
Low-normal free triiodothyronine as a predictor of post-operative atrial fibrillation after surgical coronary revascularization
Highlight box
Key findings
• Low-normal free triiodothyronine (FT3) is an independent predictor of post-operative atrial fibrillation (POAF) after model adjustment. The hospital stays of patients who developed POAF were longer.
What is known and what is new?
• Recent studies have focused on the association between thyroid function within normal range and cardiovascular diseases, especially on FT3 levels.
• This study aims to evaluate the effects of normal FT3 level on new-onset atrial fibrillation (AF) in patients with surgical coronary revascularization.
What is the implication, and what should change now?
• The findings may help to identify patients with surgical coronary revascularization at a higher risk for the development of AF and provide clinical significance in thyroid function before the operation and even redefinition of the optimal reference range.
Introduction
New-onset atrial fibrillation (AF) is the most common complications after coronary artery bypass graft (CABG) with a reported incidence of 20–40% (1). Post-operative AF (POAF) is associated with increased stroke, life-threatening ventricular arrhythmia, mortality and health-care costs (2,3). It is important to identify patients at high risk of developing AF following surgical coronary revascularization.
Thyroid function abnormalities are intimately correlated with cardiovascular diseases. Thyroid hormone consists of thyroxine (T4) and triiodothyronine (T3), and the cardiomyocytes does not convert T4 to T3. In other word, T3 is the bioactive form of thyroid hormone for cardiomyocytes and plays an important role in cardiovascular regulation (4). Hypothyroidism has been found to be linked to an increased incidence of POAF in a prospective study (5). Even in euthyroid subjects with normal serum thyroid-stimulating hormone (TSH), free-triiodothyronine (FT3), free-thyroxine (FT4), high-normal FT4 concentrations have been shown to be associated with increased risk of AF (6).
More importantly, recent studies have focused on the role of FT3 level within the normal range in cardiovascular diseases and the long-term prognosis. Low-normal FT3 level aggravated cardiac dysfunction and was associated with poor prognosis in euthyroid patients with heart failure (7,8). Recent study reported that both high and low FT3 levels were associated with AF recurrence after catheter ablation (9). Additionally, T3 administration in patients before CABG improved cardiac output but did not alter the requirement of standard postoperative therapy (10).
Until now, no studies have been published about the impact of normal FT3 levels on POAF. Accordingly, this study aims to assess the relationship between serum FT3 levels within the normal range and the risk of AF following surgical coronary revascularization. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1646/rc).
Methods
Participants
From January 2017 through October 2022, 503 eligible patients who underwent surgery coronary revascularization, including off-pump CABG and one-stop hybrid coronary revascularization, were retrospectively enrolled in our study. We excluded the patients with abnormal thyroid function, prior thyroid disease, abnormal electrolyte, atrial arrhythmia, valvular disease and cardiac pacemaker. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee of Beijing Chaoyang Hospital, Capital Medical University (No. 2019-ke-372) and individual consent for this retrospective analysis was waived.
Thyroid function
We evaluated thyroid function by measuring serum FT3, FT4 and TSH levels. Pre-operative thyroid function was tested on the first day of hospitalization. Serum FT3 level was divided into quartile groups. The normal range of FT3 level was 2.3–4.2 pg/mL.
Clinical data collection and AF diagnosis
Data on patients’ baseline characteristics including age, gender, body mass index (BMI), previous medical history including diabetes mellitus, hypertension and myocardial infarction, echocardiography indicators like left atrial diameter (LAD), left ventricular end-diastolic diameter (LVEDD), left ventricular end-systolic diameter (LVESD) and left ventricular ejection fraction (LVEF), post-operative factors including hemoglobin A1c (HbA1c), brain natriuretic peptide (BNP), post-operative ratio of neutrophile and lymphocyte (post N/L), cardiac troponin I (cTNI), lactate dehydrogenase (LDH) and pre-operative ratio of neutrophile and lymphocyte (pre-N/L) were collected by trained clinicians. The diagnostic POAF was the disappearance of P wave instead of f wave indicated by electrocardiograph monitoring within seven days after surgical coronary revascularization.
