
Surgical-decision making in the setting of unsuspected N2 disease: a cost-effectiveness analysis
Highlight box
Key findings
• When ipsilateral mediastinal nodal disease is encountered at the time of resection in lung cancer, aborting the procedure in favor of neoadjuvant therapy appears to be cost-effective in most sensitivity models.
What is known and what is new?
• Clinical staging may be inaccurate, and surgeons must be prepared for unsuspected findings at the time of lung resection.
• Taking into account both cost and oncologic effectiveness are important in our current state of healthcare economics when deciding to abort or continue with resection.
What is the implication, and what should change now?
• Aborting resection in favor of neoadjuvant therapy appears to provide the most oncologic benefit without substantial added cost. In the current era of immunotherapy and molecular testing, more detailed analyses are required.
Introduction
Appropriate therapy for non-small cell lung cancer has evolved over the past decade. Current guidelines created by the National Comprehensive Cancer Network provide evidence-based pathways for treatment based on specific clinical stage of the malignancy (1). Therapeutic options include resection, chemotherapy, immunotherapy, and radiation therapy.
Guidelines are clear for numerous scenarios. For example, stage I non-small cell lung cancer is often treated with upfront surgical resection in those that can tolerate and operation (2). Radiation therapy is a secondary option if surgical resection is not a possibility (3). If lymph nodes are returned on final pathology as positive after resection, then adjuvant therapy is warranted (2,4-8). Stage IIIa lung cancer has a different approach. If metastasis to the ipsilateral mediastinal lymph nodes (N2 nodes) is already identified prior to resection, then neoadjuvant therapy is administered prior to surgery (9). This has been studied at length in the lung cancer literature, and such a treatment algorithm has been recognized as provided the best prognosis (1,4,5,7).
There are, however, situations where the correct treatment approach is not as clear. In some scenarios, clinical staging may not be accurate prior to the development of a treatment decision. If a patient is considered stage I and brought to the operating room, what is the best course of action in the event of unsuspected N2 disease? In other words, does the surgeon abort the operation with a plan for the best recognized therapy (neoadjuvant therapy followed by reconsideration of resection), or should the resection be performed upfront as the patient has already been anesthetized and incised? While this is a rare situation, it may occur due to under-staging on clinical workup, delayed time to treatment after imaging, and in some situations due to tumor biology and micrometastases unable to be seen on imaging. Even if a rare clinical situation that is decreasing as our preoperative imaging improves, it deserves to be studied. Several previous studies have examined this question considering only survival or direct costs (6,10-12). However, there has been no assessment of the tradeoffs between costs and improvements in clinical outcomes to help identify which is the optimal approach if the patient is already in the operating room with their chest open.
The goal of this study was to examine the cost-effectiveness of proceeding with lobectomy in the setting of N2 disease followed by systemic therapy versus recommended guidelines of neoadjuvant therapy. The latter requires aborting the operation and introducing the additional costs and disutility of a second operation but with the potential of improved overall survival. We present this article in accordance with the CHEERS reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1538/rc).
Methods
A decision analysis was performed to evaluate the cost-effectiveness of continuing with resection at the time of identification of unsuspected N2 mediastinal disease versus aborting initial resection and continuing with neoadjuvant therapy prior to resection. Patients entering this simulation would be considered stage IIIa without signs of metastatic disease and are operable based on the initial decision to perform resection. The incremental cost-effectiveness ratio (ICER) was evaluated, and is defined as the cost per quality-adjusted life-year (QALY) gained. QALY is a widely used metric in cost-effectiveness literature and takes into account the quality of life as well as the number of life years gained. It is constructed by multiplying a utility weight, which varies from 0 (death) to 1 (perfect health), by the amount of time spent in each particular health state. As recommended by the Institute for Clinical and Economic Review, we used a willingness-to-pay (WTP) threshold of $150,000/QALY. In other words, the strategy of continuing with the resection would be considered cost-effective compared to the aborting strategy if the model yielded an ICER less than $150,000/QALY.
