
Hybrid ablation for persistent atrial fibrillation: a narrative review
Introduction
The history of catheter ablation in human hearts traces back to 1982 when Dr. Scheinman, Dr. Morady and colleagues reported atrioventricular junction ablation using direct current (DC) shocks (1). Subsequently, the energy source shifted from DC energy to radiofrequency (RF) energy. RF catheter ablation has been used to treat atrioventricular nodal reentrant tachycardia, accessory pathway mediated tachycardias, atrial tachycardia, atrial flutter and ventricular arrhythmias (2). Atrial fibrillation (AF) was considered untreatable with catheter ablation until Dr. Haïssaguerre and colleagues reported in 1998 that atrial ectopic beats originating from the pulmonary veins initiated AF (3). This groundbreaking report has led to the rapid development of catheter ablation as an established treatment strategy for AF. Pulmonary vein isolation (PVI) has evolved from ostial PVI to antral PVI, and further to ipsilateral PVI, expanding its indications from paroxysmal AF to persistent AF. Recently, it has been estimated that approximately 600,000 AF ablations are performed worldwide each year, and this number continues to rise (4).
Surgical treatment for AF began with the Maze procedure, first performed by Cox et al. in 1987 (5). The Maze procedure was later improved with the Maze III procedure, preserving atrial function and contraction (6). Since the Maze procedure involved repeated cut-and-sew techniques, it was associated with prolonged aortic cross-clamp times and a risk of postoperative bleeding complications. The use of ablative energy sources such as RF and cryoablation replaced cut and sew procedures, making non-incisional surgical treatment known as Cox-Maze IV procedure more common. Devices using RF-energy enabled shorter surgery times and safer treatments. In recent years, the advent of devices utilizing RF-energy has transformed surgical ablation into a safer procedure with shorter operation times (7). Surgical AF ablation has evolved, and the Cox-Maze IV procedure showed a favorable outcome in the long-term maintenance of sinus rhythm (8).
Compared to paroxysmal AF, treatment outcomes for persistent and long-standing persistent AF are not satisfactory yet. Hybrid catheter-surgical ablation is a treatment option for hard-to-treat AF, involving collaboration between cardiac surgeons and electrophysiologists. In recent years, hybrid ablation has emerged as a prominent therapeutic approach for persistent AF but has not yet become an established treatment for persistent AF. This comprehensive narrative review aims to explore the advantages and disadvantages of hybrid ablation for AF. We present this article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1671/rc).
Methods
The purpose of this narrative review is to comprehensively examine the current state of knowledge regarding hybrid ablation of AF and to synthesize the existing literature to provide insights into hybrid ablation of AF. A comprehensive literature search was conducted using PubMed. The search terms included “hybrid ablation” or “atrial fibrillation” or “catheter ablation”or “guideline on cardiology”, and the timeframe for the search was limited to articles published between 1980 and 2024. Articles were included based on peer-reviewed journals. Studies that met the criteria for English language articles and age of 19 years and older were included. A total of 138,969 articles were found using the above criteria. The selection criteria included clinically important articles for narrative review of hybrid AF ablation, and consensus was achieved through a series of structured meetings and discussions. Detailed information is shown in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | November 1, 2023 and January 7, 2024 |
| Databases and other sources searched | PubMed |
| Search terms used | “hybrid ablation”, “atrial fibrillation”, “catheter ablation”, and “guideline on cardiology” |
| Timeframe | 1980–2024 |
| Inclusion criteria | English language articles, patients age of 19 years and older |
| Selection process | S.K. (the 1st author) independently conducted the selection, and consensus was achieved through a series of structured meetings and discussions |
Progress of RF catheter ablation for AF
Currently, PVI is an established treatment for AF and demonstrates a higher maintenance rate of sinus rhythm compared to anti-arrhythmic drug therapy especially in patients with paroxysmal AF (9,10). Initially, during the inception of PVI, non-irrigation catheters without a contact sensor were used. However, in recent years, power-control irrigated catheters with contact sensors have become widely used. Three-dimensional (3D) mapping systems have been developed to predict the lesion volume based on applied RF power, contact force, and ablation duration. The CARTO system provides the ablation index as a marker of lesion volume (11). Similarly, the EnSite system provides the lesion size index (12). The Rhythmia system uses the local impedance measured by the ablation catheter as an indicator of lesion volume (13). With the evolution of these ablation catheter devices and 3D mapping systems, complications like thrombosis and cardiac tamponade have decreased, leading to improved outcomes after catheter ablation for AF. By utilizing catheters with a contact-sensor and assessing lesion volume with a 3D mapping system, particularly in paroxysmal AF cases, satisfactory results have been achieved, with approximately a 90% recurrence free rate at one-year post-ablation (14,15). High-power short-duration PVI, with electrical output raised to 50 W, has allowed for a reduction in procedural time compared to conventional power settings (16,17). Additionally, very high-power short-duration ablation, introduced recently, utilizing 90 W for 4 s in temperature-controlled mode, has shortened procedural time to around 70 minutes, with reported safety and efficacy levels comparable to 30–50 W ablation (18).
Outcome after catheter ablation for persistent AF
Undoubtedly PVI is an established treatment modality, even for persistent AF. However, sinus rhythm maintenance rate after catheter ablation for persistent AF is not as high as that for paroxysmal AF. In cases of catheter ablation for persistent AF undergoing only PVI, the recurrence rate at one year after a single procedure has been reported to be approximately 40–50% (19,20). In the recent systematic review and meta-analysis including 73 studies with 67,159 patients undergoing AF ablation, the pooled incidence of freedom from atrial arrhythmia at 5 years was 50.6% after a single ablation and 69.7% after multiple procedures. In non-paroxysmal AF, patients undergoing a single ablation demonstrated a freedom rate of 33.3%, contrasting with the higher rate of 59.7% observed in paroxysmal AF (P=0.002). For multiple ablations, the freedom rates were 60.6% for non-paroxysmal AF and 80.8% for paroxysmal AF (P<0.001) (21).
