
Analysis of the hemostatic effect of preset wrapping technique for type A aortic dissection with abnormal coagulation
Highlight box
Key findings
• The preset wrapping technique can effectively solve the unexpected bleeding problems of total aortic arch replacement in type A aortic dissection (TAAD) patients with abnormal coagulation (AC) Consequently, this technique may contribute to early postoperative recovery compared with the conventional technique.
What is known and what is new?
• Many technique to reduce anastomotic bleeding for the total arch replacement, But hemostasis is sometimes difficult to achieve after surgery for acute dissection, especially in patients with AC.
• This study represents the first investigation into the application of the preset wrapping technique in total arch replacement for TAAD with AC, and compared its operative outcomes with the conventional non-preset technique.
What is the implication, and what should change now?
• Whether TAAD combined with AC or not, the preset wrapping technique can effectively solve the bleeding problem of total aortic arch replacement and can be widely used in clinical practice.
Introduction
Type A aortic dissection (TAAD) is a critical cardiovascular emergency that requires immediate open surgery. However, total arch replacement, which is a complex procedure, necessitates hypothermic circulatory arrest during the operation, resulting in extended cardiopulmonary bypass (CPB) and operation time. These factors significantly impact the body’s coagulation function, making surgical hemostasis challenging. Furthermore, the postoperative mortality and complication rates are high, particularly in patients with preoperative abnormal coagulation (AC) (1-3). This further complicates intraoperative hemostasis and increases the risks associated with excessive bleeding and blood transfusion (4-6). Previous studies have indicated that TAAD patients with AC are prone to intractable pinhole extravasation following total arch replacement surgery (7,8). However, wrap hemostasis has proven to effectively address this bleeding issue (9,10). Therefore, we hypothesized that patients with TAAD and AC would encounter difficulties in achieving postoperative hemostasis. To address this, we implemented a preset wrapping protocol directly in TAAD surgery. In addition, we conducted a retrospective analysis of TAAD patients with AC who underwent total arch replacement surgery at our center between January 2018 and December 2022. This study reports on the hemostatic effect and early clinical efficacy of the preset bovine pericardial wrapping technique. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1581/rc).
Methods
Clinical data
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study received approval from the institutional review board of the General Hospital of Southern Theater Command (No. 2023010). The informed consent form was signed by all patients to allow the intervention and data recording.
Between January 2018 and December 2022, a total of 438 patients with TAAD underwent total arch replacement surgery at the Department of Cardiovascular Surgery, General Hospital of Southern Theater Command in Guangzhou, China. Among them, 85 patients with preoperative AC were included in this study. The study population consisted of 73 males and 12 females, with an average age of 48.8±6.8 years and a body mass index (BMI) of 26.9±1.9 kg/m2. The patients were divided into two groups based on whether the preset wrapping technique was utilized to prevent or reduce bleeding prior to the operation. The preset pericardium group included 30 patients (26 males and 4 females), while the control group comprised 55 patients (47 males and 8 females). All patients were diagnosed using aortic computed tomography angiography (CTA), bedside echocardiography, chest X-ray, and relevant laboratory tests including assessments of liver and kidney function, coagulation profile, and thrombelastography, which were conducted before the operation (Table 1).
