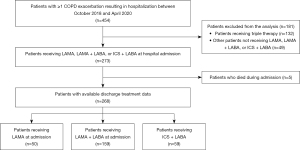
Table 1
| Characteristics | Total (N=268) | Treatment at hospital admission | P value | ||
|---|---|---|---|---|---|
| LAMA (n=50) | LAMA + LABA (n=159) | ICS + LABA (n=59) | |||
| Male, n (%) | 244 (91.0) | 45 (90.0) | 150 (94.3) | 48 (81.4) | 0.01 |
| Age (years), mean [SD] | 73 [10] | 75 [10] | 74 [10] | 72 [12] | 0.40 |
| Ethnicity, n (%) | |||||
| Chinese | 152 (56.7) | 32 (64.0) | 88 (55.3) | 32 (54.2) | 0.09 |
| Malay | 76 (28.4) | 7 (14.0) | 53 (33.3) | 16 (27.1) | |
| Indian | 16 (6.0) | 6 (12.0) | 6 (3.8) | 4 (6.8) | |
| Other | 24 (9.0) | 5 (10.0) | 12 (7.5) | 7 (11.9) | |
| Smoking history†, n (%) | N=252 | n=46 | n=151 | n=55 | |
| Current smoker | 103 (40.9) | 19 (41.3) | 61 (40.4) | 23 (41.8) | 0.78 |
| Ex-smoker | 119 (47.2) | 20 (43.5) | 75 (49.7) | 24 (43.6) | |
| Never-smoker | 30 (11.9) | 7 (15.2) | 15 (9.9) | 8 (14.5) | |
| Body mass index (kg/m2)† | N=182 | n=29 | n=115 | n=38 | |
| Median (IQR) | 21.5 (18.6–25.5) | 21.2 (18.8–24.5) | 20.9 (18.1–24.3) | 22.9 (20.1–27.2) | 0.34 |
| COPD assessment test score† | N=200 | n=36 | n=134 | n=30 | |
| Median (IQR) | 12 (7–17) | 11 (9–14) | 12 (8–16) | 11 (6–16) | 0.32 |
| <10, n (%) | 69 (34.5) | 15 (41.7) | 40 (29.9) | 14 (46.7) | 0.13 |
| ≥10, n (%) | 131 (65.5) | 21 (58.3) | 94 (70.1) | 16 (53.3) | |
| Lung function test, median (IQR)† | N=176 | n=33 | n=113 | n=30 | |
| Post-bronchodilator FEV1% predicted | 63 (49–84) | 72 (60–82) | 55 (44–72) | 68 (59–80) | 0.01 |
| Post-bronchodilator FEV1 (L) | 1.35 (0.99–1.75) | 1.57 (1.04–1.95) | 1.25 (0.93–1.56) | 1.40 (1.06–1.95) | 0.08 |
| GOLD grade†, n (%) | |||||
| 1 (>80%) | 51 (29.0) | 13 (39.4) | 25 (22.1) | 13 (43.3) | 0.06 |
| 2 (50–80%) | 78 (44.3) | 16 (48.5) | 49 (43.4) | 13 (43.3) | |
| 3 (30–<50%) | 41 (23.3) | 4 (12.1) | 34 (30.1) | 3 (10.0) | |
| 4 (<30%) | 6 (3.4) | 0 | 5 (4.4) | 1 (3.3) | |
| Blood eosinophil count (cells/µL) at hospital admission, median (IQR) | 200 (0–400) | 400 (100–750) | 100 (0–300) | 100 (0–600) | 0.23 |
| Elixhauser comorbidity score, median (IQR) | 8 (3–14) | 8 (3–21) | 8 (3–15) | 3 (3–12) | 0.12 |
| ED visits and hospitalizations for COPD (past year), n (%) | N=268 | n=50 | n=159 | n=59 | |
| 0 | 119 (44.4) | 25 (50.0) | 60 (37.7) | 34 (57.6) | 0.02 |
| 1 | 83 (31.0) | 18 (36.0) | 50 (31.4) | 15 (25.4) | |
| ≥2 | 66 (24.6) | 7 (14.0) | 49 (30.8) | 10 (16.9) | |
| Comorbidities, n (%) | |||||
| Hypertension | 121 (45.1) | 20 (40.0) | 74 (46.5) | 27 (45.8) | 0.71 |
| Hyperlipidemia | 71 (26.5) | 11 (22.0) | 42 (26.4) | 18 (30.5) | 0.60 |
| Ischemic heart disease | 96 (35.8) | 15 (30.0) | 62 (39.0) | 19 (32.2) | 0.41 |
| Congestive heart failure | 45 (16.8) | 11 (22.0) | 28 (17.6) | 6 (10.2) | 0.23 |
| Diabetes mellitus | 83 (31.0) | 13 (26.0) | 50 (31.4) | 20 (33.9) | 0.65 |
| Anxiety | 6 (2.2) | 2 (4.0) | 4 (2.5) | 0 | 0.74 |
| Depression | 8 (3.0) | 2 (4.0) | 3 (1.9) | 3 (5.1) | 0.41 |
| Chronic kidney disease | 44 (16.4) | 9 (18.0) | 26 (16.4) | 9 (15.3) | 0.93 |
