
Chest wall resections for sulcus superior tumors
Introduction
Background
Lung cancer is one of the most common types of cancer, and highly contributes to cancer-related mortality (1). Nearly 30% of patients with non-small cell lung cancer (NSCLC), which is the most common histological type of lung cancer, are diagnosed with locally advanced disease of which nearly 5% originate in the superior sulcus, also called Pancoast tumors. Treatment of such tumors is challenging due to their anatomical location, which is high in the apex of the lung (above the second rib), and due to invasion of the chest wall. More extensive tumors may also involve other structures, such as the brachial plexus, subclavian vessels and the spine, further complicating radical treatment.
Following two prospective multicenter trials which showed superior outcomes after induction chemoradiotherapy followed by resection (trimodality therapy) for patients with superior sulcus tumors (SST), this has become a guideline recommended treatment (2-5). Compared with chemoradiotherapy, this approach offers the possibility of better locoregional control, and more favorable overall survival in patients fit for surgery, even in patients diagnosed with tumors invading the spine or large vessels, those with limited mediastinal nodal involvement, or with low-volume (oligo) metastatic disease (6-8). Whether trimodality therapy is better than chemoradiotherapy followed by adjuvant immunotherapy is uncertain (9).
Rationale and knowledge gap
By definition, SSTs invade the chest wall, above the second rib. As a result, complete resection of the tumor requires pulmonary resection, en-bloc with a part of the thoracic wall. The addition of chest wall resection to anatomical pulmonary surgery, potentially results in higher morbidity and mortality when compared to a standard anatomical pulmonary resection (10). Chest wall resection may be challenging, as the thoracic outlet is a region of the body that is not easily reached, and where many important structures are located, such as the subclavian vessels, branches of the brachial plexus, phrenic nerve and stellate ganglion. Furthermore, extensive fibrosis resulting from preoperative chemoradiotherapy may further complicate resection in this area, and healing may be impaired. Intraoperative techniques vary depending on location of the tumor, and involvement of surrounding structures. Prevention of early and late morbidity is essential to preserve optimal quality of life after such extensive surgery.
Objective
In this report, we aim to provide a concise overview of the surgical treatment of SST, with particular emphasis on the chest wall resection. Pre-operative planning and work-up, surgical techniques and prevention of morbidity, are discussed.
SST
Staging and pre-operative planning
For all patients with suspected NSCLC, adequate staging is mandatory. Currently, minimum guideline recommended staging for potentially resectable NSCLC includes histological diagnosis, a contrast enhanced computed tomography (CT) scan of the chest and upper abdomen and a “whole-body” 18F-fludeoxyglucose (18F-FDG) positron emission tomography (PET)/CT scan; invasive mediastinal lymph node evaluation with endobronchial ultrasound (EBUS), esophageal ultrasound (EUS) or (video)mediastinoscopy is indicated when suspicious nodes are present on imaging, or if the tumor is FDG-negative, centrally located or is larger than 3 centimeters (2). For patients with an SST, which is considered locally advanced disease, a magnetic resonance imaging (MRI) of the brain is indicated to rule out metastases before start of curative intent treatment (2). For most SSTs, a contrast enhanced CT scan gives optimal information on tumor size and local extent in the chest wall, e.g., the number and location of the involved ribs. A CT scan detects chest wall invasion with a sensitivity and specificity of 38–87% and 40–90%, respectively (11). Although this is a wide range, sensitivity increases with the presence of symptoms, such as pain and muscle atrophy on the ipsilateral side. The sensitivity for detecting chest wall invasion with MRI is 63–90%, and is comparable with CT, although specificity is more consistently, and is reported in the range of 84–86% (12,13). To determine vascular invasion, as well as the relationship of the tumor with the brachial plexus, an MRI is of additional value (14). Although CT scan is superior in identifying vertebral bony involvement in patients with an SST in close proximity to vertebral structures, an MRI may help to differentiate between reactive inflammatory changes and true vertebral invasion (15). Pulmonary function tests and cardiovascular evaluation before the start of treatment are standard clinical practice (2).