Table 1
| Item | Preset pericardium group (n=30) | Control group (n=55) | t or χ2 value | P value |
|---|---|---|---|---|
| Gender (male/female) | 26/4 | 47/8 | 0.024 | 0.878 |
| Age (years) | 49.2±11.9 | 48.5±12.7 | 0.248 | 0.805 |
| BMI (kg/m2) | 27.4±2.8 | 26.6±3.4 | 1.100 | 0.274 |
| Time from onset to surgery (h) | 19.2±10.7 | 17.6±9.4 | 0.714 | 0.477 |
| History of hypertension | 25 (83.3) | 47 (85.5) | 0.067 | 0.795 |
| Abnormal liver function | 23 (76.7) | 44 (80.0) | 0.129 | 0.719 |
| Aspirin | 7 (23.3) | 13 (23.6) | 0.001 | 0.975 |
| DAPT | 7 (23.3) | 15 (27.3) | 0.157 | 0.692 |
| VKA | 5 (16.7) | 7 (12.7) | 0.248 | 0.618 |
| NOAC | 1 (3.3) | 2 (3.6) | 0.005 | 0.942 |
| Fibrinogen <1.5 g/L | 8 (26.7) | 16 (29.1) | 0.006 | 0.812 |
| Platelet <50×109/L | 6 (20.0) | 12 (21.8) | 0.038 | 0.845 |
| Fibrinogen (g/L) | 2.6±0.9 | 2.4±0.8 | 1.043 | 0.300 |
| INR | 1.2±0.7 | 1.3±0.9 | 0.52 | 0.599 |
| MA value (mm) | 52.1±10.3 | 53.3±11.7 | 0.471 | 0.639 |
| Platelet (×109/L) | 126.7±96.8 | 130.5±93.2 | 0.177 | 0.859 |
| K time (min) | 2.9±1.2 | 3.1±0.9 | 0.868 | 0.388 |
Values are expressed as mean ± standard deviation or number (%). BMI, body mass index; DAPT, dual anti-platelet therapy; VKA, vitamin K antagonist; NOAC, new oral anticoagulants; MA value, maximum clot firmness; INR, international normalized ratio.
The inclusion criteria of preoperative AC:
- Patients on aspirin or dual antiplatelet therapy (DAPT) prior to surgery;
- Patients receiving warfarin anticoagulation before surgery;
- Patients treated with new anticoagulants such as dabigatran and rivaroxaban before surgery;
- Patients with preoperative fibrinogen less than 1.5 g/L;
- Patients with preoperative platelet count less than 50×109/L.
Exclusion criteria: patients who underwent concomitant mitral valve surgery, coronary artery bypass grafting, or the David procedure (valve-sparing aortic root replacement), as well as those who underwent island or debranching techniques for the aortic arch, were excluded from the study.
Operation method
All patients underwent total arch replacement and endovascular stent placement in the descending thoracic aorta. The procedures were performed under combined intravenous-inhalation anesthesia, with continuous monitoring of blood pressure in the upper and lower limbs throughout the operation. The surgical steps, processes, and cerebral perfusion techniques for total arch replacement were consistent across the entire patient cohort. Arterial perfusion catheters were inserted into the femoral artery and left common carotid artery, while the right axillary artery or innominate artery was selected based on the extent of dissection involvement. A left atrial drainage tube was placed via the right superior pulmonary vein. Once the body temperature reached 32.0 °C, the ascending aorta was clamped. Cold perfusion was achieved by inserting a cold perfusion needle into the ascending aorta or by opening the ascending aorta and administering crystal cardioplegia directly through the left and right coronary arteries to induce cardiac arrest. The aortic dissection lesions were explored, and appropriate surgical procedures, such as the Bentall procedure, aortic sinus plasty, and ascending aorta replacement, were selected based on the aortic root lesions and location of the intimal tear. Taking total arch replacement and ascending aorta replacement as an example, the surgical procedure prioritized the treatment of the aortic root, reinforcing it with a “sandwich” horizontal mattress suture. When the nasopharyngeal temperature dropped to approximately 26.0 °C, lower body circulatory arrest and low-flow cerebral perfusion were initiated. Cerebral perfusion during circulatory arrest involved occlusion of the femoral artery perfusion tube, occlusion of the proximal innominate artery, and the left common carotid artery. Bilateral antegrade cerebral perfusion (BACP) with a cerebral perfusion flow rate of 8 mL/(kg·min) and perfusion through the left subclavian artery were employed. After deploying the stented elephant trunk (MicroPort Medical Co. Ltd., Shanghai, China) into the descending thoracic aorta and releasing it, the distal end of the aortic graft and proximal end of the stent were continuously anastomosed with the outer aortic membrane and pericardium strip. Subsequently, the proximal end of the aortic graft was continuously anastomosed with the reinforced base of the aortic root, and after aortic exhaust, flow perfusion through the lower body femoral artery was restored. The patient was gradually rewarmed, and the three branches of the arch vessels and three branches of the artificial blood vessels were sequentially anastomosed. Once the above procedures were completed and the surgical field showed no active bleeding at the anastomotic sites, CPB support was discontinued, and the chest was closed.