A literature search was performed utilizing two specific strategies. First, we found cost and utility input parameters that had been used in previously published cost-effectiveness analyses in the thoracic surgery literature. Specifically, we used search terms “cost effectiveness” and “lobectomy” or “Lung Surgery”. A second search was performed to obtain model inputs for variables where a cost effectiveness study has not been performed. Search terms included “cost” and “lung surgery”, “robotic surgery”, or “Thoracic Surgery”. The Cost-Effectiveness Analysis Registry sponsored by the Tufts Medical Center Institute for Clinical Research and Healthy Policy Studies was queried to supplement missing inputs utilizing “lung surgery” as the main search term. Expert opinion—by a team of thoracic surgeons and oncologists with active clinical experience in the treatment of advanced lung cancer—was used for any input parameters that we were not able to find in the published literature.
As costs and outcomes have changed drastically over time due to improved techniques and medical care, a cutoff of the year 2000 was used. For cost data, studies outside of the United States (US) were excluded due to significant variation from different health payor systems. While in our search for utility values we did not exclude studies based on geography, we assessed studies performed outside the US for their generalizability to the US healthcare system since postoperative care (specifically length of stay) is substantially longer in Europe and Asia than in the US. Only lung surgery studies focusing on patients with malignancy and adults (>18 years old) were included.
Decision trees and Markov model
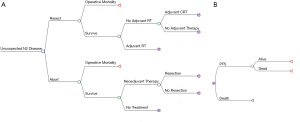
Hypothetical patients entered our model upon discovery of N2 disease at the time of resection for an initially assumed stage I non-small lung cancer (Figure 1A). An assumption was made that the resection is being performed robotically, as this has quickly become the most common approach for early-stage lung cancer in the US. In both the upfront resection and abort strategies, operative mortality was a possibility. In the upfront resection strategy, following the operation, a patient could receive adjuvant radiation therapy, adjuvant chemoradiation therapy, or no additional therapy. All treatment pathways included operative mortality as a part of the model. The patient would then enter a Markov node with progression-free survival (PFS) and death as health states (Figure 1B). This allowed us to model overall survival using a lifetime time horizon which is a critical outcome in the management of potentially curable lung cancer. In the abort resection strategy, the surgeon identified the nodal disease but opts to abort the operation prior to lung resection in favor of the recommend guidelines of neoadjuvant therapy. Following completion of the exploratory surgery, the patient may undergo neoadjuvant therapy followed by resection, neoadjuvant therapy without resection, or no additional treatment. At this point, the patient would then enter a Markov node which would track overall survival, similar to the continuation of the resection strategy. The model was developed using TreeAge Pro software (version 2022; TreeAge Inc., Williamstown, MA, USA). An annual discount rate of 3% was used for both cost and effectiveness.
Cost variables and utilities
We analyzed our model from a societal perspective, including costs and utilities not only related to the expense of treatment, surgery, and hospital stay, but also patient recovery and potentially time away from work (13-16). Upfront direct costs included the cost of an operation (cost per minute of operative time multiplied by operative time for either robotic exploration or resection), cost of hospital stay (average length of stay multiplied by the daily cost per day in hospital), and the cost of an operative mortality. Additional direct costs included the cost of radiation, chemotherapy, or chemoradiation treatment. Additional societal costs included the cost of recovery, defined as the number of days missed from work after a robotic chest operation multiplied by the average daily wage from the US Bureau of Labor Statistics. Mediastinal spread may not be identified without the use of frozen section. As every patient in the model would potentially need frozen section evaluation regardless of treatment decision, the cost of frozen analysis was not included. Surgery after neoadjuvant therapy for patients with post-systematic nodal dissection would be technically more complicated. This was accounted for with increased operative times, longer postoperative course, and worsened mortality in that treatment arm.
Quality of life was determined by way of utilities for varies aspects of the patient’s treatment. Utilities incorporated included recovery from robotic thoracic procedure, each type of systemic therapy, or radiation therapy (17). The likelihood of receiving each treatment option after the first main decision of resection or aborting was incorporated from the literature (11,13,18,19). We calculated 1-year probabilities of survival for the Markov models from published 5-year overall survival data (20,21).