According to the 8th edition of the TNM staging system for NSCLC, SSTs without distant metastases are staged IIB (T3N0), IIIA (T3N1, T4N0–1) or IIIB (T3N2 or T4N2). In general, patients with metastatic disease, especially those with multiple metastases, are not considered for surgery even though surgery may improve local control and potentially prevent debilitating pain due to brachial plexus or spine invasion. Resection in patients with limited mediastinal nodal involvement (N2) may be considered in carefully selected, fit patients, although outcomes compare unfavourably to those with N0–1 disease. Several other prognostic factors for improved survival have been identified (Table 1), e.g., radical resection (R0) and pathological complete response (pCR) to induction therapy (4,7,8,21).
Table 1
| First author | Year | Study type | Number of patients/ resected |
cT3/T4 | Guideline recommended induction CRTx | Morbidity | Surgical mortality | R0 | pCR/pCR ITT | Median OS (months) | 5 yr OS | Prognostic for OS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rusch (4) | 2007 | Prospective | 110 (ITT)/88 | 78/32 | 100% | 52% | 2.3% | 94% | 36%/29% | 33 (ITT) | 44% (ITT) | pCR |
| Kunitoh (5) | 2008 | Prospective | 76 (ITT)/57 | 56/20 | 100% | 14% (major) | 2.6% | 89% | 21%/16% | Not reached | 56% (ITT) | cT3, pCR |
| Demir (16) | 2009 | Case series | 65 | 55/10 (pT) | 15% | 26% | 6.2% | 82% | N/A | 24 | 31% | Non-N2, R0 |
| Collaud (17) | 2014 | Case series | 65 | N/A | 80% | 46% | 6% | 85% | 29% | Not reached | 69% | Response to induction, location, adjuvant treatment |
| Marulli (18) | 2015 | Case series | 56 | 32/24 | 100% | 11% (major) | 5.4% | 86% | 18% | 35 | 38% | cT3, R0, pCR |
| Waseda (19) | 2017 | Case series | 46 | 18/28 | 100% | 24% | 0% (30-day) |
96% | N/A (pCR + MPR 65%) | Not reached | 63% | N0, pathological response |
| Ushida (20) | 2019 | Case series | 60 (ITT)/54 | 46/8 | 100% | 13% (major) | 2% (90-day) |
81% | 22%/20% | Not reached | 69% | pCR |
| Bertolaccini (21) | 2023 | Case series | 100 | N/A | 9% | 20% (major) | 6.9% | 85% | N/A | 24.3 | 34% | N0, R0, posterior location |
| Ünal (8) | 2023 | Case series | 123 | 76/47 | 100% | 21% (major) | 6.5% (90-day) |
93% | 42% | 100 | 60% | MPR |
| Rzyman (22) | 2023 | Case series | 48 (ITT)/47 | N/A | 100% | 36% | 2.1% (90-day) |
89% | 28% | Not reported | 34% | Age <70 years, R0, tumor stage, pathological response |
CRTx, chemoradiotherapy; R0, radical resection margin; pCR, pathological complete response; ITT, intention to treat population; OS, overall survival; yr, year; N/A, not available; N0, no nodal disease; MPR, major pathological response.
After accurate staging, patients should be discussed in a multidisciplinary tumor board, attended by surgeons experienced in complex thoracic oncological surgery (23). For patients with an SST invading surrounding structures, such as large vessels (subclavian artery or subclavian vein), or the spine, surgery may be especially challenging. However, in a recent series of 123 patients with SSTs, nearly 40% of which were T4 tumors (TNM 7th edition), extended resections were reported as feasible and safe, and resulted in high local control rates and good overall survival (8). Therefore, in the absence of a specialised team including vascular, orthopaedic, and neurosurgical expertise, patients should be referred to a center experienced with this type of extensive surgery; the trimodality approach, including surgery, probably offers the best chance of long-term disease control and survival (24).