Control group: initially, the four-branched aortic graft (Boston Scientific Inc, Boston, MA, USA) was utilized for the artificial aortic vessels. Subsequently, for patients experiencing difficulties with suture and compression hemostasis postoperatively, autologous aortic adventitia and autologous or bovine pericardium were employed to wrap the aortic graft. This approach aimed to prevent or reduce bleeding in the surgical area. The “sandwich” method was employed to reinforce the aortic root, with bovine pericardium placed between the aortic adventitia layers. In cases where bleeding persisted at the anastomosis following the restoration of heartbeats, the lined bovine pericardium was continuously sutured to the posterior wall of the aortic adventitia. For the branch of the artificial vessel, an autologous or bovine pericardial patch was tailored as necessary and anastomosed with the cuffing of the branch vessel. Similarly, the distal anastomotic edge of the aorta was covered with a strip of bovine pericardium, which was continuously sutured to secure the wrapping material. In situations of severe bleeding accompanied by high pressure within the encapsulated annular cavity, which could lead to aneurysm-like dilatation, the lower edge of the aortic root was enclosed with an appropriate autologous or bovine pericardium. The pericardium was then turned towards the right atrial appendage to create a shunt within the wrapping cavity. Care was taken to suture shallowly to avoid damage or interference with the right coronary artery.
Preset pericardium group: the process and technique of preset wrapping were planned before the operation, ensuring a sequential suture sequence from back to front to provide a clear surgical field. Bovine pericardium was primarily used for the preset wrapping of the anterior wall, while autologous aortic tissue was predominantly employed for the posterior wall. During the operation, a team of personnel prepared the preset bovine pericardium by cutting it into appropriate sizes. Three strips of bovine pericardium were also cut, measuring 0.5, 1.0, and 1.5 cm in width, respectively. The bovine pericardium was then wrapped and sutured at the eminence of the cuff using a 4-0 prolene thread (see Figure 1 and Figure 2). Two 2 cm segments were excised from the innominate artery branch of the four-branch aortic graft, with a diameter of approximately 10 mm. These segments were inserted into two artificial vessels with 8 mm branches. After the anastomosis of the partial arch branch, a vascular sleeve with a 10 mm diameter was employed to cover the anastomotic site, preventing or reducing bleeding. Simultaneously, another group of surgeons performed the TAAD procedure. Considering the utilization of the preset wrapping technique to prevent or decrease bleeding, the posterior wall of the autologous aorta was preserved. The suturing of the posterior wall of the wrapping cavity was completed before the proximal and distal aortic anastomoses required for the replacement operation. During ascending aorta replacement, the “sandwich” method was routinely applied (11), wherein the aortic root was lined with pericardium outer pad vascular slices and bovine pericardium strips to form the proximal aortic base. The proximal bovine pericardium strip was turned over and sutured with the posterior wall of the autologous proximal aortic end. The distal aortic posterior wall anastomosis followed a continuous suture sequence involving the artificial vessels, bovine pericardium strips, distal posterior wall of the autologous aorta, and intraoperative stent vessels. After completing the posterior distal wall anastomosis, wrapping of the posterior distal wall was conducted. The anterior wall of the distal aortic anastomosis was wrapped similarly to the proximal one, with the pericardial strip turned over. During cardioversion, rewarming, examination, and suturing of any significant active bleeding, anastomosis of the wrapped anterior wall was performed, and the wrapped cavern-right atrial shunt was completed. In cases of the Bentall procedure, the integrity of the posterior wall of the proximal autologous aorta was preserved, while the root of the anterior wall of the encapsulated annular cavity was directly shunted from the right atrial appendage. To prevent the occurrence of aneurysm-like dilatation in the wrapping cavity, all patients except those undergoing the Bentall procedure received a preset artificial vessel measuring 10 mm in diameter and 2 cm in length, which was used to wrap the lumen and the superior vena cava shunt (see Figure 3).