Statistical analysis
SPSS software version 26.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis in this study. Figures were created using GraphPad Prism 9.4 (La Jolla, CA, USA). Continuous data that conformed to normal distribution were presented as mean ± standard deviation (SD) and analyzed by independent Student’s t-test while the median interquartile range (IQR) was used for non-normal distribution and performed by non-parametric test. Numerous normally distributed variables were analyzed by One-way analysis of variance (ANOVA) test followed by the Bonferroni post hoc test and the Kruskal-Wallis H test were used for nonparametric tests of multiple independent samples. Categorical variables were shown as percentages and were analyzed using Chi-squared tests or Fisher exact tests. Pearson correlation test was used to analyze the correlation between FT3 levels and age. Receiver operating characteristic (ROC) curve analysis was performed to identify the cutoff value. Kaplan-Meier curves were used to determine AF-free events. Univariate and multivariate regression analyses were performed to evaluate the value of FT3 level in POAF. A P value <0.05 was considered significant.
Results
Baseline characteristics of enrolled participants
A total of 503 patients (396 men and 107 women) were enrolled in this study. New-onset AF occurred in 120 patients (23.86%) which happened at day 2 (IQR, 2, 3). The participants were divided into two groups according to the occurrence of AF (Table 1). Age, post-operative laboratory parameters including BNP and LDH were significantly higher in the AF group compared with the non-AF group (all P<0.05). It was reported that LAD independently associated with AF following CABG (11). Consistently, the present study revealed that LAD was markedly bigger in the AF group (38.83±4.91 vs. 37.13±4.28 mm, P=0.001). Cardiac function showed by LVEF was worse in patients with AF [59% (IQR, 42%, 68%) vs. 64% (IQR, 58.25%, 68%), P=0.01]. AF patients showed longer hospital stays [23 (IQR, 20, 29) vs. 21 (IQR, 17, 25) days; P<0.001]. No significant differences were observed in gender, BMI, thyroid function and serum lipid levels.
Table 1
| Characteristics | All (n=503) | Non-AF (n=383) | AF (n=120) | P value |
|---|---|---|---|---|
| Demographic data | ||||
| Age, years | 63.01±8.67 | 62.09±8.80 | 65.93±7.56 | <0.001 |
| Male | 396 (78.73) | 304 (79.37) | 92 (76.67) | 0.53 |
| BMI, kg/m2 | 25.57±3.27 | 25.72±3.32 | 25.07±3.07 | 0.054 |
| Hypertension | 345 (68.59) | 261 (68.15) | 84 (70.00) | 0.70 |
| Diabetes mellitus | 196 (38.97) | 153 (39.95) | 43 (35.83) | 0.42 |
| Previous myocardial infarction | 36 (7.16) | 26 (6.79) | 10 (8.33) | 0.57 |
| Smoke | 282 (56.06) | 218 (56.92) | 64 (53.33) | 0.49 |
| Blood laboratory parameters | ||||
| FT3, pg/mL | 2.96±0.38 | 2.97±0.37 | 2.90±0.39 | 0.07 |
| FT4, ng/dL | 1.20±0.19 | 1.20±0.19 | 1.20±0.18 | 0.91 |
| TSH, uIU/mL | 1.85 [1.22, 2.80] | 1.77 [1.28, 2.71] | 1.64 [1.06, 2.80] | 0.55 |