Imaging techniques have improved over time, and recently, 3-dimensional reconstructions/volume rendering, and virtual reality modules have been introduced to better visualise pulmonary anatomy, the primary tumor, and its local extension. These techniques may help to optimise pre-operative planning, and may facilitate informed patient consent before surgery (25). Although promising, the use of software to reconstruct 3D images is not widely used in clinical practice, partly due to a lack of experience by radiologists and other specialists (26). The use of 3D-printing in the preoperative planning for SST has not yet extensively been investigated, but might be of additional value in planning of complex, high-risk thoracic resections compared to conventional CT scans and MRI, and may even reduce operating room time (27,28). Although promising, this technique currently is only available in a few highly specialized thoracic centers.
Imaging is key in preoperative planning in thoracic surgery, e.g., to determine how many ribs are involved and the best surgical approach, but also to help with informing the patient about what to expect after surgery. For example, most SST tumors involve the T1-nerve root, which once resected, results in numbness of the ulnar side of the forearm. Sometimes, the stellate ganglion is involved in the tumor mass, and removal results in permanent ipsilateral myosis, and ptosis (Horner-syndrome). Vertebral resection and reconstruction frequently require the use of an osteosynthetic material, with a risk of hard-to-treat and persisting infection. Patients with a reconstruction of the subclavian artery with prosthetic material, need life-long antiplatelet therapy.
Surgical technique and prevention of morbidity
The standard surgical procedure for SSTs involves a pulmonary (anatomical) resection (e.g., lobectomy + mediastinal lymph node dissection) with en-bloc resection of the chest wall and involved adjacent structures. In case of poor pulmonary function, a wedge resection or (multi-)segmentectomy can be considered to preserve vital lung capacity. The preferred surgical approach strongly depends on the location of the tumor and involved structures, but also on the surgeons’ preference.
Most commonly used approaches are the posterolateral (Shaw-Paulson), anterior [transmanubrial (Grunewald) or transclavicular (Dartevelle), and their modifications] and hemi-clamshell incision. Sometimes these approaches (posterolateral and anterior) are combined to gain adequate access to the thoracic outlet and important structures. The anterior approach may be preferred for patients with vascular involvement because good vascular exposure can be obtained, but transection of the clavicle or manubrium is necessary to gain access, which introduces additional morbidity (29,30). The classic Shaw-Paulson thoracotomy, also known as the posterolateral approach, facilitates excellent exposure of the posterior chest wall and lateral part of the spine (31). It also offers good access to the nerve roots (C8, T1) and inferior trunk of the brachial plexus, and is the most appropriate incision through which to perform anatomical pulmonary resection and lymphadenectomy. The posterolateral incision also allows an intercostal muscle flap to be easily dissected from the ribs and fixed on the bronchial stump to reduce the risk of bronchopleural fistula formation. The hemi-clamshell incision, in which the lateral thoracotomy level may vary according to the size of the tumor, also gives excellent access to the anterior part of the mediastinum and great vessels (32). For patients with spinal involvement, a one-day procedure is possible, but we and others prefer a staged two-day approach, in which a dorsal approach for vertebral resection and posterolateral thoracotomy for chest wall and pulmonary resection performed on two consecutive days (33).
Minimally invasive surgical techniques are disseminating rapidly including for thoracic surgery. Video-assisted thoracic surgery (VATS) and robotic-assisted thoracic surgery (RATS) pulmonary resections are safe and feasible and have proved to result in comparable long-term survival and improved peri-operative outcomes when compared to an open approach in patients with NSCLC (34-38). Minimally invasive surgery, or a combined approach with open surgery (hybrid approach), is a technique that is increasingly being investigated for complex pulmonary oncological resections. Currently, there are case reports, but no robust evidence for its use in surgery for SST (39-41).