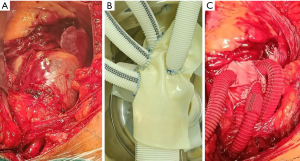
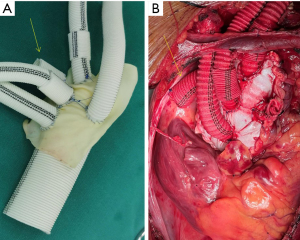
Conservative strategies and observation indicators
The medical treatment strategy for coagulation disorders was based on the 2017 American Guidelines for the Management of Blood in Adult Patients undergoing Cardiac Surgery (EACTS/EACTA). Additionally, the specific medical treatment strategy was formulated according to the circumstances of our center (12,13). The following methods were implemented:
- Platelet and desmopressin infusions were administered to patients on aspirin and DAPT, as well as patients with decreased platelet count and function.
- Consideration was given to appropriate infusions of prothrombin complex concentrate (PCC) and fresh frozen plasma (FFP) for patients with elevated international normalized ratio (INR) due to warfarin anticoagulation;
- Patients with fibrinogen less than 1.5 g/L should consider fibrinogen product infusion;
- Recombinant activated factor VII (rFVIIa) was considered for non-surgical bleeding that was difficult to correct;
- Tranexamic acid drugs were selected as anti-fibrinolytic agents;
- When conditions allowed, efforts were made to maintain normal core temperature and internal environment during the operation to prevent coagulation dysfunction resulting from low temperature and internal environment disruption.
- For all procedures, we routinely employed two human fibrin adhesive adhesives, an absorbable hemostatic collagen sponge, and a Johnson Ethicon hemostatic gauze to assist with hemostasis.
The following indicators were observed:
- Demographic information such as age, gender, time of onset, number of cases of hypertension, BMI index, number of cases of abnormal liver function, number of cases with preoperative anticoagulation and antiplatelet therapy, and coagulation indexes in both groups prior to the operation;
- Surgical conditions in both groups, including surgical methods, operation time, CPB duration, and cardiac arrest duration;
- The amount of packed red blood cells (PRBC), FFP, platelets, PCC, and rFVIIa used during and immediately after the operation, as well as the volume of drainage within 24 hours after the operation in both groups;
- Early postoperative conditions, including the duration of intensive care unit (ICU) stay, mechanical ventilation time, number of re-thoracotomies, bedside hemofiltration, incidences of mediastinal infection, neurologic dysfunction, and in-hospital mortality (including automatic discharge).
Postoperative follow-up
The patients were primarily followed up through telephone consultations or outpatient clinic visits. Thoracoabdominal CTA and ultrasonic cardiogram were repeated 3–6 months after the operation to assess surgical efficacy, distal dissection remodeling, and progress.
Statistical methods
Continuous data were presented as mean (standard deviation) or median (interquartile range) depending on the distribution’s normality, while categorical data were reported as counts and percentages. We compared the baseline characteristics, preoperative, intraoperative, perioperative, and postoperative recovery-related clinical data between the preset pericardium and control groups using the Chi-squared test for categorical variables, independent t-tests for parametric continuous variables, and Mann-Whitney tests for nonparametric continuous variables. All statistical tests were two-tailed, and a significance level of P<0.05 was applied. Data management and statistical analyses were performed using IBM SPSS Statistics version 22.2.
Results
Colligation condition during operation
No significant differences were observed between the two groups regarding Bentall procedure, operation time, and cardiac arrest. However, the mean CPB time was reduced by 68 minutes, and the mean operative time was reduced by 115 minutes in the preset pericardium group compared to the control group, as shown in Table 2. In the control group, 22 patients (40%) were treated with all of these hemostatic agents, multiple stitches and repeated pressure with gauze. However, not all bleeding can be prevented. Diffuse oozing, suture hole bleeding, and anastomosis site bleeding were occurred in 33 patients (60%), of which 22 cases (40%) were local compressive maneuver to manage it, and the remaining 11 cases (20%) were applied a pericardial hood and shunt.