Various additional ablations for improving treatment outcomes of persistent AF have been reported, such as linear ablation (i.e., roof line ablation or mitral isthmus ablation), complex fractionated atrial electrogram (CFAE) ablation, low voltage ablation, and posterior wall isolation. However, the effectiveness of these approaches remains uncertain.
The STAR AF II study, a randomized controlled trial, compared different ablation strategies for persistent AF, including PVI alone, PVI plus linear ablation, and PVI plus CFAE ablation. After 18 months, all three groups had a recurrence rate of approximately 50%. The study couldn’t establish the superiority of the additional ablation methods in preventing the recurrence of atrial tachyarrhythmias (22).
Low voltage zone ablation targets atrial scar identified by voltage maps in 3D mapping systems or cardiac magnetic resonance imaging (MRI). However, reports regarding its effectiveness have been inconsistent. While there is data supporting its effectiveness, there are also studies that fail to demonstrate its efficacy (23). For instance, the STABLE-SR-II trial, a multicenter randomized controlled trial involving 300 patients with persistent AF, allocated patients in a 1:1 ratio to either PVI alone or PVI with additional low voltage zone ablation identified by a 3D mapping system (24). The recurrence rate at 18 months was 67% in both groups, with no significant difference demonstrated. Similarly, the DECAAF II trial, a multicenter randomized controlled trial comprising 834 patients with persistent AF, assigned patients in a 1:1 ratio to either PVI alone or PVI with ablation targeting scars identified by cardiac MRI (25). In both groups, the recurrence rates were approximately 45%, and no superiority of low voltage area ablation identified by MRI was observed.
Posterior wall isolation for persistent AF
AF trigger sites are most commonly located in the pulmonary veins, but in the treatment of persistent and long-standing AF, PVI alone is often insufficient, and the treatment of non-PV foci is needed. Frequent sites for non-PV foci include the superior vena cava, crista terminalis, ligament of Marshall, coronary sinus, left atrial appendage, and inter-atrial septum in addition to the left atrial posterior wall (26). The embryological origin of the left atrial posterior wall is similar to that of the pulmonary veins and is one of the most common sites of non-PV foci. However, the effectiveness of left atrial posterior wall isolation is not well-established. Recent reports show that adding posterior wall isolation to PVI does not significantly improve sinus rhythm maintenance rates in patients with persistent AF (27). Lee and colleagues conducted a study where they randomized 217 patients with persistent AF into two groups: PVI alone and PVI plus posterior wall isolation. In the group with PV isolation alone, 50.5% maintained sinus rhythm without anti-arrhythmic drugs after a mean follow-up of 16 months, while in the group with PVI and posterior wall isolation, maintenance of sinus rhythm was 55.9%, showing no significant difference (28). The CAPLA trial, a multi-center randomized study involving 338 patients with persistent AF, also showed no significant difference in arrhythmia-free rates without anti-arrhythmic drugs after a single procedure at 12 months between the PVI only group (53.6%) and the PVI plus posterior wall isolation group (52.4%) (19). The conflicting data on the effectiveness of posterior wall isolation may be attributed to the durability of left atrial posterior wall lines. According to recent systematic reviews on left atrial posterior wall isolation, while the acute success rate exceeds 90%, the reconnection rate of the left atrial posterior wall line in the second procedure is approximately 60% (29).
Performing left atrial posterior wall isolation from the endocardial side with RF ablation and confirming electrical isolation through bidirectional block using pacing and voltage mapping on the 3D mapping system may be insufficient for confirming the completion of posterior wall isolation. In some studies, residual potentials were observed on the epicardial side of left atrial posterior wall even after confirmation of posterior wall isolation by endocardial mapping, which could conduct into the left atrium and contribute to the recurrence of AF. After posterior left atrial wall isolation, there have been reports suggesting that residual potentials in the unipolar voltage maps may be associated with the recurrence of AF (30,31). Creating transmural lesions in the left atrial posterior wall can be challenging due to the presence of the septo-pulmonary bundle and the proximity of the esophagus (32). This may result in insufficient RF energy application (to avoid complications such as left atrial-esophageal fistulas) and failure to achieve permanent posterior wall isolation.
Alternative techniques such as cryothermal or pulsed field ablation for left atrial posterior wall isolation are also reported, which may improve the effectiveness and safety of posterior wall isolation. Aryana and colleagues examined the efficacy of posterior wall isolation using cryoballoon for persistent AF. In their analysis of 168 patients in the PV isolation-alone group and 222 patients in the PV plus posterior wall isolation group, the group with posterior wall isolation had a significantly lower recurrence rate of AF (33). In a study involving 25 patients with persistent AF, pulsed field ablation was used to achieve PVI plus left atrial posterior wall isolation, showing a 100% acute success rate for both PV and posterior wall isolation. The long-term durability of the posterior wall was also 100% (34).
Outcomes after hybrid ablation for AF
Cardiac surgeons and electrophysiologists contribute independently but complementary to hybrid ablation. The surgical ablation offers the advantages of excising or closing the left atrial appendage and addressing interventions on the ligament of Marshall. The excision or closure of the left atrial appendage is a highly reliable strategy for preventing cardioembolic stroke in AF patients, particularly those for whom discontinuation of anticoagulants is desirable (35,36). Lesion durability created during surgical ablation will be discussed later.