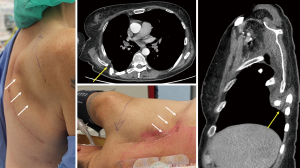
For tumors invading the chest wall, a complete (R0) resection contributes to improved survival, and the size of chest wall resection strongly correlates with postoperative morbidity. In most patients with SST, three or fewer ribs have to be partially removed to obtain radical surgical margins. For patients with posterior located tumors, this can be performed without the need for chest wall reconstruction. However, in patients with an anterior tumor invading larger parts of the chest wall, requiring three or more ribs to be removed, or more than four ribs for posterior tumors with risk of scapular impingement (Figure 1), restoration of the integrity of the chest wall is recommended to preserve chest wall stability, mechanics and respiratory function. This is in line with the recent expert consensus on resection of chest wall tumors and chest wall reconstruction, in which rigid implants for chest wall reconstruction is recommended once the maximum diameter of the chest wall defect exceeds 5 centimeters (42). Patients with SST invading the spine represent a challenging group, especially for those whose tumor invades the vertebral corpus and spinal canal. Curative intent treatment with partial or complete, single or multilevel vertebrectomy, has been reported with considerable 5-year overall survival rates (43–61%), and acceptable and manageable morbidity in high volume, specialized centers (33,43).
For chest wall reconstruction, several materials are available. Preferably, the material is semi-rigid, i.e. stiff enough for structural integrity, but still flexible enough to allow chest wall movement, and inert, such that it induces a limited inflammatory response. In addition, the material should ideally be radiolucent or non-scattering to allow for detection of (loco)regional recurrences with conventional imaging techniques (CT or MRI) during follow-up. Although synthetic resorbable meshes are used [e.g., Vicryl (Ethicon, NJ, USA)], mostly to cover small defects, we prefer the use of synthetic non-resorbable meshes for larger defects to obtain permanent stability [polytetrafluoroethylene (PTFE), polypropylene]. Their use is safe, allows for perfect sizing, and low infection rates are reported (<7%) (44). However, reconstruction can be difficult due to fibrotic tissue resulting from induction chemoradiotherapy, which may hamper proper fixation and ingrowth. Due to its location in the apex and removal of at least the first rib, solid fixation may further be complicated by the limited sites for insertion of the non-resorbable sutures. Rigid reconstruction with titanium ribs, biologic implants, or titanium meshes are rarely needed in patients with chest wall resection in the context of SST (45). With the use of synthetic meshes, a relatively high infection rate (6–22%) in chest wall reconstruction in non-contaminated defects is reported, with up to 42% requirement of removal of synthetic mesh (46). Therefore, biological meshes, or biosynthetic materials, in which biologic and synthetic components are combined, deserve consideration, in particular for patients at high risk for infection (47,48). 3D-printing techniques for reconstructive and restorative use, to replace resected structures such as chest wall or vascular structures with biomaterial, will facilitate surgery for complex thoracic tumors and overcome the disadvantages of synthetic material (26-28).
Impaired healing as a result from radiotherapy, extensive surgery and patient related factors, such as smoking history and diabetes, prompt aggressive perioperative measures to reduce the incidence of infection, e.g., antibiotic prophylaxis. Enhanced recovery programs are now available for thoracic surgery, promoting faster recovery and helping to minimize complications and morbidity, including infections and thoracotomy pain (49). With this approach, low morbidity and mortality can be achieved in patients with extended chest wall resections and reconstructions.
Conclusions
Table 2 summarizes key issues form this review. Trimodality therapy consisting of chemoradiotherapy followed by surgery is a guideline recommended treatment for patients with NSCLC originating from the superior sulcus. The optimal surgical approach depends on the exact location, extension to surrounding structures and surgeons’ preference. Chest wall reconstruction is seldom indicated. All patients should be discussed in a multidisciplinary tumor board attended by thoracic surgeons experienced in complex thoracic surgery.