Statistical analysis
Deterministic sensitivity analyses included one-way sensitivity analyses of critical variables in the model and a three-way sensitivity analysis taking into account perioperative variables that would be substantially affected if a non-robotic approach was substituted for an open thoracotomy. One-way sensitivity analyses were performed using the most likely impactful variables along with variables that could be easily modified for a patient undergoing lung resection. Easily modifiable variables included length of stay after operation, operative time, days off work after surgery, and the utility for recovery after surgery (which is directly related to improved pain control). We also included the best-practice therapies in both groups. For upfront resection, that would be receiving adjuvant chemoradiation. Probability of receiving chemoradiation, and the survival probability for those that receive adjuvant chemoradiation were included. For the abort group, receiving neoadjuvant therapy followed by resection is best-practice. The probability of receiving that therapy and then the survival probability for that group were included in sensitivity analysis. We conducted a probabilistic sensitivity analysis (PSA) utilizing 10,000 simulations to assess the impact of variation in all parameters on model results simultaneously. Finally, a three-way sensitivity analysis was performed to assess whether an open approach may have different results compared to the minimally-invasive approach analyzed in these models. Pain, length of stay, and recovery time were chosen as impactful variables often different when an open approach is utilized.
Results
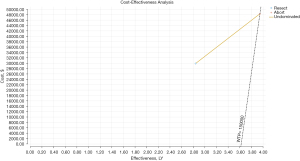
In the base-case analysis for the decision model, upfront resection with unsuspected N2 disease was estimated to cost $29,873 and result in 2.84 QALYs (Table 1). Aborting initial resection at the discovery of N2 disease with the possibility of additional treatment was estimated to cost $40,415 and yield 3.95 QALYs. This resulted in an incremental cost of $10,542, and incremental effectiveness of 1.11 QALYs and an ICER an $9,526/QALY for aborting the operation compared to continuing with the resection. These results are plotted in quadrant 1 of a base-case cost-effectiveness plane and are acceptable based on a WTP of $150,000 (Figure 2).
Table 1
| Strategy | Cost (USD) | Incr cost (USD) | Eff (QALYs) | Incr Eff (QALYs) | ICER (cost/QALYs) |
|---|---|---|---|---|---|
| Resect | $29,873 | – | 2.84 | – | – |
| Abort | $40,415 | $10,542 | 3.95 | 1.11 | $9,526 |
USD, United States dollar; Incr, incremental; QALYs, quality-adjusted life-years; Eff, effectiveness; ICER, incremental cost-effectiveness ratio.
In one-way sensitivity analyses, varying the length of stay from 1–14 days had a moderate impact on the ICER (Figure 3), but at no point did it approach the WTP threshold. Small changes in the mortality probabilities for the best therapy groups had large effects on the ICER, although again did not approach the WTP. An increase in the annual rate of death of just 1% increased the ICER for aborting initial resection by almost 50%. A detailed one-way sensitivity analysis was performed for the probability of death after aborting, receiving neoadjuvant therapy, and then resection. The base-case probability was 0.11. As this approached 0.15, the WTP of $150,000 was met. If the probability of mortality probability reached 0.152, aborting surgery was no longer cost-effective and that approach was dominated by initial resection.
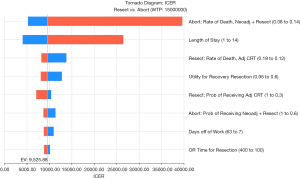
Figure 4 shows the results of a two-way sensitivity analysis of the probability of death after best therapy, and the likelihood of receiving that therapy in the abort strategy (Figure 4). As the likelihood of receiving the best therapy in the abort surgery group increased, that approach remained cost effective at a WTP of $150,000 despite increases in mortality rate. If 100% of patients in the abort group received neoadjuvant therapy followed by resection, then the probability of death could reach 0.177 while remaining cost-effective at a WTP of $150,000, and would not become dominated until a death rate of 0.181.

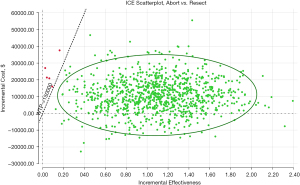
In the PSA, aborting initial surgery was the optimal approach in 99.7% of samples as seen in the cost-effectiveness acceptability curve (Figure 5). On the ICE scatter plot shown in Figure 6, 86.7% of samples fell in quadrant 1 while maintaining a WTP of less than $150,000. A small subset (0.2%) falls above the WTP threshold. The remaining 13.0% of samples fall in quadrant 2, indicating that aborting resection was the dominant strategy.