| Asthma | 47 (17.5) | 7 (14.0) | 14 (8.8) | 26 (44.1) | <0.01 |
| Bronchiectasis | 21 (7.8) | 3 (6.0) | 16 (10.1) | 2 (3.4) | 0.21 |
| Cardiac arrhythmias | 48 (17.9) | 17 (34.0) | 28 (17.6) | 3 (5.1) | <0.01 |
| Pulmonary tuberculosis | 3 (1.1) | 0 | 3 (1.9) | 0 | 0.99 |
| Obstructive sleep apnea | 6 (2.2) | 1 (2.0) | 4 (2.5) | 1 (1.7) | 0.92 |
| Pneumonia | 63 (23.5) | 8 (16.0) | 44 (27.7) | 11 (18.6) | 0.14 |
†, data were not available for all patients included in this study. LAMA, long-acting muscarinic antagonist; LABA, long-acting beta2-agonist; ICS, inhaled corticosteroid; SD, standard deviation; IQR, interquartile range; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; L, liters; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ED, emergency department.
Treatment regimen on admission and discharge
At the time of hospital admission, 50 patients (18.7%) were receiving LAMA, 159 (59.3%) were receiving LAMA + LABA, and 59 (22.0%) were receiving ICS + LABA. At hospital discharge, 28 patients (10.4%) were receiving LAMA, 153 (57.1%) were receiving LAMA + LABA, 42 (15.7%) were receiving ICS + LABA, and 42 patients (15.7%) were receiving triple therapy. Two patients were discharged with short-acting beta2-agonist (SABA) + short-acting muscarinic antagonist (SAMA) and one patient with ICS. Treatment regimen at discharge, stratified according to treatment regimen on admission, is shown in Table 2. In total, 205 patients (76.5%) remained on the same treatment regimen at hospital discharge as they were receiving at admission.
Table 2
| Admission | Discharge | ||||||
|---|---|---|---|---|---|---|---|
| ICS + LABA | LAMA + LABA | LAMA | Triple therapy | Others | SABA/SAMA | Total | |
| ICS + LABA | 41 (69.5)† | 7 (11.9) | 1 (1.7) | 10 (16.9) | 0 | 0 | 59 (100.0) |
| LAMA + LABA | 0 | 137 (86.2)† | 0 | 19 (11.9) | 1 (0.6) | 2 (1.3) | 159 (100.0) |
| LAMA | 1 (2.0) | 9 (18.0) | 27 (54.0)† | 13 (26.0) | 0 | 0 | 50 (100.0) |
| Total | 42 (15.7) | 153 (57.1) | 28 (10.4) | 42 (15.7) | 1 (0.4) | 2 (0.7) | 268 (100.0) |
Data are reported as n (%). †, cases where admission and discharge treatments are the same. ICS, inhaled corticosteroid; LABA, long-acting beta2-agonist; LAMA, long-acting muscarinic antagonist; SABA, short-acting beta2-agonist; SAMA, short-acting muscarinic antagonist.
Expected impact of the GOLD 2019 and 2023 strategies on existing real-world practice
Overall, 226 patients would have met the criteria for treatment escalation according to either the GOLD 2019 or 2023 strategy. Of these patients, only 31 (13.7%) received treatment escalations that would be in line with GOLD 2019 recommendations and 34 (15.0%) received GOLD 2023-concordant treatment on discharge. Treatment escalations that would and would not be concordant with GOLD 2019 or 2023 are shown in Figure 4, and treatment escalation patterns according to maintenance treatment at hospital admission are shown in Figure 5.