On the other hand, endocardial catheter ablation excels in detailed evaluation of intracardiac electrogram, with 3D mapping systems and multi-electrodes catheters. The thoracoscopic approach of surgical ablation involves limited access, making comprehensive mapping challenging. Endocardial ablation, in contrast, allows for mapping of all areas on the right and left atrial endocardium, aiding in clarifying incomplete lesions of epicardial ablation and completing the lesion sets such as PVI, posterior wall isolation, mitral isthmus line or superior vena cava isolation. Catheter ablation can treat non-PV foci originating from the coronary sinus ostium and atrial septum by point ablation rather than anatomical ablation (i.e., electrical isolation or linear ablation). Benefits of hybrid ablation, which combines surgical ablation and catheter ablation, are summarized in Figure 1.
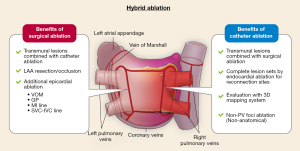
Treatment outcomes after hybrid ablation have been reported in several studies. In a single-center prospective study from the Netherlands conducted in 2019, a single-stage hybrid ablation was performed on 50 patients with persistent AF or failed catheter ablation. This procedure involved thoracoscopic PVI, left atrial roof and bottom line ablation, followed by catheter ablation during the same session. If the surgical ablation site was not isolated, additional ablation was performed to complete the isolation of pulmonary veins and left atrial posterior wall. For cases with AF lasting even after the completion of pulmonary veins and left atrial posterior wall, CFAE ablation was done, followed by cavo-tricuspid isthmus ablation. The atrial tachy-arrhythmia-free rate off anti-arrhythmic drugs at 12 months after a single procedure was 76% (38 out of 50 patients) (37).
The CONVERGE trial, published in 2020, was conducted in 27 hospitals in the United States and the United Kingdom and included 153 patients (58% with persistent AF and 42% with long-standing persistent AF). This was a randomized controlled trial that allocated patients in a 2:1 ratio to hybrid ablation and catheter ablation groups. The hybrid ablation involved epicardial ablation for PVI and posterior left atrial wall isolation, followed by catheter ablation during the same session. Additional ablation was performed as needed to complete electrical isolation of pulmonary veins and the posterior wall, followed by cavo-tricuspid isthmus ablation. In the catheter ablation group, PVI, roof line ablation, CFAE ablation, and cavo-tricuspid isthmus ablation were performed. The atrial tachy-arrhythmia-free rate at 12 months, off antiarrhythmic drugs, after a single procedure was 53.5% in the hybrid ablation group and 32.0% in the catheter ablation group (P=0.0128) (38).
In 2020, Haywood and colleagues published the outcomes of hybrid ablation in 166 patients (41% with persistent AF and 59% with long-standing persistent AF) from four European centers. In this study, catheter ablation was performed as a staged procedure, with a median duration of 92.5 days from surgical ablation to catheter ablation. At the 18-month average follow-up, 56% (93 out of 166 patients) were free from atrial tachyarrhythmia off anti-arrhythmic drugs. An analysis of 56 patients with implantable loop recorders at a mean of 14 months showed that 60% of patients remained fully arrhythmia-free, and 80% had an AF burden of less than 5%. This study is important as it demonstrated a reduction in AF burden even in cases presenting with AF recurrence (39). In 2022, Bhatia and colleagues presented the outcomes of hybrid ablation in 64 patients (12.5% with paroxysmal AF, 25% with persistent AF, and 62.5% with long-standing persistent AF). Epicardial ablation included PVI, posterior wall isolation, excision of the ligament of Marshall, and left atrial appendage closure. Out of the 64 patients, 36 experienced a recurrence of AF, but the remaining 28 did not. Regardless of recurrence, all 64 patients underwent endocardial ablation. Catheter ablation treatment was a patient-tailored procedure, including ablation in low voltage zones (n=6), ablation of AF drivers (n=21), and treatment at sites of reconnection following surgical ablation. As a result, the atrial tachyarrhythmia-free rate of anti-arrhythmic drugs at 12 months was 73% (40). Up to this point, the outcomes of hybrid ablation studies have been single-arm or have involved variations in lesion sets. A recent meta-analysis examined 34 studies comparing hybrid and catheter ablation for persistent or longstanding persistent AF. The analysis included a total of 520 articles, with the selected studies comprising a patient population of 49,759. Hybrid ablation, which combines a thoracoscopic epicardial and transvenous endocardial approach, demonstrated superior efficacy in maintaining sinus rhythm compared to catheter ablation (70.7% vs. 49.9%, P<0.001). The conclusion emphasizes the necessity for large randomized controlled trials directly comparing hybrid and catheter ablation to draw definitive conclusions (41).
The HARTCAP-AF trial, published in 2023, is a randomized controlled trial that directly compared hybrid ablation and catheter ablation with the same lesion set. In this trial, 19 patients with an average duration of AF of 22 months underwent hybrid ablation. Surgical ablation included PVI, posterior wall isolation, and left atrial appendage resection or closure, followed by additional catheter ablation treatment to confirm the isolation of pulmonary veins and posterior walls and provide additional ablation for clinically documented atrial tachycardia or flutter if necessary. For the 22 patients with an average duration of AF of 33 months who underwent catheter ablation, the same lesion set treatment was performed. The primary endpoint was freedom from any atrial tachyarrhythmias lasting more than 5 minutes off anti-arrhythmic drugs after 12 months. In the hybrid ablation group, the rate was 89%, compared to 41% in the catheter ablation group (P=0.002) (42). The CEASE-AF trial is a multi-center, randomized controlled trial, investigating the effectiveness of hybrid ablation compared to endocardial catheter ablation in patients with persistent and longstanding persistent AF. The study involved 154 patients, with 102 undergoing hybrid ablation and 52 undergoing catheter ablation. Primary effectiveness, evaluated as freedom from AF/atrial flutter/atrial tachycardia >30 s through 12 months without anti-arrhythmic drugs, was significantly higher in the hybrid ablation group (71.6%) compared to the catheter ablation group (39.2%) with an absolute benefit increase of 32.4%. Major complications were similar between groups. This more recent study showed that hybrid ablation is more effective than catheter ablation in maintenance of sinus rhythm in persistent and long-standing persistent AF without a significant increase in procedural risk (43).