A three-way sensitivity analysis was performed to evaluate whether a different surgical approach, namely open thoracotomy, may have alternate final results. This assumption was based on the lower utility due to pain and recovery, and higher postoperative costs from length of stay and operative time. The three variables chosen were the ones most likely affected by an open approach—length of stay, operative time, and time off work postoperatively. Despite increasing all three variables to results well above the maximum national averages (length of stay of 20 days, operating room time of 400 minutes, and 60 days off work postoperatively), aborting the initial surgery was still determined to be the most cost-effective while remaining under a WTP threshold of $150,000.
Discussion
This study evaluated the cost-effectiveness of two treatment modalities of advanced lung cancer. While guidelines are clear as to the best therapy when the advanced stage is known prior to the initiation of treatment, there is currently no guidance on what pathway to take if unsuspected nodal metastasis is found at the time of operation. Our main finding is that it is cost-effective to abort the initial operation, even when best case therapy of neoadjuvant chemotherapy followed by a second operation may not occur in every scenario.
Our analysis considered the various treatment pathways that a patient may take after aborting the initial resection. This included best-case therapy of treatment with neoadjuvant chemotherapy followed by resection, neoadjuvant therapy without resection, and no further treatment. Based on the literature, approximately 25% of patients will not receive neoadjuvant therapy followed by resection for stage IIIa disease. Despite this large percentage of patients receiving care that leads to worse overall survival, aborting the initial operation led to an ICER of $9,528.88/QALY, far under the WTP threshold of $150,000.
On sensitivity analysis, we found that the mortality rate for aborting initial resection, neoadjuvant therapy, and then resection was extremely influential in the ICER. While the base-case estimate was 11.3%/year (or a 5-year mortality rate of 45%), increasing the annual mortality to just 15% increased the ICER up to the WTP of $150,000. Even if 100% of patients received that best-case therapy, rather than the literature-estimated 75%, the annual mortality only had to increase to 17.7% before reaching the WTP. Operative-related variables such as length of stay and operative time did not have nearly as large an impact on the ICER, and even when increased to the extremes of what was clinically reasonable, did not reverse the overall cost-effectiveness result.
These results indicate that for treatment of advanced lung cancer, the most important aspect when considering cost-effectiveness is the actual effectiveness of the treatment in relation to overall survival. As new systemic therapies are developed that impact prognosis including targeted treatment and immunotherapy, it will only take a small improvement in a treatment given as adjuvant therapy to potentially alter the overall outcome these results (15,22,23). Unfortunately, many of newer immunotherapy treatments are very expensive, despite moderate increases in QALYs. In a recent analysis from Canada, atezolizumab and nivolumab were compared to docetaxel as second-line therapy, with ICERs of $142,074 and $158,875 respectively (24). Docetaxel was cost-effective on 100% of simulations up to a WTP threshold of nearly $100,000. As immunotherapy may be quite effective, it is very expensive. It will be appropriate in future studies to determine the best scenarios where immunotherapy will be cost-effective based on appropriate WTP thresholds. For example, minor improvements in disease free survival in patients with low PD-L1 (<1%) may not lead to appropriate ICER values, while high PD-L1 (>50%) may prove cost-effective.
Whether surgery itself adds any benefit to systemic therapy in the treatment of stage IIIa non-small cell lung cancer has been controversial. Samson and colleagues performed a cost-effectiveness analysis utilizing the National Cancer Database as a foundation, comparing chemoradiation therapy to chemoradiation plus surgery in any sequence (25). While they only took into account direct costs and the perspective of the payer, they found an ICER of $17,618/QALY in favor of the addition of surgery, well under any standard WTP thresholds. While the addition of resection nearly doubled the cost, the increased effectiveness was substantial. Similar to our study however, minor changes in overall survival, in this case evaluated in by modifying 30-day surgical survival, led to changes in the overall cost-effectiveness on two-way sensitivity analysis.