Table 2
| Item | Preset pericardium group (n=30) | Control group (n=55) | t or χ2 value | P value |
|---|---|---|---|---|
| Operation time (min) | 380.1±67.8 | 495.5±81.3 | 6.616 | <0.001* |
| CPB time (min) | 223.5±44.2 | 291.5±56.8 | 5.681 | <0.001* |
| Cardiac arrest time (min) | 109.3±38.6 | 106.8±36.3 | 0.297 | 0.767 |
| Bentall | 8 (26.7) | 13 (23.6) | 0.096 | 0.757 |
| PRBC (mL) | 485.1±376.2 | 815.3±739.9 | 2.284 | 0.025* |
| FFP (mL) | 413.8±295.1 | 746.1±703.4 | 2.467 | 0.016* |
| Platelet count (U) | 0.7±0.4 | 1.1±0.6 | 3.272 | 0.002* |
| PCC (kIU) | 17.3±5.8 | 22.8±9.1 | 2.991 | 0.004* |
| rFVIIa (kIU) | 17.9±8.6 | 35.1±11.5 | 7.164 | <0.001* |
| Fibrinogen (g) | 2.0±1.2 | 3.2±1.9 | 3.131 | 0.002* |
Values are expressed as mean ± standard deviation or number (%). *, P<0.05. CPB, cardiopulmonary bypass; PRBC, red blood cells concentrate; FFP, fresh frozen plasma; PCC, prothrombin complex concentrate; rFVIIa, recombinant activated factor VII.
The use of blood products and coagulation-related drugs during operation and early after operation
The preset pericardium group received fewer transfusions of blood products (PRBC, FFP, and platelets) and used fewer coagulation-related drugs (PCC, fibrinogen, and rFVIIa) compared to the control group (P<0.05), as shown in Table 2.
Early postoperative recovery
In comparison to the control group, the preset pericardium group exhibited a mean reduction of 575 mL in the 24-hour postoperative mediastinal drainage volume. The probability of hemofiltration was significantly lower, and the duration of mechanical ventilation and ICU stay was significantly shorter, as shown in Table 3.
Table 3
| Item | Preset pericardium group (n=30) | Control group (n=55) | t or χ2 value | P value |
|---|---|---|---|---|
| 24 h drainage after surgery (mL) | 605.2±295.6 | 1,180.3±458.7 | 6.193 | <0.001* |
| Re-exploration | 2 (6.7) | 9 (16.4) | 1.620 | 0.203 |
| Neurologic dysfunction | 1 (3.3) | 3 (5.5) | 0.194 | 0.659 |
| ICU time (h) | 105.3±39.4 | 129.5±43.9 | 2.516 | 0.014* |
| Treated with CRRT | 5 (16.7) | 21 (38.2) | 4.232 | 0.039* |
| Mechanical ventilation time (h) | 29.7±18.8 | 43.2±32.1 | 2.111 | 0.038* |
| Mediastinum infection | 5 (16.7) | 9 (16.4) | 0.001 | 0.971 |
| In hospital death | 2 (6.7) | 7 (12.7) | 0.753 | 0.384 |
Values are expressed as mean ± standard deviation or number (%). *, P<0.05. ICU, intensive care unit; CRRT, continuous renal replacement therapy.
Follow-up
A total of 81 patients were followed up, with 4 patients from the control group lost to follow-up. The overall follow-up rate was 95.3%, with a follow-up duration ranging from 7 to 62 months, averaging 23±6.8 months. During the follow-up period, there were 2 deaths (3.9%) in the control group and 1 death (3.3%) in the preset pericardium group. No significant mediastinal infections were reported in either group.