These clinical studies are observational studies or randomized controlled trials involving a small number of cases from experienced centers. The current recommendation for surgical ablation in the European Society of Cardiology guidelines is Class IIa or IIb. For patients with a history of failed catheter ablation for anti-arrhythmic drug-refractory AF, it has a class IIa indication (44). In the latest 2023 American College of Cardiology (ACC)/American Heart Association (AHA)/American College of Clinical Pharmacy (ACCP)/Heart Rhythm Society (HRS) guideline for the diagnosis and management of AF, concomitant surgical ablation during cardiac surgery is a class IIa recommendation, while hybrid ablation has a class IIb recommendation (45). On the other hand, there is no specific recommendation regarding hybrid ablation in the Japanese Circulation Society (JCS)/Japanese Heart Rhythm Society (JHRS) guideline. Concomitant surgical ablation is a class I recommendation, and stand-alone surgical ablation has a class IIa recommendation for patients with symptomatic AF without structural heart disease and those with AF after catheter ablation (46). Further investigations involving a larger number of patients are needed in the future to assess the effectiveness of hybrid ablation.
Timing of catheter ablation (simultaneous or staged catheter ablation)
Several differences exist between performing hybrid ablation in a single stage (i.e., simultaneous endocardial ablation) or in separated stages (i.e., staged endocardial ablation). The advantage of simultaneous surgical and endocardial catheter-based ablation is that cardiac surgeons and electrophysiologists can collaborate in real time, and the treatment can be completed in a single procedure. However, disadvantages include longer anesthesia time, performing catheter ablation in an operating room, an unusual environment for electrophysiologists, and difficulties in evaluating accurate potentials due to postoperative myocardial edema. Staged endocardial catheter-based ablation offers the advantages of accurate potential evaluation since mapping can be performed after the acute edema from surgical ablation has resolved. However, the disadvantage is that patients require treatment in two stages. After the first surgical ablation, patients are discharged, and some cases may present with palpitations due to an incomplete lesion set or reconnection before the staged catheter ablation.
Durability of epicardial ablation for persistent AF
Recurrence patterns after surgical ablation include not only AF recurrence but also atrial tachycardia and atrial flutter recurrence (47). Cases requiring the cavo-tricuspid isthmus line/mitral isthmus line ablation are relatively common (39). Representative cases are shown in Figures 2,3. Previous reports on staged catheter ablation after surgical ablation for AF highlighted that the roof line as most common reconnection site after epicardial ablation. In evaluations of endocardial mapping performed 3–8 weeks after endocardial ablation, pulmonary vein reconnection was observed in 28.1% of cases, and the reconnection rate in the left atrial posterior wall was 59.4% (34). The durability of surgical PVI and left atrial posterior wall isolation shows room for improvement.
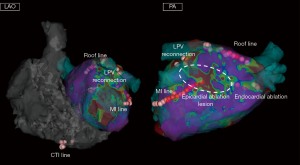
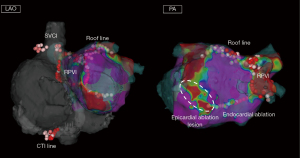
Another study that conducted staged catheter ablation reported that only 25% did not show pulmonary vein or posterior wall reconnection after surgical PVI and posterior wall isolation. The roof line had the highest reconnection rate at 36%, followed by right pulmonary vein reconnection at 28%, left pulmonary vein reconnection at 22%, and bottom line reconnection at 14% (39). As for lesion durability after endocardial catheter ablation using RF energy, limited chronic posterior wall isolation line durability is shown. The PeAF-Box study investigated the lesion durability of endocardial PVI and posterior wall isolation using ablation catheters with RF energy. Twenty-four patients underwent circumferential PVI and posterior wall isolation with RF energy. At the 6-month reassessment, the rate of reconnection of PVI line and posterior wall isolation line is 15% and 54%, respectively (48). In current practice, high-power short-duration ablation or pulsed field catheter-based ablation may be considered superior in terms of the durability of PVI. On the other hand, regarding left atrial posterior wall isolation, surgical ablation might offer significant benefits. In order to avoid atrio-esophageal fistula formation, the floor line often receives insufficient RF application when performing endocardial posterior wall isolation. However, reconnection of the floor line close to the esophagus after epicardial posterior wall isolation is less common, which allows for easy re-isolation of the posterior wall from the endocardial side. Thus, combined epicardial-endocardial posterior wall isolation may result in more durable transmural lesions, which contribute to a high postoperative sinus rhythm maintenance rate. Data regarding lesion durability after surgical ablation is available. However, there is no data regarding the long-term lesion durability after staged catheter ablation, which needs to be investigated in the future.