| HbA1c, % | 6.75±1.39 | 6.75±1.44 | 6.75±1.21 | 0.99 |
| Uric acid, μmol/L | 323.98±105.80 | 327.79±103.84 | 312.07±111.31 | 0.16 |
| Creatinine, μmol/L | 88.32±38.48 | 87.71±41.46 | 90.28±26.95 | 0.52 |
| Hcy, mmol/L | 16 [13, 20] | 16 [13, 21] | 19 [15, 22] | 0.07 |
| Lipoprotein (a), mg/dL | 9.1 [4.9, 19.43] | 8.8 [4.70, 17.58] | 8.20 [4.40, 17.10] | 0.82 |
| BNP, pg/mL | 131 [54.09, 315] | 115.50 [47.25, 316.80] | 207 [72, 498] | 0.007 |
| Pre-N/L | 2.24 [1.68, 2.91] | 2.06 [1.62, 2.89] | 2.38 [1.65, 3.14] | 0.54 |
| Post-N/L | 7.6 [5.42, 12.17] | 6.98 [5.23, 11.30] | 7.80 [5.20, 12.17] | 0.70 |
| cTNI, ng/mL | 0.73 [0.28, 2.50] | 0.80 [0.27, 2.76] | 1.02 [0.47, 6.93] | 0.059 |
| LDH, U/L | 204 [170, 257] | 191 [166, 239.25] | 236 [205, 296] | <0.001 |
| Echocardiography | ||||
| LAD, mm | 37.54±4.49 | 37.13±4.28 | 38.83±4.91 | 0.001 |
| LVEDD, mm | 49 [46, 53] | 48.5 [45, 52] | 51 [46, 56] | 0.09 |
| LVESD, mm | 31 [28, 36] | 31 [28, 35] | 33 [29, 43] | 0.033 |
| LVEF, % | 63 [56, 68] | 64 [58.25, 68] | 59 [42, 68] | 0.01 |
| Hospital stays, days | 21 [18, 26] | 21 [17, 25] | 23 [20, 29] | <0.001 |
Data are presented as mean ± standard deviation or median [interquartile range] or n (%). AF, atrial fibrillation; BMI, body mass index; FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid-stimulating hormone; HbA1c, hemoglobin A1c; Hcy, homocysteine; BNP, brain natriuretic peptide; pre-N/L, preoperative ratio of neutrophile and lymphocyte; post-N/L, post-operative ratio of neutrophile and lymphocyte; cTNI, cardiac troponin I; LDH, lactate dehydrogenase; LAD, left atrial diameter; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction.
Characteristics of patients according to FT3 quartile
We further divided participants into four groups according to serum FT3 levels. Baseline characteristics of subgroups according to the quartile range of FT3 among those with normal FT3 levels are shown in Table 2. Among patients with normal range of FT3 levels, we performed the analysis of the low-normal FT3 group [the lowest quartile of FT3 levels, quartile 1 (Q1)] with those of remaining patients (quartiles 2–4) in addition to comparisons among the four quartiles. The FT3 Q1 group included older age (65.41±8.24 vs. 61.78±8.64 years), lower BMI (25.02±3.59 vs. 25.85±3.06 kg/m2) and fewer male patients (69.41% vs. 83.48%) than FT3 Q2–4 group (all P<0.05). More importantly, the occurrence of AF was markedly higher in the Q1 group compared with the Q2–4 group (31.18% vs. 20.12%, P=0.006). Additionally, longer hospital stays were observed in the Q1 group [23 (IQR, 19, 28.5) vs. 21 (IQR, 17, 25) days, P=0.001]. Free T4 level in the Q1 group was lower than those in the Q2–4 group. Patients in the FT3 Q1 group had a worse cardiac function, showed by decreased LVEF and increased BNP and severe inflammatory response showed by inflammatory biomarker LDH (all P<0.05). There were no differences in hypertension and cardiac structure parameters between the Q1 group and Q2–4 group.