Discussion
TAAD is characterized by rapid onset and progression, and if a blood vessel ruptures, it can pose a life-threatening situation, necessitating immediate surgical intervention. When TAAD patients also present with AC, such as preoperative use of anticoagulant and antiplatelet medications, they are susceptible to bleeding or transfusion-related complications following total arch replacement surgery (14). In some cases, patients with acute aortic syndrome may initially be misdiagnosed as acute coronary syndrome and receive high-dose DAPT until being diagnosed with TAAD during coronary angiography. Refractory hypoxemia has been reported to occur after significant blood transfusions. Therefore, targeted treatment to restore coagulation function is crucial and necessary, as the patient’s AC function and postoperative hemostasis directly impact the clinical outcome (1-3,6).
For TAAD patients with AC prioritized to 2018, our center prioritizes a relatively straightforward surgical treatment strategy focused on the dissection, such as ascending aorta replacement alone (15). Total arch replacement surgery is typically indicated for TAAD patients with arch tears, arch aneurysms, circumferential dissection resulting from arch dissection, left common carotid artery dissection, or Marfan syndrome (16). The procedure often involves the use of a four-branched graft, resulting in extensive incisions and anastomoses, as well as selective cerebral perfusion under hypothermic circulatory arrest. The complexity of this surgery leads to prolonged CPB and operation times, which inevitably affect the body’s coagulation function and increase postoperative bleeding (17). Achieving hemostasis during total arch replacement in TAAD patients poses significant challenges, often resulting in intractable bleeding (18,19). Prioritized to 2018, patients with AC undergoing total arch replacement often required the use of artificial vascular patches or pericardial patches for anastomotic covering to address intractable bleeding (9,20). These interventions necessitated intraoperative design of wrapping schemes, demanding surgeons with advanced suturing skills. Prolonged hemostasis during forced wrapping, as well as the extended CPB and operation times, continually deplete the body’s coagulation function, leading to consumption coagulopathy and extensive blood transfusions (21).
Conversely, the preset wrapping technique involves suturing the bovine pericardium to the four-branched graft, but without increasing the operation time. Suturing the posterior wall of the proximal encapsulated annular cavity can be completed in approximately 2–4 minutes before opening the aortic wall clamp. Thus, the aortic occlusion time did not significantly differ between the two groups. Furthermore, with the confidence of the preset technique in hemostasis, the time of anastomosis and Cardiac arrest was reduced to 50 to 60 minutes. Upon opening the aortic wall clamp, the anterior wall wrapping and shunt were completed in approximately 12–15 minutes. The preset wrapping process is designed in a back-to-front fashion, providing full exposure. Consequently, the preset bovine pericardial wrapping technique does not significantly extend the suture time required for vascular anastomosis. After completing the vascular anastomosis and the cavo-right shunt necessary for total arch replacement, intraoperative examination often reveals minimal active bleeding, allowing for closure of the chest. Compared to the control group, the preset pericardium group experienced significantly shorter CPB and operation times, indicating a reduced hemostasis duration.
In 1978, Cabrol et al. first proposed the connection of the encapsulated lumen of the aortic root to the right atrium for shunting and hemostasis (10). Wrapping hemostasis diverts the oozing blood from the arterial cavity, the primary cause of refractory bleeding, into the venous cavity, with minimal hemodynamic pressure within the wrapping cavity (9,22). Additionally, the short hemostasis duration during the operation and CPB minimizes consumption of the body’s coagulation function. In this study, the preset pericardium group exhibited reduced drainage within 24 hours after surgery, less blood transfusion, and lower usage of coagulation drugs, thereby reducing the risks associated with excessive blood transfusion and bleeding, such as acute renal failure and prolonged mechanical ventilation (5).