Table 2
| Guideline recommended treatment for patients with superior sulcus tumors is induction concurrent chemoradiotherapy followed by anatomical resection |
| Surgery for superior sulcus tumors is complex due to their anatomical location in the apex of the lung, adjacent to important structures such as brachial plexus, subclavian vessels and spine |
| 3D-reconstruction techniques may help pre-operative planning and reduce operating room time |
| Reconstruction of the chest wall is rarely needed, but 3D-printing of patient tailored implants with biomaterial is a promising new technique |
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Erik de Loos, José Ribas de Campos and Jean Daemen) for the series “Chest Wall Resections and Reconstructions” published in Journal of Thoracic Disease. The article has undergone external peer review.
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-828/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-828/coif). The series “Chest Wall Resections and Reconstructions” was commissioned by the editorial office without any funding or sponsorship. M.D. reports institutional research grants and personal honorarium from Varian Medical Systems. C.D. reports institutional research support from Astra Zeneca and Bristol Myers Squibb, institutional payments for advisory boards from Astra Zeneca, Bristol Myers Squibb, and Merck Sharp & Dohme. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this article and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv1-iv21. [Crossref] [PubMed]
- Ramnath N, Dilling TJ, Harris LJ, et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e314S-40S.
- Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Clin Oncol 2007;25:313-8. [Crossref] [PubMed]
- Kunitoh H, Kato H, Tsuboi M, et al. Phase II trial of preoperative chemoradiotherapy followed by surgical resection in patients with superior sulcus non-small-cell lung cancers: report of Japan Clinical Oncology Group trial 9806. J Clin Oncol 2008;26:644-9. [Crossref] [PubMed]
- Vos CG, Hartemink KJ, Blaauwgeers JL, et al. Trimodality therapy for superior sulcus tumours: evolution and evaluation of a treatment protocol. Eur J Surg Oncol 2013;39:197-203. [Crossref] [PubMed]
- Kwong KF, Edelman MJ, Suntharalingam M, et al. High-dose radiotherapy in trimodality treatment of Pancoast tumors results in high pathologic complete response rates and excellent long-term survival. J Thorac Cardiovasc Surg 2005;129:1250-7. [Crossref] [PubMed]
- Ünal S, Winkelman JA, Heineman DJ, et al. Long-Term Outcomes After Chemoradiotherapy and Surgery for Superior Sulcus Tumors. JTO Clin Res Rep 2023;4:100475. [Crossref] [PubMed]
- Spigel DR, Faivre-Finn C, Gray JE, et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J Clin Oncol 2022;40:1301-11. Erratum in: J Clin Oncol 2022;40:1965. [Crossref] [PubMed]
- Towe CW, Servais EL, Grau-Sepulveda M, et al. Impact of Chest Wall Resection on Mortality After Lung Resection for Non-Small Cell Lung Cancer. Ann Thorac Surg 2022;114:2023-31. [Crossref] [PubMed]
- Quint LE, Francis IR. Radiologic staging of lung cancer. J Thorac Imaging 1999;14:235-46. [Crossref] [PubMed]
- Padovani B, Mouroux J, Seksik L, et al. Chest wall invasion by bronchogenic carcinoma: evaluation with MR imaging. Radiology 1993;187:33-8. [Crossref] [PubMed]
- Webb WR, Gatsonis C, Zerhouni EA, et al. CT and MR imaging in staging non-small cell bronchogenic carcinoma: report of the Radiologic Diagnostic Oncology Group. Radiology 1991;178:705-13. [Crossref] [PubMed]