Table 2
| Characteristics | Q1 (n=170) | Q2 (n=219) | Q3 (n=103) | Q4 (n=11) | P* | Q2–4 (n=333) | P** |
|---|---|---|---|---|---|---|---|
| Demographic data | |||||||
| Age, years | 65.41±8.24 | 62.67±8.18 | 60.42±9.21 | 56.91±9.46 | <0.001 | 61.78±8.64 | <0.001 |
| Male | 118 (69.41) | 170 (77.63) | 97 (94.17) | 11 (100.00) | <0.001 | 278 (83.48) | <0.001 |
| BMI, kg/m2 | 25.02±3.59 | 25.89±3.09 | 25.88±3.01 | 24.69±3.15 | 0.035 | 25.85±3.06 | 0.007 |
| AF | 53 (31.18) | 42 (19.18) | 23 (22.33) | 2 (18.18) | 0.046 | 67 (20.12) | 0.006 |
| Hypertension | 116 (68.24) | 149 (68.04) | 73 (70.87) | 7 (66.64) | 0.94 | 229 (68.77) | 0.90 |
| Diabetes mellitus | 79 (46.47) | 86 (39.27) | 29 (28.16) | 2 (18.18) | 0.011 | 117 (35.14) | 0.014 |
| Previous myocardial infarction | 15 (8.82) | 13 (5.94) | 7 (6.80) | 1 (9.09) | 0.73 | 21 (6.31) | 0.30 |
| Smoke | 79 (46.47) | 121 (55.25) | 74 (71.84) | 8 (72.73) | <0.001 | 203 (60.96) | 0.002 |
| Blood laboratory parameters | |||||||
| FT4, ng/dL | 1.15±0.20 | 1.22±0.18 | 1.23±0.17 | 1.47±0.26 | <0.001 | 1.23±0.18 | <0.001 |
| TSH, uIU/mL | 1.75 [1.11, 3.06] | 1.90 [1.29, 2.63] | 1.69 [1.19, 2.63] | 2.17 [1.33, 3.37] | 0.63 | 1.79 [1.28, 2.70] | 0.99 |
| HbA1c, % | 6.60 [5.80, 8.45] | 6.2 [5.70, 7.28] | 6.1 [5.65, 7.25] | 5.75 [5.30, 6.50] | 0.003 | 6.10 [5.70, 7.20] | 0.003 |
| Uric acid, μmol/L | 315.32±108.13 | 328.71±109.90 | 327.21±91.60 | 334.18±117.20 | 0.63 | 328.43±104.47 | 0.19 |
| Creatinine, μmol/L | 96.50±54.46 | 84.41±24.27 | 84.37±30.10 | 76.35±17.35 | 0.007 | 84.13±25.99 | 0.006 |
| Hcy, mmol/L | 17 [13, 21] | 17 [13.25, 24] | 15 [12, 19.50] | 17.5 [11, 23.50] | 0.62 | 16 [13, 22] | 0.24 |
| Lipoprotein (a), mg/dL | 7.2 [4.85, 16.25] | 8.2 [4.13, 21.23] | 9.7 [6.45, 18.60] | 10.45 [2.43, 17.13] | 0.18 | 9 [4.60, 19.20] | 0.14 |
| BNP, pg/mL | 209.9 [86.5, 532.5] | 111.85 [47, 320.75] | 117 [33.84, 279] | 251.5 [31.5, 530.5] | <0.001 | 114 [45.96, 315] | 0.001 |
| Pre-N/L | 2.3 [1.69, 3.07] | 2.09 [1.65, 2.84] | 2.06 [1.48, 3.11] | 1.54 [1.37, 1.99] | 0.216 | 2.06 [1.56, 2.89] | 0.098 |
| Post-N/L | 6.64 [5.14, 9.43] | 7.24 [4.85, 12.17] | 8.55 [5.56, 12.66] | 7.10 [5.67, 14.51] | 0.23 | 7.34 [5.34, 12.56] | 0.25 |
| cTNI, ng/mL | 1.05 [0.37, 6.48] | 0.62 [0.22, 2.61] | 1.02 [0.42, 2.01] | 0.86 [0.13, 2.13] | 0.44 | 0.73 [0.28, 2.02] | 0.28 |
| LDH, U/L | 211 [179.50, 268] | 198.5 [166, 255.75] | 188 [171, 212] | 225.50 [160.50, 239.25] | 0.003 | 194 [167, 242] | 0.001 |
| Echocardiography | |||||||
| LAD, mm | 37.28±4.76 | 37.70±4.41 | 37.76±4.17 | 36.36±4.92 | 0.61 | 37.67±4.35 | 0.38 |
| LVEDD, mm | 48 [44, 53] | 49 [46, 53] | 50 [47, 51] | 45 [43, 57.75] | 0.10 | 49 [46, 53] | 0.07 |
| LVESD, mm | 31 [29, 36] | 32 [28, 36] | 30 [28.5, 33] | 33 [27.5, 47.5] | 0.75 | 32 [28, 36] | 0.38 |
| LVEF, % | 63 [51, 67] | 63 [56.25, 68] | 65 [60, 68.50] | 56.5 [35.25, 66.50] | 0.18 | 64 [57, 68] | 0.031 |
| Hospital stays, days | 23 [19, 28.50] | 21 [18, 26] | 19 [17, 24] | 20 [16, 25] | <0.001 | 21 [17, 25] | 0.001 |
Data are presented as mean ± standard deviation or median [interquartile range] or n (%). *, comparisons between FT3 quartile groups; **, comparisons between FT3 quartile 1 and quartile 2–4 group. FT3, free triiodothyronine; BMI, body mass index; AF, atrial fibrillation; FT4, free thyroxine; TSH, thyroid-stimulating hormone; HbA1c, hemoglobin A1c; Hcy, homocysteine; BNP, brain natriuretic peptide; pre-N/L, preoperative ratio of neutrophile and lymphocyte; post-N/L, post-operative ratio of neutrophile and lymphocyte; cTNI, cardiac troponin I; LDH, lactate dehydrogenase; LAD, left atrial diameter; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction.