Posterior wall isolation of hybrid AF ablation
In hybrid AF ablation, there are several ways to thoracoscopically isolate the posterior wall, including (I) the posterior box (single ring); (II) PVI with connecting roof and floor lines; and (III) posterior left atrial ablation. For the creation of a posterior box, two devices are commonly used to create this lesion set. The COBRA fusion (AtriCure) is a non-clamping, flexible device that wraps around the pulmonary venous antrum in a circumferential fashion. Non-irrigated RF energy is delivered in alternating unipolar and bipolar cycles to furnish as continuous a lesion set as possible. The Gemini-S (Medtronic) ablation system is an irrigated RF clamp that uses irrigated bipolar energy to enhance the creation of transmural lesions. For both devices, multiple applications around the pulmonary venous antrum are generally needed to achieve a contiguous lesion set. Technical differences exist between the two devices. Extensive dissection of the oblique and transverse pericardial sinuses is required with use of a clamp-based device, whereas less dissection in this region is needed when a non-clamping device is used. Also, completion of the epicardial lesion set can be achieved with a unilateral thoracoscopic approach with a non-clamping device, whereas a bilateral thoracoscopic approach is often needed with use of a clamp-based device. In the third ablation strategy (posterior left atrial ablation), a small subxiphoid incision is made to create a pericardial window for passage of the pericardioscope and ablation tool. The posterior left atrial epicardial surface is ablated using the EPI-sense device (AtriCure), which delivers unipolar energy and has a built-in vacuum to maximize contact with the epicardial surface. This approach requires a single access site, making it potentially advantageous over other techniques that require multiple access ports (49).
There is a difference between thoracoscopic and subxiphoid hybrid AF ablation. Thoracoscopic hybrid ablation provides direct visualization of the heart, enabling precise lesion creation and yielding high success rates, especially when combined with endocardial catheter ablation. However, its invasiveness, involving thoracoscopic surgery, introduces additional risks compared to catheter-based methods. In contrast, subxiphoid hybrid ablation offers a less invasive alternative, minimizing postoperative pain through a smaller incision below the xiphoid process. The single-port access simplifies the procedure, but two limitations are associated with the subxiphoid approach. Firstly, anatomic constraints imposed by the transverse and oblique sinuses restrict the extent of posterior left atrial ablation, particularly affecting the roof aspect. Secondly, achieving PVI is challenging due to the inability to perform circumferential ablation, constrained by pericardial reflections. Consequently, additional endocardial ablation is frequently necessary to accomplish PVI with this approach (50).
Complications associated with hybrid ablation
The complication rate of hybrid ablation is reported to be around 7–20%, which is 2.0–3.6-fold higher compared to catheter ablation (37-40,49). Furthermore, the majority of complications associated with hybrid ablation occur during the surgical part. Complications that may occur include bleeding, pulmonary vein injury, irreversible phrenic nerve injury, pleural effusion, pericarditis, stroke, pericardial effusion requiring drainage, conversion to sternotomy and bradycardia requiring pacemaker implantation. According to the meta-analysis, published in 2019, including 34 studies with 49,759 patients, hybrid ablation showed a slightly higher overall complication rate than catheter ablation. Specific complication rates of hybrid ablation and catheter ablation included bleeding requiring transfusion (1.6% vs. 0.4%, P<0.001), cardiac tamponade (1.7% vs. 0.7%, P=0.049), hospital mortality (1.1% vs. 0.5%, P=0.007), pacemaker implantation (1.0% vs. 0.4%, P=0.041), phrenic nerve injury (1.2% vs. 0.4%, P=0.002), pneumothorax (1.0% vs. 0.4%, P=0.033), and PV stenosis not requiring stenting (1.0% vs. 0.4%, P=0.007) (41). However, recent reports suggest that, with the advancement of treatment devices and increased treatment experience in recent years, the serious complication rate has decreased in the contemporary study of hybrid ablation. In the HARTCAP-AF study published in 2023, the incidence of major complications was 5% for both hybrid ablation and catheter ablation, with no statistically significant difference observed. The median radiation dose was 31 cGycm2 in the hybrid ablation group compared to 67 cGycm2 in the catheter ablation group, showing a statistically significant difference (P<0.001) (42). Similarly, in the CEASE-AF trial published in 2023, the rate of major complication was 7.8% in hybrid ablation and 5.8% in catheter ablation, which did not show a statistically significant difference (43).
Intervention for left atrial appendage in hybrid ablation
When performing hybrid ablation, it is common to include left atrial appendage resection or closure in addition to the surgical ablation. This intervention on the left atrial appendage is one of the most crucial procedures in hybrid ablation. The left atrial appendage is the most frequent site for the formation of intracardiac thrombi, and the resection or closure of the left atrial appendage in non-valvular AF patients is the most reliable prevention against AF-related strokes.
Ohtsuka et al. presented follow-up data for thoracoscopic left atrial appendage closure in 201 non-valvular AF patients. They reported a 100% success rate in the removal of the left atrial appendage in all patients. Despite a high average CHA2DS2-VASc score of 4.7, the rate of cardioembolic stroke occurrence without anticoagulation therapy was 0.25 events per 100 patient-years (51). No major complications were observed, ensuring the safety of the procedure (52). A more recent randomized control study, the LAAOS III study, demonstrated that left atrial appendage occlusion reduces ischemic stroke or systemic embolism risk in AF patients undergoing cardiac surgery. This analysis examined left atrial appendage occlusion’s impact on stroke risk concerning oral anticoagulant (OAC) therapy variation. Among the 3,027 discharged patients, 63.5% received vitamin K antagonists, and 18.5% received direct OACs. The consistent reduction in stroke risk with left atrial appendage occlusion persisted across OAC-use categories: hazard ratios of 0.70, 0.63, and 0.76. Importantly, left atrial appendage occlusion conferred thromboembolism reduction independently of OAC use (36). In the latest 2023 ACC/AHA/ACCP/HRS guideline, left atrial appendage closure in patients with AF and CHA2DS2-VASc ≥2 undergoing cardiac surgery is a class I recommendation (45).