Compared to “the streaking” technique using artificial aortic vessels, wrapping hemostasis introduces additional foreign bodies into the mediastinum, which may potentially increase the risk of mediastinal infection in patients (23). However, in this study, no statistically significant difference in the rate of mediastinal infection was observed between the two groups. It is important to note the following considerations regarding considerable active bleeding: (I) to avoid increasing cardiac preload due to excessive left-to-right shunting and the incidence of pseudoaneurysms in the wrapping cavity, needle sutures should be used to control bleeding. (II) The encapsulated annular cavity, wrapped by the preset pericardium, consists of the artificial aortic vessel on the inner wall, a bovine pericardial patch on the outer anterior wall, and autologous aortic tissue on the outer posterior wall, resulting in a predominance of foreign bodies within the cavity. If postoperative bleeding is minimal, blood flow within the cavity will be relatively slow, allowing for gradual restoration of the body’s coagulation function. Over time, thrombosis may form or even occlude the encapsulated annular cavity. Postoperative aortic CTA (median 23 days) revealed a dark area around the artificial vessels in the ascending aorta, indicating the presence of the encapsulated annular cavity (see Figure 4). While a low-grade fever accompanying thrombus absorption may occur within the encapsulated annular cavity postoperatively, no such cases were observed in this study. This may be attributed to the composition of the encapsulated annular cavity, which predominantly consists of foreign bodies, making hematoma absorption more challenging.

The International Registry of Acute Aortic Dissection (IRAD) has reported a surgical intervention rate of approximately 90% for TAAD, with surgery typically performed within 4 hours of onset (24). However, an investigation into the current treatment of aortic dissection in China revealed a lower surgical intervention rate of around 50% for TAAD, with surgery often delayed by approximately 15 hours from onset (25). Postoperative bleeding and hemostasis pose significant challenges in total arch replacement surgery for TAAD (20,21). Therefore, the preset bovine pericardial wrap hemostasis technique is particularly suitable for TAAD patients with preoperative AC, providing an improved method of postoperative hemostasis for total arch replacement surgery in TAAD patients.
Study limitations
Firstly, this study is a retrospective single-center study, and its results may be influenced by nonrandomized grouping. Secondly, the sample size of 85 cases is relatively small, with the preset pericardium group comprising only 30 cases compared to the control group’s 55 cases. Thirdly, the results may be influenced by the different operators performing TAAD surgeries at our center. Furthermore, when multiple studies are conducted, variations among different centers and operators are expected. Given these potential biases, drawing conclusive findings can be challenging. This study primarily focuses on the direct hemostatic effect of the technique, and the clinical efficacy, management of adverse complications, and long-term follow-up effects should be further investigated in future studies with larger sample sizes.
Conclusions
Considering the potential for postoperative bleeding in TAAD patients with AC, we proactively developed the preset wrapping technique. This approach, characterized by reverse thinking, has shown promising results in reducing both the duration of CPB and the overall operation time. Importantly, it has also demonstrated a significant decrease in postoperative bleeding, blood transfusion requirements, and the need for hemostatic drugs. By implementing this technique, we aim to enhance the standard of care for TAAD patients with AC.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1581/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1581/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1581/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1581/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study received approval from the institutional review board of the General Hospital of Southern Theater Command (No. 2023010). The informed consent form was signed by all patients to allow the intervention and data recording.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lin CY, Wu MY, Tseng CN, et al. Delayed sternal closure for intractable bleeding after acute type A aortic dissection repair: outcomes and risk factors analyses. J Cardiothorac Surg 2022;17:184. [Crossref] [PubMed]
- Zindovic I, Sjögren J, Bjursten H, et al. The Coagulopathy of Acute Type A Aortic Dissection: A Prospective, Observational Study. J Cardiothorac Vasc Anesth 2019;33:2746-54. [Crossref] [PubMed]
- Li SW, Yang YW, Lu JK, et al. Risk factors for coagulopathy after Stanford type A acute aortic dissection repair. Chinese Journal of Clinical Thoracic and Cardiovascular Surgery 2018;25:670-5.