- Bonomo L, Ciccotosto C, Guidotti A, et al. Lung cancer staging: the role of computed tomography and magnetic resonance imaging. Eur J Radiol 1996;23:35-45. [Crossref] [PubMed]
- Mihoubi Bouvier F, Thomas De Montpréville V, Besse B, et al. Can MRI differentiate surrounding vertebral invasion from reactive inflammatory changes in superior sulcus tumor? Eur Radiol 2021;31:8991-9. [Crossref] [PubMed]
- Demir A, Sayar A, Kocaturk CI, et al. Surgical treatment of superior sulcus tumors: results and prognostic factors. Thorac Cardiovasc Surg 2009;57:96-101. [Crossref] [PubMed]
- Collaud S, Machuca T, Mercier O, et al. Long-term outcome after resection of non-small cell lung cancer invading the thoracic inlet. Ann Thorac Surg 2014;98:962-7. [Crossref] [PubMed]
- Marulli G, Battistella L, Perissinotto E, Breda C, Favaetto AG, Pasello G, et al. Results of surgical resection after induction chemoradiation for Pancaost tumours. Interact Cardiovasc Thorac Surg 2015;20:805-11. [Crossref] [PubMed]
- Waseda R, Klikovits T, Hoda MA, et al. Trimodality therapy for Pancoast tumors: T4 is not a contraindication to radical surgery. J Surg Oncol 2017;116:227-35. [Crossref] [PubMed]
- Uchida S, Yoshida Y, Ohe Y, et al. Trimodality therapy for superior sulcus tumour: experience of a single institution over 19 years. Eur J Cardiothorac Surg 2019;56:167-73. [Crossref] [PubMed]
- Bertolaccini L, Casiraghi M, Galetta D, et al. Surgical management of superior sulcus tumors: A twenty-year experience of an oncological high volume referral centre. Front Oncol 2023;12:1080765. [Crossref] [PubMed]
- Rzyman W, Łazar-Poniatowska M, Dziedzic R, et al. Trimodality Treatment of Superior Sulcus Non-Small Cell Lung Cancer: An Institutional Series of 47 Consecutive Patients. Curr Oncol 2023;30:4551-62. [Crossref] [PubMed]
- Dickhoff C, Dahele M. The multidisciplinary lung cancer team meeting: increasing evidence that it should be considered a medical intervention in its own right. J Thorac Dis 2019;11:S311-4. [Crossref] [PubMed]
- Tricard J, Filaire M, Vergé R, et al. Multimodality therapy for lung cancer invading the chest wall: A study of the French EPITHOR database. Lung Cancer 2023;181:107224. [Crossref] [PubMed]
- Sadeghi AH, Maat APWM, Taverne YJHJ, et al. Virtual reality and artificial intelligence for 3-dimensional planning of lung segmentectomies. JTCVS Tech 2021;7:309-21. [Crossref] [PubMed]
- George E, Barile M, Tang A, et al. Utility and reproducibility of 3-dimensional printed models in pre-operative planning of complex thoracic tumors. J Surg Oncol 2017;116:407-15. [Crossref] [PubMed]
- Gillaspie EA, Matsumoto JS, Morris NE, et al. From 3-Dimensional Printing to 5-Dimensional Printing: Enhancing Thoracic Surgical Planning and Resection of Complex Tumors. Ann Thorac Surg 2016;101:1958-62. [Crossref] [PubMed]
- Pavan Kalyan BG, Kumar L. 3D Printing: Applications in Tissue Engineering, Medical Devices, and Drug Delivery. AAPS PharmSciTech 2022;23:92. [Crossref] [PubMed]
- Grunenwald D, Spaggiari L. Transmanubrial osteomuscular sparing approach for apical chest tumors. Ann Thorac Surg 1997;63:563-6. [Crossref] [PubMed]
- Dartevelle PG, Chapelier AR, Macchiarini P, et al. Anterior transcervical-thoracic approach for radical resection of lung tumors invading the thoracic inlet. J Thorac Cardiovasc Surg 1993;105:1025-34.