While the primary goal of interventions on the left atrial appendage is to prevent intracardiac thrombi and related cardioembolic strokes, there may be additional benefits. The left atrial appendage serves as a non-PV foci source (53). Inamura et al. published a paper regarding the origin of non-PV foci in 2,967 patients who underwent ablation for paroxysmal or persistent AF (26). They identified non-PV foci in 564 patients (19.2%) using a high-dose isoproterenol challenge, with 113 patients having a left atrial origin of non-PV foci. Among these 113 patients, 14 had left atrial appendage-origin non-PV foci, suggesting the possibility of treating left atrial appendage-origin AF triggers by resecting the left atrial appendage. The BELIEF trial was a randomized controlled trial involving 173 patients with long-standing persistent AF, and it demonstrated the effectiveness of empirical electrical isolation of the left atrial appendage in reducing AF recurrence (54). Improvements in blood pressure control have also been reported after left atrial appendage resection. When compared to percutaneous left atrial appendage closure, surgical left atrial appendage exclusion significantly lowered systolic blood pressure at 3 and 12 months (55,56). A downregulation of the renin-aldosterone-angiotensin system has been suggested as a mechanism for the blood pressure-lowering effect, and this improvement in comorbidities, such as blood pressure, might contribute to the prevention of AF recurrence.
Conclusions
Hybrid ablation is a novel therapeutic option that combines minimally invasive surgical ablation with endocardial catheter-based ablation. Existing literature shows favorable outcomes and higher complication rates of hybrid ablation compared to catheter ablation. PVI can be performed by both surgical and catheter ablation; however, the durability of posterior wall isolation is better in hybrid ablation. Additionally, the advantages of hybrid ablation include left atrial appendage excision or additional interventions for the vein of Marshall or mitral isthmus line, contributing to the favorable outcomes of hybrid ablation. Nevertheless, hybrid ablation is not recommended for all AF patients because it is more invasive compared to catheter ablation, and some patients with persistent AF maintain sinus rhythm with catheter ablation alone. At present, hybrid ablation remains a treatment option performed in experienced centers for selected patients. More clinical data are required to determine which patients are suitable candidates for hybrid ablation.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Hiroshi Kubota) for the series “Surgical Treatment of Arrhythmias” published in Journal of Thoracic Disease. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1671/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1671/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1671/coif). The series “Surgical Treatment of Arrhythmias” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Scheinman MM, Morady F, Hess DS, et al. Catheterinduced ablation of the atrioventricular junction to control refractory supraventricular arrhythmias. JAMA 1982;248:851-5.
- Bolling SF, Morady F, Calkins H, et al. Current treatment for Wolff-Parkinson-White syndrome: results and surgical implications. Ann Thorac Surg 1991;52:461-8. [Crossref] [PubMed]
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659-66. [Crossref] [PubMed]
- Ditac G, Cottinet PJ, Quyen Le M, et al. Carbon footprint of atrial fibrillation catheter ablation. Europace 2023;25:331-40. [Crossref] [PubMed]
- Cox JL, Schuessler RB, D'Agostino HJ Jr, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991;101:569-83.
- Cox JL, Jaquiss RD, Schuessler RB, et al. Modification of the maze procedure for atrial flutter and atrial fibrillation. II. Surgical technique of the maze III procedure. J Thorac Cardiovasc Surg 1995;110:485-95. [Crossref] [PubMed]
- Voeller RK, Bailey MS, Zierer A, et al. Isolating the entire posterior left atrium improves surgical outcomes after the Cox maze procedure. J Thorac Cardiovasc Surg 2008;135:870-7. [Crossref] [PubMed]
- Khiabani AJ, MacGregor RM, Bakir NH, et al. The long-term outcomes and durability of the Cox-Maze IV procedure for atrial fibrillation. J Thorac Cardiovasc Surg 2022;163:629-641.e7. [Crossref] [PubMed]
- Poole JE, Bahnson TD, Monahan KH, et al. Recurrence of Atrial Fibrillation After Catheter Ablation or Antiarrhythmic Drug Therapy in the CABANA Trial. J Am Coll Cardiol 2020;75:3105-18. [Crossref] [PubMed]
- Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon Ablation as Initial Therapy for Atrial Fibrillation. N Engl J Med 2021;384:316-24. [Crossref] [PubMed]
- Das M, Loveday JJ, Wynn GJ, et al. Ablation index, a novel marker of ablation lesion quality: prediction of pulmonary vein reconnection at repeat electrophysiology study and regional differences in target values. Europace 2017;19:775-83. [Crossref] [PubMed]