Comparison with GOLD 2019 strategy
A total of 50 patients were receiving LAMA at the time of admission. Forty of these patients (80.0%) had a blood eosinophil count ≥100 cells/µL and would have been eligible for escalation to ICS + LABA as per GOLD 2019. Only one patient (2.5%) was escalated to ICS + LABA, 12 patients (30.0%) were escalated directly to triple therapy, and the remaining 27 patients (67.5%) were kept on LAMA. Ten patients receiving LAMA at hospital admission had a blood eosinophil count <100 cells/µL. As per GOLD 2019, these patients would have been eligible for escalation to LAMA + LABA; three of these patients (30.0%) were escalated to LAMA + LABA, six (60.0%) were kept on LAMA, and one patient (10.0%) was escalated to triple therapy.
There were 117 patients receiving LAMA + LABA at hospital admission and with a blood eosinophil count ≥100 cells/µL. According to GOLD 2019, these patients would have been eligible for escalation to triple therapy. While 17 of these patients (14.5%) were escalated to triple therapy, 97 (82.9%) were kept on LAMA + LABA. Three patients (2.6%) were prescribed other treatment regimens (SABA/SAMA or ICS).
Lastly, there were 59 patients receiving ICS + LABA at hospital admission; 10 (16.9%) were escalated to triple therapy, which would have been concordant with GOLD 2019, while 41 (69.5%) were kept on ICS + LABA. Eight patients (13.6%) were prescribed other treatment regimens [LAMA (n=1) or LAMA + LABA (n=7)] at hospital discharge. Among the seven patients who were prescribed LAMA + LABA, the median (IQR) blood eosinophil count was 0 (0–100) cells/µL [lower than the group median of 100 (0–300) cells/µL] and the incidence of pneumonia was 28.6% (n=2).
Comparison with GOLD 2023 strategy
Amongst the 50 patients on LAMA monotherapy at admission, 25 (50.0%) had a blood eosinophil count of ≥300 cells/µL and eight (32.0%) were escalated to triple therapy in accordance with GOLD 2023. Of the remaining 25 patients with blood eosinophil counts <300 cells/µL, five (20.0%) were escalated to LAMA + LABA, in line with GOLD 2023. A total of 13 (26.0%) patients on LAMA at admission were discharged with GOLD 2023-concordant treatment, whilst 37 (74.0%) were discordant.
The GOLD 2023 treatment escalation recommendations for patients who experience exacerbations whilst receiving ICS + LABA or LAMA + LABA remain in line with the GOLD 2019 strategy. As such, the 117 patients who received LAMA + LABA at hospital admission, with a blood eosinophil count ≥100 cells/µL, would similarly have been eligible for escalation to triple therapy according to GOLD 2023 recommendations. Of the 59 patients receiving ICS + LABA at hospital admission, 10 (16.9%) were escalated to triple therapy, which would also have been concordant with GOLD 2023.
Comparison between patients with GOLD-concordant treatment escalations vs. those with GOLD-discordant treatment
The results from the pooled analysis comparing characteristics of patients who received GOLD-concordant treatment escalations vs. those who did not are presented in Table 3. A lower measured post-bronchodilator FEV1 was associated with concordant treatment escalations [median (IQR): 1.26 (0.80–1.57) vs. 1.42 (1.05–1.87) L; P=0.028], as was an increased number of hospital admissions or ED visits in the past year [median (IQR): 1 (0–2) vs. 1 (0–1); GOLD 2019: P=0.048; GOLD 2023: P=0.049]. There were no significant differences between the escalation and the non-escalation groups in terms of age, BMI, CAT score, eosinophil count, or Elixhauser comorbidity score. Hypertension (GOLD 2019: 67.7% vs. 39.5%, P=0.003; GOLD 2023: 61.8% vs. 40.1%, P=0.005), ischemic heart disease (GOLD 2019: 51.6% vs. 30.8%, P=0.02; GOLD 2023: 47.1% vs. 31.3%, P=0.03), and anxiety (GOLD 2019: 9.7% vs. 1.5%, P=0.04; GOLD 2023: 8.8% vs. 1.6%, P=0.03) were more prevalent among patients who were escalated in line with GOLD recommendations. Of note, pneumonia in the past year was prevalent, but the prevalence rate was not significantly different between patients who were escalated in line with GOLD 2019 or 2023 recommendations (GOLD 2019: 19.4%; GOLD 2023: 17.6%) and those who were not (GOLD 2019: 22.6%; GOLD 2023: 22.9%).