It is important to note that N2 disease is a very heterogeneous problem. A patient with bulky multi-station mediastinal disease would be staged the same as a patient with a left upper lobe malignancy and solitary aortopulmonary window lymph node. Current treatment guidelines do not differentiate different “subtypes” of N2 disease, and therefore recommendations tend to be the same. Several assumptions were made in this analysis. Prognosis is provided in the literature for stage IIIa disease as one solitary group, so the heterogeneity seen in N2 disease was not considered. Preoperative workup and staging were also assumed to have met the standards of care, as that data is not available. This is in line with other frequently published analyses from large datasets such as the National Cancer Database where that information is not available.
There are several limitations to this study. Only overall survival was utilized as a final outcome in the Markov analysis after each treatment arm. In real-world clinical practice, disease-free survival is often considered, and additional therapies may be given after initial recurrence leading to prolonged survival. While our model assumes that if there is a recurrence, additional treatment will not be successful, it is not far-fetched as the overall survival from a recurrence of stage III lung cancer is quite poor. This decision was due to the lack of widely accepted disease-free survival data for the individual treatment arms. Secondly, the only cost associated with PFS was an annual computed tomography (CT) scan. While this is the most likely situation, there may be other costs if additional imaging such as a positron emission tomography (PET) scan are required. Histology was also not considered, as all cancers were evaluated together as non-small cell lung cancer. In the modern era, treatment effect is often directly correlated to cancer histology and specific biomarkers.
Finally, an obvious limitation is the change in standard of care over the last 12 months. With results of Checkmate-816, IMpower 010, Keynote091, Keynote-671, among others, treatment pathways for stage IIa–IIIa NSCLC have changed substantially (26,27). This analysis was initiated prior to the acceptance of immunochemotherapy as standard treatment, and when chemoradiation was still recommended therapy for IIIa disease. The results are still an important window into cost-effective treatment strategies when unsuspected mediastinal disease is discovered.
Conclusions
Stage IIIa non-small cell lung cancer is a complex oncologic scenario which requires the decision making of a multidisciplinary team of physicians. As preoperative imaging is imprecise, overall stage may suddenly increase at the time of a planned resection. It is important to evaluate both cost, quality of life, and survival when making treatment decisions in this setting. Further studies utilizing costs and utilities on more granular treatments will refine this model and help physicians choose the optimal treatment algorithm.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CHEERS reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1538/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1538/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1538/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 5.2021). Accessed July 19, 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Yang CF, Kumar A, Gulack BC, et al. Long-term outcomes after lobectomy for non-small cell lung cancer when unsuspected pN2 disease is found: A National Cancer Data Base analysis. J Thorac Cardiovasc Surg 2016;151:1380-8. [Crossref] [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-313S.
- Andre F, Grunenwald D, Pignon JP, et al. Survival of patients with resected N2 non-small-cell lung cancer: evidence for a subclassification and implications. J Clin Oncol 2000;18:2981-9. [Crossref] [PubMed]
- Boffa DJ, Hancock JG, Yao X, et al. Now or later: evaluating the importance of chemotherapy timing in resectable stage III (N2) lung cancer in the National Cancer Database. Ann Thorac Surg 2015;99:200-8. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS. Survival of patients with unsuspected N2 (stage IIIA) nonsmall-cell lung cancer. Ann Thorac Surg 2008;86:362-6; discussion 366-7. [Crossref] [PubMed]