- Whitaker J, Fish J, Harrison J, et al. Lesion Index-Guided Ablation Facilitates Continuous, Transmural, and Durable Lesions in a Porcine Recovery Model. Circ Arrhythm Electrophysiol 2018;11:e005892. [Crossref] [PubMed]
- Sulkin MS, Laughner JI, Hilbert S, et al. Novel Measure of Local Impedance Predicts Catheter-Tissue Contact and Lesion Formation. Circ Arrhythm Electrophysiol 2018;11:e005831. [Crossref] [PubMed]
- Taghji P, El Haddad M, Phlips T, et al. Evaluation of a Strategy Aiming to Enclose the Pulmonary Veins With Contiguous and Optimized Radiofrequency Lesions in Paroxysmal Atrial Fibrillation: A Pilot Study. JACC Clin Electrophysiol 2018;4:99-108. [Crossref] [PubMed]
- Phlips T, Taghji P, El Haddad M, et al. Improving procedural and one-year outcome after contact forceguided pulmonary vein isolation: the role of interlesion distance, ablation index, and contact force variability in the 'CLOSE'-protocol. Europace 2018;20:f419-27. [Crossref] [PubMed]
- O'Brien J, Obeidat M, Kozhuharov N, et al. Procedural efficiencies, lesion metrics, and 12-month clinical outcomes for Ablation Index-guided 50W ablation for atrial fibrillation. Europace 2021;23:878-86. [Crossref] [PubMed]
- Müller J, Nentwich K, Berkovitz A, et al. Acute oesophageal safety and long-term follow-up of AI-guided high-power short-duration with 50 W for atrial fibrillation ablation. Europace 2023;25:1379-91. [Crossref] [PubMed]
- O'Neill L, El Haddad M, Berte B, et al. Very High-Power Ablation for Contiguous Pulmonary Vein Isolation: Results From the Randomized POWER PLUS Trial. JACC Clin Electrophysiol 2023;9:511-22. [Crossref] [PubMed]
- Kistler PM, Chieng D, Sugumar H, et al. Effect of Catheter Ablation Using Pulmonary Vein Isolation With vs Without Posterior Left Atrial Wall Isolation on Atrial Arrhythmia Recurrence in Patients With Persistent Atrial Fibrillation: The CAPLA Randomized Clinical Trial. JAMA 2023;329:127-35. [Crossref] [PubMed]
- Fink T, Schlüter M, Heeger CH, et al. Stand-Alone Pulmonary Vein Isolation Versus Pulmonary Vein Isolation With Additional Substrate Modification as Index Ablation Procedures in Patients With Persistent and Long-Standing Persistent Atrial Fibrillation: The Randomized AlsterLost-AF Trial (Ablation at St. Georg Hospital for LongStanding Persistent Atrial Fibrillation). Circ Arrhythm Electrophysiol 2017;10:e005114. [Crossref] [PubMed]
- Ngo L, Lee XW, Elwashahy M, et al. Freedom from atrial arrhythmia and other clinical outcomes at 5 years and beyond after catheter ablation of atrial fibrillation: a systematic review and meta-analysis. Eur Heart J Qual Care Clin Outcomes 2023;9:447-58. [Crossref] [PubMed]
- Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812-22. [Crossref] [PubMed]
- Moustafa A, Karim S, Kahaly O, et al. Low voltage area guided substrate modification in nonparoxysmal atrial fibrillation: A systematic review and meta-analysis. J Cardiovasc Electrophysiol 2023;34:455-64. [Crossref] [PubMed]
- Yang G, Zheng L, Jiang C, et al. Circumferential Pulmonary Vein Isolation Plus Low-Voltage Area Modification in Persistent Atrial Fibrillation: The STABLE-SR-II Trial. JACC Clin Electrophysiol 2022;8:882-91. [Crossref] [PubMed]
- Marrouche NF, Wazni O, McGann C, et al. Effect of MRI-Guided Fibrosis Ablation vs Conventional Catheter Ablation on Atrial Arrhythmia Recurrence in Patients With Persistent Atrial Fibrillation: The DECAAF II Randomized Clinical Trial. JAMA 2022;327:2296-305. [Crossref] [PubMed]
- Inamura Y, Nitta J, Inaba O, et al. Presence of nonpulmonary vein foci in patients with atrial fibrillation undergoing standard ablation of pulmonary vein isolation: Clinical characteristics and long-term ablation outcome. Int J Cardiol Heart Vasc 2021;32:100717. [Crossref] [PubMed]
- Sau A, Kapadia S, Al-Aidarous S, et al. Temporal Trends and Lesion Sets for Persistent Atrial Fibrillation Ablation: A Meta-Analysis With Trial Sequential Analysis and Meta-Regression. Circ Arrhythm Electrophysiol 2023;16:e011861. [Crossref] [PubMed]
- Lee JM, Shim J, Park J, et al. The Electrical Isolation of the Left Atrial Posterior Wall in Catheter Ablation of Persistent Atrial Fibrillation. JACC Clin Electrophysiol 2019;5:1253-61. [Crossref] [PubMed]
- Thiyagarajah A, Kadhim K, Lau DH, et al. Feasibility, Safety, and Efficacy of Posterior Wall Isolation During Atrial Fibrillation Ablation: A Systematic Review and Meta-Analysis. Circ Arrhythm Electrophysiol 2019;12:e007005. [Crossref] [PubMed]
- Arai T, Fukamizu S, Kitamura T, et al. Residual potential at the epicardial left atrium after conventional left atrial posterior wall isolation for persistent atrial fibrillation: A case report. J Arrhythm 2020;36:808-10. [Crossref] [PubMed]
- Kujiraoka H, Hojo R, Arai T, et al. Association between residual unipolar voltage and arrhythmia recurrence after left atrial posterior wall isolation for persistent atrial fibrillation. J Cardiovasc Electrophysiol 2023;34:1622-9. [Crossref] [PubMed]
- Rao S, Kwasnik A, Tung R. Direct Epicardial Recordings in the Region of the Septopulmonary Bundle: Anatomy "Behind" Posterior Wall Activation. JACC Clin Electrophysiol 2020;6:1214-6. [Crossref] [PubMed]