Accumulating incidence of AF in quartiles of FT3 level by using Kaplan-Meier analysis
The lower FT3 group (FT3 Q1) had higher AF occurrence in all patients. A big data analysis suggested that FT3 levels were significantly decreased with age (12). Another study demonstrated a decrease of FT4 conversion to FT3 with age, indicating that the observation may be part of the aging process (13). The present study reported that serum FT3 levels were negatively correlated with age (r=−0.24, P<0.001). Thus, we further performed subgroup analysis based on age and found that the incidence of AF was markedly increased in FT3 Q1 group in older patients (age ≥65 years). However, we further conducted the interaction test and the results showed that there was no significant interaction in age and FT3 in multivariable regression analysis [HR =1.27, 95% confidence interval (CI): 0.59, 2.67, P=0.54]. The above results revealed that patients with lower FT3 levels had a significantly higher AF occurrence, especially in older patients (Figure 1).

Clinical predictors of AF in patients with normal thyroid function
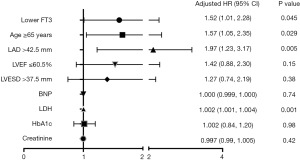
Based on the above results, ROC analysis was performed to identify the cutoff value of age, LAD, LVESD and LVEF. In univariate analysis, lower FT3, age ≥65 years, LAD >42.5 mm, LVEF ≤60.5%, LVESD >37.5 mm, BNP and LDH were significantly associated with POAF (Table 3). After adjusting the model, low-normal FT3 remained an independent predictor of POAF. Moreover, age ≥65 years, LAD >42.5 mm and LDH were also independent predictors of POAF (Table 3, Figure 2).
Table 3
| Variables | Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Lower FT3 (2.3–2.8 pg/mL) | 1.61 | 1.12, 2.31 | 0.009 | 1.52 | 1.01, 2.28 | 0.045 | |
| Age ≥65 years | 1.79 | 1.24, 2.58 | 0.002 | 1.57 | 1.05, 2.35 | 0.029 | |
| LAD >42.5 mm | 2.63 | 1.75, 3.96 | <0.001 | 1.97 | 1.23, 3.17 | 0.005 | |
| LVEF ≤60.5% | 1.75 | 1.22, 2.50 | 0.002 | 1.42 | 0.88, 2.30 | 0.15 | |
| LVESD >37.5 mm | 1.97 | 1.34, 2.89 | 0.001 | 1.27 | 0.74, 2.19 | 0.38 | |
| BNP | 1.001 | 1.000, 1.001 | 0.014 | 1.000 | 0.999, 1.000 | 0.74 | |
| LDH | 1.002 | 1.001, 1.004 | <0.001 | 1.002 | 1.001, 1.004 | 0.001 | |
| HbA1c | 0.99 | 0.87, 1.14 | 0.91 | 1.002 | 0.84, 1.20 | 0.98 | |
| Creatinine | 1.001 | 0.997, 1.005 | 0.56 | 0.997 | 0.99, 1.005 | 0.42 | |
HR, hazard ratio; CI, confidence interval; FT3, free triiodothyronine; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; BNP, brain natriuretic peptide; LDH, lactate dehydrogenase; HbA1c, hemoglobin A1c.
Discussion
In the present study, FT3 level in Q1 (≤2.8 pg/mL) was associated with an increased risk of POAF and was an independent predictor of POAF, with thyroid function within the normal range. The hospital stays of patients who occurred AF were longer. Serum FT3 level was negatively correlated with age.