Table 3
| Characteristics | Escalated in line with GOLD 2019 | Escalated in line with GOLD 2023 | |||||
|---|---|---|---|---|---|---|---|
| Yes (n=31) | No (n=195) | P value | Yes (n=34) | No (n=192) | P value | ||
| Age (years), mean [SD] | 74 [11] | 73 [10] | 0.61 | 74 [11] | 73 [10] | 0.62 | |
| Body mass index† | n=19 | n=134 | n=19 | n=134 | |||
| Median (IQR) | 21.9 (18.6–23.1) | 21.7 (18.6–26.0) | 0.62 | 21.9 (18.6–23.1) | 21.7 (18.6–26.0) | 0.62 | |
| COPD assessment test score† | n=24 | n=139 | n=24 | n=139 | |||
| Median (IQR) | 11 (7–18) | 12 (8–16) | 0.74 | 11 (7–18) | 12 (8–16) | 0.73 | |
| Post-bronchodilator FEV1% predicted† | n=22 | n=125 | n=22 | n=125 | |||
| Median (IQR) | 57.5 (42.5–74.3) | 66.0 (50–85) | 0.15 | 57.5 (42.5–74.3) | 66.0 (50–85) | 0.15 | |
| Post-bronchodilator FEV1 measured (L)† | n=22 | n=125 | n=22 | n=125 | |||
| Median (IQR) | 1.26 (0.80–1.57) | 1.42 (1.05–1.87) | 0.028 | 1.26 (0.80–1.57) | 1.42 (1.05–1.87) | 0.028 | |
| Eosinophil count (cells/µL), median (IQR) | 200 (100–600) | 200 (100–400) | 0.51 | 200 (100–600) | 200 (100–400) | 0.53 | |
| Elixhauser comorbidity score, median (IQR) | 8 (3–16) | 8 (3–14) | 0.16 | 8 (3–16) | 8 (3–14) | 0.17 | |
| ED and hospitalizations for COPD (past year), median (IQR) | 1 (0–2) | 1 (0–1) | 0.048 | 1 (0–2) | 1 (0–1) | 0.049 | |
| Hospitalizations for COPD (past year), n (%) | |||||||
| 0 | 10 (32.3) | 103 (52.8) | 0.068 | 10 (29.4) | 103 (53.6) | 0.070 | |
| 1 | 11 (35.5) | 57 (29.2) | 14 (41.2) | 54 (28.1) | |||
| ≥2 | 10 (32.3) | 35 (17.9) | 10 (29.4) | 35 (18.2) | |||
| Comorbidities, n (%) | |||||||
| Hypertension | 21 (67.7) | 77 (39.5) | 0.003 | 21 (61.8) | 77 (40.1) | 0.005 | |
| Hyperlipidemia | 9 (29.0) | 54 (27.7) | 0.88 | 9 (26.5) | 54 (28.1) | 0.88 | |
| Ischemic heart disease | 16 (51.6) | 60 (30.8) | 0.02 | 16 (47.1) | 60 (31.3) | 0.03 | |
| Congestive heart failure | 5 (16.1) | 31 (15.9) | 0.97 | 5 (14.7) | 31 (16.1) | 0.97 | |
| Diabetes mellitus | 9 (29.0) | 47 (24.1) | 0.56 | 9 (26.5) | 47 (24.5) | 0.57 | |
| Anxiety | 3 (9.7) | 3 (1.5) | 0.04 | 3 (8.8) | 3 (1.6) | 0.03 | |
| Depression | 3 (9.7) | 5 (2.6) | 0.08 | 3 (8.8) | 5 (2.6) | 0.08 | |
| Chronic kidney disease | 8 (25.8) | 28 (14.4) | 0.11 | 8 (23.5) | 28 (14.6) | 0.21 | |
| Pneumonia in the past year | 6 (19.4) | 44 (22.6) | 0.69 | 6 (17.6) | 44 (22.9) | 0.65 | |
| Asthma | 6 (19.4) | 39 (20.0) | 0.93 | 6 (17.6) | 39 (20.3) | 0.90 | |
| Bronchiectasis | 1 (3.2) | 13 (6.7) | 0.46 | 1 (2.9) | 13 (6.8) | 0.48 | |
| Cardiac arrhythmias | 6 (19.4) | 29 (14.9) | 0.52 | 6 (17.6) | 29 (15.1) | 0.50 | |
| Pulmonary tuberculosis | 0 | 2 (1.0) | 0.55 | 0 | 2 (1.0) | 0.53 | |
| Obstructive sleep apnea | 0 | 5 (2.6) | 0.37 | 0 | 5 (2.6) | 0.36 | |
†, data were not available for all patients included in this study. GOLD, Global Initiative for Chronic Obstructive Lung Disease; SD, standard deviation; IQR, interquartile range; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; L, liters; ED, emergency department.