- Aryana A, Baker JH, Espinosa Ginic MA, et al. Posterior wall isolation using the cryoballoon in conjunction with pulmonary vein ablation is superior to pulmonary vein isolation alone in patients with persistent atrial fibrillation: A multicenter experience. Heart Rhythm 2018;15:1121-9. [Crossref] [PubMed]
- Reddy VY, Anic A, Koruth J, et al. Pulsed Field Ablation in Patients With Persistent Atrial Fibrillation. J Am Coll Cardiol 2020;76:1068-80. [Crossref] [PubMed]
- Whitlock RP, Belley-Cote EP, Paparella D, et al. Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke. N Engl J Med 2021;384:2081-91. [Crossref] [PubMed]
- Connolly SJ, Healey JS, Belley-Cote EP, et al. Oral Anticoagulation Use and Left Atrial Appendage Occlusion in LAAOS III. Circulation 2023;148:1298-304. [Crossref] [PubMed]
- Al-Jazairi MIH, Rienstra M, Klinkenberg TJ, et al. Hybrid atrial fibrillation ablation in patients with persistent atrial fibrillation or failed catheter ablation. Neth Heart J 2019;27:142-51. [Crossref] [PubMed]
- DeLurgio DB, Crossen KJ, Gill J, et al. Hybrid Convergent Procedure for the Treatment of Persistent and Long-Standing Persistent Atrial Fibrillation: Results of CONVERGE Clinical Trial. Circ Arrhythm Electrophysiol 2020;13:e009288. [Crossref] [PubMed]
- Haywood GA, Varini R, Osmancik P, et al. European multicentre experience of staged hybrid atrial fibrillation ablation for the treatment of persistent and longstanding persistent atrial fibrillation. Int J Cardiol Heart Vasc 2020;26:100459. [Crossref] [PubMed]
- Bhatia NK, Shah RL, Deb B, et al. Mapping Atrial Fibrillation After Surgical Therapy to Guide Endocardial Ablation. Circ Arrhythm Electrophysiol 2022;15:e010502. [Crossref] [PubMed]
- van der Heijden CAJ, Vroomen M, Luermans JG, et al. Hybrid versus catheter ablation in patients with persistent and longstanding persistent atrial fibrillation: a systematic review and meta-analysis†. Eur J Cardiothorac Surg 2019;56:433-43. [Crossref] [PubMed]
- van der Heijden CAJ, Weberndörfer V, Vroomen M, et al. Hybrid Ablation Versus Repeated Catheter Ablation in Persistent Atrial Fibrillation: A Randomized Controlled Trial. JACC Clin Electrophysiol 2023;9:1013-23. [Crossref] [PubMed]
- Doll N, Weimar T, Kosior DA, et al. Efficacy and safety of hybrid epicardial and endocardial ablation versus endocardial ablation in patients with persistent and longstanding persistent atrial fibrillation: a randomised, controlled trial. EClinicalMedicine 2023;61:102052. [Crossref] [PubMed]
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373-498. [Crossref] [PubMed]
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024;149:e1-e156. [Crossref] [PubMed]
- Nogami A, Kurita T, Abe H, et al. JCS/JHRS 2019 Guideline on Non-Pharmacotherapy of Cardiac Arrhythmias. Circ J 2021;85:1104-244. Erratum in: Circ J 2021;85:1692-700. [Crossref] [PubMed]
- Kataoka S, Kato K, Tanaka H, et al. Atrial tachycardia originating from an incompletely isolated box lesion in a patient undergoing thoracoscopic left atrial appendectomy and surgical ablation for long-standing persistent atrial fibrillation. J Cardiol Cases 2018;18:25-8. [Crossref] [PubMed]
- Worck R, Sørensen SK, Johannessen A, et al. Posterior wall isolation in persistent atrial fibrillation feasibility, safety, durability, and efficacy. J Cardiovasc Electrophysiol 2022;33:1667-74. [Crossref] [PubMed]
- Bisleri G, Pandey AK, Verma S, et al. Combined Minimally Invasive Surgical and Percutaneous Catheter Ablation of Atrial Fibrillation: JACC Review Topic of the Week. J Am Coll Cardiol 2023;81:606-19. [Crossref] [PubMed]
- Syed FF, Oral H. Electrophysiological Perspectives on Hybrid Ablation of Atrial Fibrillation. J Atr Fibrillation 2015;8:1290. [Crossref] [PubMed]
- Ohtsuka T, Nonaka T, Hisagi M, et al. Thoracoscopic stapler-and-loop technique for left atrial appendage closure in nonvalvular atrial fibrillation: Mid-term outcomes in 201 patients. Heart Rhythm 2018;15:1314-20. [Crossref] [PubMed]
- Ohtsuka T, Ninomiya M, Nonaka T, et al. Thoracoscopic stand-alone left atrial appendectomy for thromboembolism prevention in nonvalvular atrial fibrillation. J Am Coll Cardiol 2013;62:103-7. [Crossref] [PubMed]
- Santangeli P, Marchlinski FE. Techniques for the provocation, localization, and ablation of non-pulmonary vein triggers for atrial fibrillation. Heart Rhythm 2017;14:1087-96. [Crossref] [PubMed]
- Di Biase L, Burkhardt JD, Mohanty P, et al. Left Atrial Appendage Isolation in Patients With Longstanding Persistent AF Undergoing Catheter Ablation: BELIEF Trial. J Am Coll Cardiol 2016;68:1929-40. [Crossref] [PubMed]
- Lakkireddy D, Turagam M, Afzal MR, et al. Left Atrial Appendage Closure and Systemic Homeostasis: The LAA HOMEOSTASIS Study. J Am Coll Cardiol 2018;71:135-44. [Crossref] [PubMed]
- Turagam MK, Vuddanda V, Verberkmoes N, et al. Epicardial Left Atrial Appendage Exclusion Reduces Blood Pressure in Patients With Atrial Fibrillation and Hypertension. J Am Coll Cardiol 2018;72:1346-53. [Crossref] [PubMed]