POAF is the common complication in patients following CABG. It has been reported that POAF is associated with an increased risk of poor prognosis (3). It is important to identify risk factors of POAF in order to better prevent POAF.
Thyroid dysfunction has a great influence on cardiovascular diseases. It is well known that AF is a recognized manifestation of the hyperthyroid state (14). Recent studies have demonstrated that hypothyroidism led to AF. In animal study, hypothyroidism leads to increased AF vulnerability due to shortening of atrial effective refractory period and the fact that thyroid replacement therapy could maintain cardiac electrophysiology and prevent structural remodeling with hypothyroidism (15,16). Moreover, hypothyroidism was found to be associated with an increased risk of AF following cardiac injury (5). Particularly, increasing clinical studies have focused on the association between thyroid function within the normal range and AF. Anderson et al. reported that higher FT4 levels within the normal range were associated with the increased risk of AF (17). A prospective study of 477 individuals found that high-normal TSH level had higher incidence of atrial tachyarrhythmia recurrence after catheter ablation of AF (18). Additional prospective cohort study found that low T3 syndrome was correlated with poor prognosis in heart failure patients (8). Previous systematic review demonstrated that preoperative factors including older age, creatinine level, worse cardiac function, hypertension and perioperative factors like perfusion time, use of inotropes and re-operation were associated with the risk of POAF following CABG (19). Other study monitored the dynamic levels of FT3 and found that decreased tendency in FT3 values was prominent on the postoperative second day, which is the most common day for arrhythmia following cardiac surgery (20). Interestingly, CABG-related euthyroid sick syndrome exerted detrimental effects on in-hospital adverse cardiovascular outcomes (21). Recently, off-pump CABG and one-stop hybrid coronary revascularization become the common effective ways of surgical coronary revascularization, which avoid the negative effects of cardiopulmonary bypass.
Thus, the present study aimed to investigate the association between preoperative thyroid function within the normal range, and POAF in patients with surgical coronary revascularization. This study excluded abnormal thyroid function in order to control potential confounders. There were 503 patients enrolled in this study. AF occurred in 120 patients (23.68%) which was consistent with the previous study (22). In addition, the patients who experienced AF had longer hospital stays. Low FT3 level predicted POAF in patients with CABG while the study did not confine normal FT3 level (23). Considering the fact that normal thyroid function is correlated with cardiovascular diseases, the present study explored the association between normal thyroid hormone levels and POAF following surgical coronary revascularization and help to provide a basis on redefinition of the optimal reference range. Our study observed that the incidence of AF was significantly higher in FT3 Q1 group. Other study found that decreased tendency in FT3 values was prominent on the postoperative second day (20), our study also reported that AF occurred at day 2. These results indicated that lower FT3 level was strongly associated with AF.
To make the HR comparable, the continuous predictor variables were dichotomized for age, LAD, LVESD and LVEF. Analysis of big data derived from normal thyroid function test showed that FT3 levels were significantly decreased with age (12). Our study also found that serum FT3 level was negatively correlated with age (r=−0.24, P<0.001) and patients in FT3 Q1 was older than Q2–4 group (65.41±8.24 vs. 61.78±8.64, P<0.001). Advancing age was associated with a step-wise increased prevalence of AF (24). The present study also revealed that age ≥65 years was an independent predictor of POAF, which was similar with the reports of Seo et al. (19). Further study was performed to evaluate the association between FT3 level and POAF based on age. The results demonstrated that low-normal FT3 was significantly associated with increased risk of AF, especially in older patients. Univariate analysis revealed that FT3 in Q1 was a predictor of POAF. After adjusting for the factors that significantly in univariate analysis, multivariable COX analysis showed that FT3 in Q1 was still an independent predictor of POAF. In addition, a systematic review reported that intraoperative factors like perfusion time and use of inotropes were associated with AF following CABG (19), our study involved postoperative factors including inflammatory and myocardial injury markers which reflex operative-related status in the study and found that postoperative LDH level was also independently associated with increased risk of POAF following surgical coronary revascularization.