- Shaw RR, Paulson DL, Kee JL. Treatment of Superior Sulcus Tumor by Irradiation Followed by Resection. Ann Surg 1961;154:29-40. [Crossref] [PubMed]
- Lardinois D, Sippel M, Gugger M, et al. Morbidity and validity of the hemiclamshell approach for thoracic surgery. Eur J Cardiothorac Surg 1999;16:194-9. [Crossref] [PubMed]
- Collaud S, Waddell TK, Yasufuku K, et al. Long-term outcome after en bloc resection of non-small-cell lung cancer invading the pulmonary sulcus and spine. J Thorac Oncol 2013;8:1538-44. [Crossref] [PubMed]
- Zhang O, Alzul R, Carelli M, et al. Complications of Robotic Video-Assisted Thoracoscopic Surgery Compared to Open Thoracotomy for Resectable Non-Small Cell Lung Cancer. J Pers Med 2022;12:1311. [Crossref] [PubMed]
- Yang HX, Woo KM, Sima CS, et al. Long-term Survival Based on the Surgical Approach to Lobectomy For Clinical Stage I Nonsmall Cell Lung Cancer: Comparison of Robotic, Video-assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann Surg 2017;265:431-7. [Crossref] [PubMed]
- Kent MS, Hartwig MG, Vallières E, et al. Pulmonary Open, Robotic, and Thoracoscopic Lobectomy (PORTaL) Study: An Analysis of 5721 Cases. Ann Surg 2023;277:528-33. [Crossref] [PubMed]
- Gao Y, Jiang J, Xiao D, et al. Robotic-assisted thoracic surgery following neoadjuvant chemoimmunotherapy in patients with stage III non-small cell lung cancer: A real-world prospective cohort study. Front Oncol 2022;12:969545. [Crossref] [PubMed]
- Huang J, Tian Y, Li C, et al. Robotic-assisted thoracic surgery reduces perioperative complications and achieves a similar long-term survival profile as posterolateral thoracotomy in clinical N2 stage non-small cell lung cancer patients: a multicenter, randomized, controlled trial. Transl Lung Cancer Res 2021;10:4281-92. [Crossref] [PubMed]
- Mariolo AV, Casiraghi M, Galetta D, et al. Robotic Hybrid Approach for an Anterior Pancoast Tumor in a Severely Obese Patient. Ann Thorac Surg 2018;106:e115-6. [Crossref] [PubMed]
- Uchida S, Suzuki K, Fukui M, et al. Hybrid robotic lobectomy with thoracic wall resection for superior sulcus tumor. Gen Thorac Cardiovasc Surg 2022;70:756-8. [Crossref] [PubMed]
- Kostic M, Sarsam M, Bottet B, et al. Post-immunotherapy combined operative technique with an anterior surgical approach and robot-assisted lobectomy for an anterior superior sulcus tumor—case report. J Vis Surg 2022;8:5.
- Wang L, Yan X, Zhao J, et al. Expert consensus on resection of chest wall tumors and chest wall reconstruction. Transl Lung Cancer Res 2021;10:4057-83. [Crossref] [PubMed]
- Collaud S, Fadel E, Schirren J, et al. En Bloc Resection of Pulmonary Sulcus Non-small Cell Lung Cancer Invading the Spine: A Systematic Literature Review and Pooled Data Analysis. Ann Surg 2015;262:184-8. [Crossref] [PubMed]
- Lo Iacono G, Mazzella A, Mohamed S, et al. The Role of Surgery in Primary Chest Wall Tumors: Over 20 Years' Experience in Resection and Reconstruction. Cancers (Basel) 2023;15:2153. [Crossref] [PubMed]
- Bennett DT, Weyant MJ. Extended chest wall resection and reconstruction in the setting of lung cancer. Thorac Surg Clin 2014;24:383-90. [Crossref] [PubMed]
- Weyant MJ, Bains MS, Venkatraman E, et al. Results of chest wall resection and reconstruction with and without rigid prosthesis. Ann Thorac Surg 2006;81:279-85. [Crossref] [PubMed]
- Gonfiotti A, Viggiano D, Vokrri E, et al. Chest wall reconstruction with implantable cross-linked porcine dermal collagen matrix: Evaluation of clinical outcomes. JTCVS Tech 2022;13:250-60. [Crossref] [PubMed]
- Miller DL, Durden FL. Chest Wall Reconstruction Utilizing Ovine-derived Reinforced Tissue Matrix. Ann Thorac Surg 2023;115:1266-72. [Crossref] [PubMed]
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]