- Thomas DC, Arnold BN, Rosen JE, et al. The Significance of Upfront Knowledge of N2 Disease in Non-small Cell Lung Cancer. World J Surg 2018;42:161-71. [Crossref] [PubMed]
- Tsitsias T, Boulemden A, Ang K, et al. The N2 paradox: similar outcomes of pre- and postoperatively identified single-zone N2a positive non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:882-7. [Crossref] [PubMed]
- Rosell R, Gómez-Codina J, Camps C, et al. Preresectional chemotherapy in stage IIIA non-small-cell lung cancer: a 7-year assessment of a randomized controlled trial. Lung Cancer 1999;26:7-14. [Crossref] [PubMed]
- Detterbeck F. What to do with "Surprise" N2?: intraoperative management of patients with non-small cell lung cancer. J Thorac Oncol 2008;3:289-302. [Crossref] [PubMed]
- Ferguson MK. Optimal management when unsuspected N2 nodal disease is identified during thoracotomy for lung cancer: cost-effectiveness analysis. J Thorac Cardiovasc Surg 2003;126:1935-42. [Crossref] [PubMed]
- Kim HK, Choi YS, Kim J, et al. Outcomes of unexpected pathologic N1 and N2 disease after video-assisted thoracic surgery lobectomy for clinical stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2010;140:1288-93. [Crossref] [PubMed]
- Cipriano LE, Romanus D, Earle CC, et al. Lung cancer treatment costs, including patient responsibility, by disease stage and treatment modality, 1992 to 2003. Value Health 2011;14:41-52. [Crossref] [PubMed]
- Heiden BT, Mitchell JD, Rome E, et al. Cost-Effectiveness Analysis of Robotic-assisted Lobectomy for Non-Small Cell Lung Cancer. Ann Thorac Surg 2022;114:265-72. [Crossref] [PubMed]
- Sheehan DF, Criss SD, Chen Y, et al. Lung cancer costs by treatment strategy and phase of care among patients enrolled in Medicare. Cancer Med 2019;8:94-103. [Crossref] [PubMed]
- Simianu VV, Gaertner WB, Kuntz K, et al. Cost-effectiveness Evaluation of Laparoscopic Versus Robotic Minimally Invasive Colectomy. Ann Surg 2020;272:334-41. [Crossref] [PubMed]
- Kent MS, Hartwig MG, Vallières E, et al. Pulmonary Open, Robotic, and Thoracoscopic Lobectomy (PORTaL) Study: An Analysis of 5721 Cases. Ann Surg 2023;277:528-33. [Crossref] [PubMed]
- Brascia D, De Iaco G, Schiavone M, et al. Resectable IIIA-N2 Non-Small-Cell Lung Cancer (NSCLC): In Search for the Proper Treatment. Cancers (Basel) 2020;12:2050. [Crossref] [PubMed]
- Rajaram R, Correa AM, Xu T, et al. Locoregional Control, Overall Survival, and Disease-Free Survival in Stage IIIA (N2) Non-Small-Cell Lung Cancer: Analysis of Resected and Unresected Patients. Clin Lung Cancer 2020;21:e294-301. [Crossref] [PubMed]
- Abrão FC, Moreira FR, de Abreu IRLB, et al. Real-Life Long-Term Cohort of Patients With Stage IIIA Non-Small-Cell Lung Cancer: Overall Survival Related to Patients' Characteristics and Multiple Treatment Models. JCO Glob Oncol 2021;7:1572-85. [Crossref] [PubMed]
- Mitzman B, Varghese TK Jr, Kuchta K, et al. National guideline concordance and outcomes for pathologic N2 disease in non-small cell lung cancer. J Thorac Dis 2022;14:1360-73. [Crossref] [PubMed]
- Wolff HB, Alberts L, van der Linden N, et al. Cost-effectiveness of stereotactic body radiation therapy versus video assisted thoracic surgery in medically operable stage I non-small cell lung cancer: A modeling study. Lung Cancer 2020;141:89-96. [Crossref] [PubMed]
- Wu D, Li J, Wang Y, et al. Cost-effectiveness analysis of neoadjuvant versus adjuvant chemotherapy for cT2-4N0-1 non-small cell lung cancer patients during initial treatment phase. Cost Eff Resour Alloc 2021;19:44. [Crossref] [PubMed]
- Ondhia U, Conter HJ, Owen S, et al. Cost-effectiveness of second-line atezolizumab in Canada for advanced non-small cell lung cancer (NSCLC). J Med Econ 2019;22:625-37. [Crossref] [PubMed]
- Samson P, Patel A, Robinson CG, et al. The Role of Surgical Resection in Stage IIIA Non-Small Cell Lung Cancer: A Decision and Cost-Effectiveness Analysis. Ann Thorac Surg 2015;100:2026-32; discussion 2032. [Crossref] [PubMed]
- Wakelee H, Liberman M, Kato T, et al. Perioperative Pembrolizumab for Early-Stage Non-Small-Cell Lung Cancer. N Engl J Med 2023;389:491-503. [Crossref] [PubMed]
- Forde PM, Spicer J, Lu S, et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med 2022;386:1973-85. [Crossref] [PubMed]