In a randomized double-blind study, FT3 supplementation increased cardiac output and decreased the incidence of POAF after cardiac operations in 5 days, but did not decrease POAF at day first (10,25). Additionally, intravenous T3 did not change hemodynamic variables (26). In clinical practice, preoperative FT3 supplementation in patients with low-normal FT3 concentration is in debate and needs to be identified by large randomized cohort studies.
POAF is correlated with poor prognosis in euthyroid patients after CABG. Our study demonstrated that the occurrence of AF was high in low-normal FT3 group. And patients with AF had longer hospital stays. Interestingly, the longer hospital stays were observed in FT3 Q1 group. Furthermore, a recent study reported that low FT3 was an independent predictor of all-cause mortality in heart failure patients (27). Based on the above results, long-term follow-up is needed to be performed in this study to evaluate the association between normal thyroid function and prognosis in patients with surgery coronary artery revascularization.
This study has several limitations. Not all patients admitted to the hospital had thyroid function test, patients who did not have the evaluation of thyroid function were excluded in this study, which leads to the relatively smaller sample size. The present study did not analyze the association between FT3 level and long-term prognosis in patients with surgical coronary revascularization. Additionally, the dynamic postoperative FT3 levels should be tested and future studies were needed to explore the therapeutic effects of FT3 administration in patients with surgical coronary revascularization.
Conclusions
FT3 level in Q1 (≤2.8 pg/mL) is associated with an increased risk of POAF and is an independent predictor of POAF, with thyroid function within the normal range. Moreover, the hospital stays of patients who developed AF were longer. Serum FT3 level was negatively correlated with age and hospital stays. These findings provide clinical significance in thyroid function before the operation and even redefinition of the optimal reference range.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1646/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1646/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1646/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1646/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee of Beijing Chaoyang Hospital, Capital Medical University (No. 2019-ke-372) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Woldendorp K, Farag J, Khadra S, et al. Postoperative Atrial Fibrillation After Cardiac Surgery: A Meta-Analysis. Ann Thorac Surg 2021;112:2084-93. [Crossref] [PubMed]
- Gerçek M, Börgermann J, Gummert J, et al. Five-year-outcome of new-onset perioperative atrial fibrillation after left atrial appendage amputation concomitant with cardiac surgery. Clin Res Cardiol 2023;112:1800-11. [Crossref] [PubMed]
- Oraii A, Masoudkabir F, Pashang M, et al. Effect of postoperative atrial fibrillation on early and mid-term outcomes of coronary artery bypass graft surgery. Eur J Cardiothorac Surg 2022;62:ezac264. [Crossref] [PubMed]
- Bruinstroop E, van der Spek AH, Boelen A. Role of hepatic deiodinases in thyroid hormone homeostasis and liver metabolism, inflammation, and fibrosis. Eur Thyroid J 2023;12:e220211. [Crossref] [PubMed]
- Bowdish ME, Bagiella E, Giustino G, et al. Prospective Study of Risk Factors for Postoperative Atrial Fibrillation After Cardiac Surgery. J Surg Res 2024;294:262-8. [Crossref] [PubMed]
- Baumgartner C, da Costa BR, Collet TH, et al. Thyroid Function Within the Normal Range, Subclinical Hypothyroidism, and the Risk of Atrial Fibrillation. Circulation 2017;136:2100-16. [Crossref] [PubMed]
- Chen YY, Shu XR, Su ZZ, et al. A Low-Normal Free Triiodothyronine Level Is Associated with Adverse Prognosis in Euthyroid Patients with Heart Failure Receiving Cardiac Resynchronization Therapy. Int Heart J 2017;58:908-14. [Crossref] [PubMed]