Discussion
Since 2019, the GOLD strategy suggests considering blood eosinophil count to guide treatment adjustments in patients with COPD and high exacerbation risk (7,8). This could potentially affect many patients, as prior to GOLD 2019, treatment escalation was commonly managed without relying on blood eosinophil count (1,7). By analyzing patients who were being managed before or around the time that the GOLD 2019 eosinophil-directed algorithm was published, we aimed to elucidate the proportion of patients in whom treatment change would have been indicated based on the eosinophil-directed algorithm.
We found that among the 268 patients admitted to CGH between October 2018 and early 2020 with an exacerbation of COPD, 226 (84%) would have been eligible for treatment escalation under the new strategies, indicating that implementation of the updated GOLD 2019 or GOLD 2023 strategy would affect the majority of COPD patients who were admitted for exacerbations. This study also found that the majority of patients did not undergo any change in maintenance treatment at discharge from hospitalized exacerbation, irrespective of the initial treatment the patient received on admission. This suggests widespread therapeutic inertia in the real world, which refers to a failure to initiate or intensify therapy when therapeutic goals (i.e., prevention of exacerbation) are not reached (10). Our findings demonstrate a large potential treatment gap between real-world practice and treatment recommendations. This emphasizes the need for systematic, coordinated interventions to modify clinical practice to facilitate the alignment of real-world practice with updated guidelines and treatment recommendations, which may ultimately encourage more individualized treatment based on specific treatable traits. Subsequent studies comparing the real-world treatment patterns with the latest available treatments using the GOLD 2023 recommendations are needed to evaluate the true prevalence, challenges in implementation and extent of therapeutic inertia, and assess potential barriers to treatment escalation as recommended.
Despite general acceptance of clinical practice recommendations among clinicians, discordance with real-world practice has been similarly observed across different countries and diverse healthcare settings. A study conducted in Spain found that more than 50% of patients were receiving treatments that were not concordant with the GOLD strategy (11). Similarly, prescribing practices for COPD treatment amongst primary care doctors in the United Kingdom were found to be largely discordant with GOLD and National Institute for Health and Care Excellence recommendations (12). In a study conducted in Pennsylvania, United States, non-concordance to GOLD was reported in 67% of the study sample (13). Aligning prescribing practices to treatment recommendations is associated with cost savings from reduced pharmaceutical spending (11,13), as most cases of non-concordance are due to unnecessary use of ICS or triple therapy. Our study adds to existing literature by demonstrating that undertreatment might be common and may account for non-concordance to treatment recommendations. This may lead to poorer disease control with increased healthcare utilization, hospital admissions, and ultimately increased healthcare expenditure. When indicated, escalating treatment as recommended to prevent exacerbations leads to overall cost savings amongst patients with COPD (14).
Our study found that even when hospitalized for an exacerbation, few patients had maintenance therapy changed at discharge. There are several possible reasons that may account for non-escalation of therapy. Firstly, patients may be averse to changing their current treatment regimen, as a change in medication can often lead to increased costs and/or confusion. Escalation of therapy is also often associated with an increased number/type of inhaler devices, which is known to adversely affect inhaler adherence and persistence. This can potentially be mitigated using single-device combination therapies (15). Physicians may be hesitant in adding ICS to treatment regimens due to concerns of pneumonia or often decide to focus on acute management and defer decisions regarding long-term therapy to outpatient follow-up visits when the patient is more stable. Additionally, there may be a preference to defer these decisions to the primary physician who may have a better understanding of the patient’s condition and/or better rapport with the patient. Nevertheless, encouraging guideline-concordant treatment escalations during an exacerbation represents a window of opportunity to optimize the long-term management of patients with COPD. Examples of this include both non-pharmacological (disease-specific education, smoking cessation, psychosocial support) and pharmacological measures (escalation of inhaler therapy), which can help to reduce the risk of recurrent exacerbations.