- Li M, Xu S, Yan Y, et al. Association of biomarkers related to preoperative inflammatory and coagulation with postoperative in-hospital deaths in patients with type A acute aortic dissection. Sci Rep 2021;11:18775. [Crossref] [PubMed]
- Gupta R. Red Blood Cell Transfusion and Cardiac Surgery-Associated Acute Kidney Injury. JACC Basic Transl Sci 2022;7:639-41. [Crossref] [PubMed]
- Chen FT, Chou AH, Wu VC, et al. Effect of massive blood transfusion on late outcomes after surgical repair of acute type A aortic dissection. Medicine (Baltimore) 2019;98:e17816. [Crossref] [PubMed]
- Patlolla SH, Saran N, Dearani JA, et al. Outcomes and risk factors of late failure of valve-sparing aortic root replacement. J Thorac Cardiovasc Surg 2022;164:493-501.e1. [Crossref] [PubMed]
- Zheng HJ, Zhang XP, Yu SJ, et al. A modified prosthesis eversion technique for proximal anastomosis in ascending aorta replacement. J Thorac Dis 2023;15:4596-605. [Crossref] [PubMed]
- Chen LW, Wu XJ, Dai XF. Transverse Pericardial Sinus Closure in Acute Type A Aortic Dissection Operation. Ann Thorac Surg 2017;104:e351-3. [Crossref] [PubMed]
- Cabrol C, Pavie A, Mesnildrey P, et al. Long-term results with total replacement of the ascending aorta and reimplantation of the coronary arteries. J Thorac Cardiovasc Surg 1986;91:17-25.
- Tang Y, Liao Z, Han L, et al. Long-term results of modified sandwich repair of aortic root in 151 patients with acute type A aortic dissection. Interact Cardiovasc Thorac Surg 2017;25:109-13. [Crossref] [PubMed]
- Boer C, Meesters MI, et al. 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. J Cardiothorac Vasc Anesth 2018;32:88-120. [Crossref] [PubMed]
- Erdoes G, Ahmed A, Kurz SD, et al. Perioperative hemostatic management of patients with type A aortic dissection. Front Cardiovasc Med 2023;10:1294505. [Crossref] [PubMed]
- Guan X, Li J, Gong M, et al. The hemostatic disturbance in patients with acute aortic dissection: A prospective observational study. Medicine (Baltimore) 2016;95:e4710. [Crossref] [PubMed]
- Cai ZX, Zhang WD, Che Q, et al. Operation and treatment strategy for type A aortic dissection with abnormal coagulation. Journal of Clinical Cardiology 2019;35:463-7.
- Sun LZ, Li JR. Progress and challenge of Stanford type A aortic dissection in China. Zhonghua Wai Ke Za Zhi 2017;55:241-4. [Crossref] [PubMed]
- Guan XL, Wang XL, Liu YY, et al. Changes in the Hemostatic System of Patients With Acute Aortic Dissection Undergoing Aortic Arch Surgery. Ann Thorac Surg 2016;101:945-51. [Crossref] [PubMed]
- Boldyrev SY, Barbukhatty KO, Porhanov VA. Surgical Treatment of Acute Type A Aortic Dissection with 18-Litre Bleeding. Aorta (Stamford) 2021;9:30-2. [Crossref] [PubMed]
- Lee JH. Prevention and management of difficult hemostasis in acute type A aortic dissection repair. Asian Cardiovasc Thorac Ann 2023;31:15-9. [Crossref] [PubMed]
- Galea J, Manché A. Method of Hemorrhage Control From the Aorta After Repair of a Dissected Aortic Aneurysm. Ann Thorac Surg 2017;103:e299-300. [Crossref] [PubMed]
- Liu Y, Han L, Li J, et al. Consumption coagulopathy in acute aortic dissection: principles of management. J Cardiothorac Surg 2017;12:50. [Crossref] [PubMed]
- Kao CL, Chang JP. Perigraft-to-right atrial shunt for aortic root hemostasis. Tex Heart Inst J 2003;30:205-7.
- Li DH, Cao Y, Y, He LX, et al. Treatment of patients with incision infection involving extracardiac bovine pericardial patch after cardiac surgery. Infect Inflamm Rep 2014;15:112-113.
- Pape LA, Awais M, Woznicki EM, et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends From the International Registry of Acute Aortic Dissection. J Am Coll Cardiol 2015;66:350-8. [Crossref] [PubMed]
- Wang W, Duan W, Xue Y, et al. Clinical features of acute aortic dissection from the Registry of Aortic Dissection in China. J Thorac Cardiovasc Surg 2014;148:2995-3000. [Crossref] [PubMed]