- Kannan L, Shaw PA, Morley MP, et al. Thyroid Dysfunction in Heart Failure and Cardiovascular Outcomes. Circ Heart Fail 2018;11:e005266. [Crossref] [PubMed]
- Wei SB, Wang W, Liu N, et al. U-shaped association between serum free triiodothyronine and recurrence of atrial fibrillation after catheter ablation. J Interv Card Electrophysiol 2018;51:263-70. [Crossref] [PubMed]
- Klemperer JD, Klein I, Gomez M, et al. Thyroid hormone treatment after coronary-artery bypass surgery. N Engl J Med 1995;333:1522-7. [Crossref] [PubMed]
- Zhang H, Qiao H, Yang B, et al. Development and validation of a diagnostic model based on left atrial diameter to predict postoperative atrial fibrillation after off-pump coronary artery bypass grafting. J Thorac Dis 2023;15:3708-25. [Crossref] [PubMed]
- Wang D, Yu S, Ma C, et al. Reference intervals for thyroid-stimulating hormone, free thyroxine, and free triiodothyronine in elderly Chinese persons. Clin Chem Lab Med 2019;57:1044-52. [Crossref] [PubMed]
- Strich D, Karavani G, Edri S, et al. TSH enhancement of FT4 to FT3 conversion is age dependent. Eur J Endocrinol 2016;175:49-54. [Crossref] [PubMed]
- Salem JE, Shoemaker MB, Bastarache L, et al. Association of Thyroid Function Genetic Predictors With Atrial Fibrillation: A Phenome-Wide Association Study and Inverse-Variance Weighted Average Meta-analysis. JAMA Cardiol 2019;4:136-43. [Crossref] [PubMed]
- Li J, Liu Z, Zhao H, et al. Alterations in atrial ion channels and tissue structure promote atrial fibrillation in hypothyroid rats. Endocrine 2019;65:338-47. [Crossref] [PubMed]
- Zhang Y, Dedkov EI, Teplitsky D, et al. Both hypothyroidism and hyperthyroidism increase atrial fibrillation inducibility in rats. Circ Arrhythm Electrophysiol 2013;6:952-9. [Crossref] [PubMed]
- Anderson JL, Jacobs V, May HT, et al. Free thyroxine within the normal reference range predicts risk of atrial fibrillation. J Cardiovasc Electrophysiol 2020;31:18-29. [Crossref] [PubMed]
- Morishima I, Okumura K, Morita Y, et al. High-Normal Thyroid-Stimulating Hormone Shows a Potential Causal Association With Arrhythmia Recurrence After Catheter Ablation of Atrial Fibrillation. J Am Heart Assoc 2018;7:e009158. [Crossref] [PubMed]
- Seo EJ, Hong J, Lee HJ, et al. Perioperative risk factors for new-onset postoperative atrial fibrillation after coronary artery bypass grafting: a systematic review. BMC Cardiovasc Disord 2021;21:418. [Crossref] [PubMed]
- Donmez K, Akca B, Erdil N. Is there a relationship between thyroid hormone change and postoperative arrhythmia in patients undergoing coronary bypass surgery? A prospective randomized controlled trial. Azerbaijan Journal of Cardiovascular Surgery 2022;3:36-42.
- Wang J, Yuan W, Dong R, et al. Predictors for euthyroid sick syndrome and its impact on in-hospital clinical outcomes in high-risk patients undergoing coronary artery bypass grafting. Perfusion 2019;34:679-88. [Crossref] [PubMed]
- Filardo G, Damiano RJ Jr, Ailawadi G, et al. Epidemiology of new-onset atrial fibrillation following coronary artery bypass graft surgery. Heart 2018;104:985-92. [Crossref] [PubMed]
- Cerillo AG, Bevilacqua S, Storti S, et al. Free triiodothyronine: a novel predictor of postoperative atrial fibrillation. Eur J Cardiothorac Surg 2003;24:487-92. [Crossref] [PubMed]
- Gao P, Gao X, Xie B, et al. Aging and atrial fibrillation: A vicious circle. Int J Cardiol 2024;395:131445. [Crossref] [PubMed]
- Klemperer JD, Klein IL, Ojamaa K, et al. Triiodothyronine therapy lowers the incidence of atrial fibrillation after cardiac operations. Ann Thorac Surg 1996;61:1323-7; discussion 1328-9. [Crossref] [PubMed]
- Bennett-Guerrero E, Jimenez JL, White WD, et al. Cardiovascular effects of intravenous triiodothyronine in patients undergoing coronary artery bypass graft surgery. A randomized, double-blind, placebo- controlled trial. Duke T3 study group. JAMA 1996;275:687-92.
- De Matteis G, Covino M, Burzo ML, et al. Prognostic role of hypothyroidism and low free-triiodothyronine levels in patients hospitalized with acute heart failure. Intern Emerg Med 2021;16:1477-86. [Crossref] [PubMed]