Limitations of this study include its single-center design with a small sample size, which may limit the generalizability of the findings. The small sample size also precluded further subgroup analyses (comparison of patient characteristics between escalation and non-escalation groups according to initial therapy). Furthermore, ICS + LABA is not encouraged as a treatment option for patients with stable COPD in the updated GOLD 2023 strategy (9). This may further affect the generalizability of findings in current real-world practice. Eosinophil count was recorded as the first measure during the hospital admission for exacerbation in this study, which is a single reading taken at an arbitrary timepoint and may not accurately reflect the eosinophilic status of the patients. Prior studies have also shown that eosinophil count levels are affected by multiple factors including diurnal variations and fluctuating disease states, co-existing illnesses, and treatment (16,17). As such, patients may have had different eosinophil counts at other timepoints prior to, or during admission and physicians may choose to make treatment changes based on stable eosinophil counts (i.e., in a stable outpatient setting). Further to this, approximately 18% of patients in this study also had a record of concomitant asthma which may have impacted prescribing choices (e.g., addition of ICS to long-acting bronchodilator regimens irrespective of eosinophil count, or retention of ICS-containing regimens despite low eosinophils). The majority of patients (91.0%) in this analysis were male, although previous studies have described similar demographics and therefore, this may be representative of the general population in Singapore (18). There were also missing data for some key variables, which were not recorded in the CGH COPD data warehouse. Missing data is a common limitation of retrospective studies, as they rely on the accurate reporting and recording of results by clinicians (19). Although these missing data are reflective of the variation observed in clinical documentation in real-world practice, this may have affected the data analysis (20). Data obtained from the data warehouse were captured from CGH inpatient admissions, ED visits, specialist outpatient clinics, and follow-ups via the CGH telehealth program. Visits to private medical institutions were not captured in this analysis meaning these data are potentially an underestimate of healthcare use. Post-discharge data were not included in this analysis and the treatment escalations that may have occurred at the follow-up outpatient visit would not have been captured in this analysis. In addition, for patients with multiple records of hospitalization for COPD within the eligibility window, the most recent hospitalization episode was selected; therefore, patients receiving ICS + LABA or LAMA + LABA with multiple records of hospitalization, who were captured in this analysis and described as not receiving guideline-concordant treatment escalation, may have in fact received guideline-concordant treatment escalations during an earlier hospitalization, but may not have had sufficient time for the new treatment to become effective, and thus were discharged from the hospital with the same treatment regimen. Finally, clinical outcomes such as repeat exacerbations were not assessed following treatment change. Further studies are required to assess if using eosinophil levels to guide treatment escalation will have a positive effect on clinical outcomes.
Conclusions
The findings from this study suggest that a significantly greater proportion of patients may be eligible for treatment escalation according to the GOLD 2019 and 2023 strategies compared with real-world clinical practice. This highlights a gap between real-world practice and updated treatment recommendations, both by GOLD 2019 and GOLD 2023. There may be a need for systematic, coordinated interventions to modify clinical practice to be more aligned with updated treatment recommendations.
Acknowledgments
Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing, and referencing) was provided by Rebecca Cunningham of Apollo, OPEN Health Communications and was funded by GSK.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1769/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1769/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1769/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1769/coif). X.X., P.B., A.A.N.R., and D.M. are employees of, and/or hold stock/shares in GSK. A.Y. reports that the development of the data warehouse was funded by GSK. A.T. and A.Y. reports that medical writing was funded by GSK. A.T. has received honoraria from GSK for involvement in the GSK Singapore COPD Diagnosis Program Advisory Board and AstraZeneca for involvement in the AstraZeneca COPD advisory board and chairing of a sponsored webinar and COPD event. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was granted an exemption by the Singhealth Centralised Institutional Review Board (2018/2698). No direct subject contact or primary collection of individual human subject data occurred; therefore, individual consent for this retrospective cohort analysis was waived. In accordance with Singhealth personal data protection policies, the data were submitted to a trusted third party for deidentification and/or anonymization prior to analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- GOLD. Global Initiative for Chronic Obstructive Lung Disease (GOLD) - global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2018 report). 2018. [cited 2024 Jan 10]. Available online: https://goldcopd.org/wp-content/uploads/2017/11/GOLD-2018-v6.0-FINAL-revised-20-Nov_WMS.pdf
- Nici L, Mammen MJ, Charbek E, et al. Pharmacologic Management of Chronic Obstructive Pulmonary Disease. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med 2020;201:e56-69. [Crossref] [PubMed]
- Bafadhel M, Peterson S, De Blas MA, et al. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomised trials. Lancet Respir Med 2018;6:117-26. [Crossref] [PubMed]
- Vedel-Krogh S, Nielsen SF, Lange P, et al. Blood Eosinophils and Exacerbations in Chronic Obstructive Pulmonary Disease. The Copenhagen General Population Study. Am J Respir Crit Care Med 2016;193:965-74. [Crossref] [PubMed]
- Singh D, Agusti A, Martinez FJ, et al. Blood Eosinophils and Chronic Obstructive Pulmonary Disease: A Global Initiative for Chronic Obstructive Lung Disease Science Committee 2022 Review. Am J Respir Crit Care Med 2022;206:17-24. [Crossref] [PubMed]
- Pavord ID, Lettis S, Anzueto A, et al. Blood eosinophil count and pneumonia risk in patients with chronic obstructive pulmonary disease: a patient-level meta-analysis. Lancet Respir Med 2016;4:731-41. [Crossref] [PubMed]
- GOLD. Global Initiative for Chronic Obstructive Lung Disease (GOLD) - global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2020 report). 2020. [cited 2024 Jan 10]. Available online: https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.1wms.pdf
- Singh D, Agusti A, Anzueto A, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: the GOLD science committee report 2019. Eur Respir J 2019;53:1900164. [Crossref] [PubMed]
- GOLD. Global Initiative for Chronic Obstructive Lung Disease (GOLD) – global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2023 report). 2023. [cited 2023 Jun 15]. Available online: https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf
- Lebeau JP, Cadwallader JS, Aubin-Auger I, et al. The concept and definition of therapeutic inertia in hypertension in primary care: a qualitative systematic review. BMC Fam Pract 2014;15:130. [Crossref] [PubMed]
- Miravitlles M, Solé A, Aguilar H, et al. Economic Impact of Low Adherence to COPD Management Guidelines in Spain. Int J Chron Obstruct Pulmon Dis 2021;16:3131-43. [Crossref] [PubMed]
- Price D, West D, Brusselle G, et al. Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns. Int J Chron Obstruct Pulmon Dis 2014;9:889-904. [Crossref] [PubMed]
- Asche CV, Leader S, Plauschinat C, et al. Adherence to current guidelines for chronic obstructive pulmonary disease (COPD) among patients treated with combination of long-acting bronchodilators or inhaled corticosteroids. Int J Chron Obstruct Pulmon Dis 2012;7:201-9. [Crossref] [PubMed]
- Tkacz J, Evans KA, Touchette DR, et al. PRIMUS - Prompt Initiation of Maintenance Therapy in the US: A Real-World Analysis of Clinical and Economic Outcomes Among Patients Initiating Triple Therapy Following a COPD Exacerbation. Int J Chron Obstruct Pulmon Dis 2022;17:329-42. [Crossref] [PubMed]
- Bosnic-Anticevich S, Chrystyn H, Costello RW, et al. The use of multiple respiratory inhalers requiring different inhalation techniques has an adverse effect on COPD outcomes. Int J Chron Obstruct Pulmon Dis 2017;12:59-71. [Crossref] [PubMed]
- Durrington HJ, Gioan-Tavernier GO, Maidstone RJ, et al. Time of Day Affects Eosinophil Biomarkers in Asthma: Implications for Diagnosis and Treatment. Am J Respir Crit Care Med 2018;198:1578-81. [Crossref] [PubMed]
- Kostikas K, Brindicci C, Patalano F. Blood Eosinophils as Biomarkers to Drive Treatment Choices in Asthma and COPD. Curr Drug Targets 2018;19:1882-96. [Crossref] [PubMed]
- Tiew PY, Ko FWS, Pang SL, et al. Environmental fungal sensitisation associates with poorer clinical outcomes in COPD. Eur Respir J 2020;56:2000418. [Crossref] [PubMed]
- Talari K, Goyal M. Retrospective studies - utility and caveats. J R Coll Physicians Edinb 2020;50:398-402. [Crossref] [PubMed]
- Wells BJ, Chagin KM, Nowacki AS, et al. Strategies for handling missing data in electronic health record derived data. EGEMS (Wash DC) 2013;1:1035. [Crossref] [PubMed]


