
Cerebral protection in acute type A aortic dissection surgery: a systematic review and meta-analysis
Highlight box
Key findings
• Antegrade cerebral perfusion (ACP) and retrograde cerebral perfusion (RCP) are both safe and acceptable techniques to use in emergent settings.
What is known and what is new?
• Two main methods to reduce cerebrovascular events in acute type A aortic dissection surgeries are ACP and RCP.
• In this study, we found that transient neurologic deficit (TND) risk in the bilateral ACP (bACP) group was higher than the unilateral ACP (uACP) group, intensive care unit-stay time was longer in the uACP group compared to bACP, circulatory arrest time during ACP was longer than during RCP, and core temperature was higher in ACP.
What is the implication, and what should change now?
• Cardiothoracic surgeons concerned about TND may find it beneficial to utilize uACP.
Introduction
Acute type A aortic dissection (ATAAD) still challenges physicians and warrants emergent surgical management. It is a disruption of the tunica intima, resulting in blood flow into the tunica media, and specifically involves the ascending aorta (proximal to the brachiocephalic artery), regardless of primary entry tear location according to the Stanford classification (1). The mortality rate in ATAAD patients who underwent surgery is about 26% compared to 58% among patients who received non-surgical treatment (2). Among those who underwent surgery, the incidence of postoperative neurological impairment is around 10–30% (3). For decades, various brain protection methods have been proposed to reduce cerebrovascular events in surgeries (4). Besides, postoperative brain malperfusion or advanced age are the leading causes of mortality and morbidity in ATAAD (5,6). In most cases of brain protection, a circulatory arrest (CA) phase is required. This phase is accompanied by increases in the incidence rate of cerebrovascular events, mortality, and hospital stay (7,8). Hence, there is a significant urgency to enhance neuroprotection methods during these surgeries.
Two main methods to reduce cerebrovascular events in ATAAD surgeries are antegrade cerebral perfusion (ACP) and retrograde cerebral perfusion (RCP). These two methods improve brain protection during CA. For instance, ACP regulates temperature or cerebral and systemic circulation blood flow, whereas RCP drains possible emboli from the cerebral circulation. Nevertheless, the optimal perfusion strategy in ATAAD surgeries remains unclear. ACP was proposed as a more physiological strategy than RCP for intraoperative brain protection, but whether ACP or RCP is associated with better clinical outcomes is still debatable (9). Besides, embolization and thrombosis are possible side effects of ACP. In the RCP method, the extent to which the brain can tolerate malperfusion is not fully understood, and more studies are needed to determine the complications of RCP.
We conducted a systematic review and meta-analysis to compare the ACP and RCP methods during ATAAD surgery. We evaluated and analyzed related endpoints to investigate the neurologic events and mortality in ACP and RCP techniques. We present this article in accordance with the PRISMA reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1039/rc) (10).
Methods
Search strategy
In this study, we searched databases PubMed, Embase, Scopus, Cochrane Library, Web of Science, Ovid, ProQuest, and hand searching through Google Scholar from January 1998 until March 29th, 2023, based on the following keywords and strategy designed by the authors: “aortic dissection”, “retrograde”, “antegrade”, “cerebral”, “perfusion”, “brain protection”.
Inclusion & exclusion criteria
The inclusion criteria for selected studies should consist of at least one of the following: (I) studies reporting the outcomes associated with RCP and ACP [e.g., cardiopulmonary bypass (CPB) time, CA time, operative mortality, permanent neurologic dysfunction (PND), transient neurologic deficit (TND), intensive care unit (ICU)-stay time, and cross-clamp time (CCT)]; (II) studies reporting the outcomes mentioned previously in patients with ATAAD; and (III) original peer-reviewed articles.
Studies with any of the following criteria were excluded from this review: (I) case reports, review articles, conference abstracts, letters, comments, non-English articles, and book chapters; (II) studies on surgeries other than acute aortic dissection (e.g., patients with aortic aneurysms or chronic aortic dissection); and (III) studies with unavailable full-length texts.
Quality assessment
Two authors (A.N. and S.H.) independently evaluated the quality of the included studies using the Joanna Briggs Institute (JBI) critical appraisal tools. Any disputes were addressed through consensus.
Study selection
Based on the keywords and the eligibility criteria, the following steps were taken by two authors independently: (I) identifying the titles related to the study concept; (II) removing duplicates; (III) screening the titles and abstracts of the studies as a result of the primary search; (IV) evaluating the full text of the included studies in the earlier step; (V) assessing the quality of the included studies using JBI critical appraisal tools; and (VI) including for data collection.
Data collection and outcome definitions
Two authors (A.N. and S.H.) screened studies based on inclusion and exclusion criteria and then extracted the data of demographic, intervention, and outcome separately. Any disputes were addressed through consensus. We removed duplicate studies, and studies that met the inclusion criteria of this systematic review were included by assessing the title, abstract, and full text using EndNote® software (version X10, Thomson Reuters, Philadelphia, USA).
Operative mortality was defined as all deaths occurring during the hospitalization within 30 days of surgery and all deaths happening after discharge from the hospital within 30 days. PND is defined as the presence of permanent neurologic deficits that are focal or global and persisting at discharge from the hospital, and TND is known as the presence of delirium, agitation, obtundation, postoperative confusion, or transient Parkinson’s without any neurologic signs. The study protocol was approved by the ethics committee of Tabriz University of Medical Sciences (No. IR.TBZMED.REC.1401.662).
Missing data evaluation
Considering the characteristics of this systematic review and meta-analysis, where data was gathered from case-control studies, it should be noted that not all studies had identical variables. To address this disparity, the handling of missing data and adjustments made to the denominator for calculating percentage values were carefully examined. In situations where data was missing for routine variables like demographic information, it was assumed that the missing data occurred randomly.
Statistical analysis
Forest plots were used to represent the clinical outcomes. For dichotomous variables, risk ratios (RR), and 95% confidence intervals (CIs) were calculated from included studies. For continuous variables, mean difference (MD) and 95% CIs were calculated from included studies. The heterogeneity across the studies was calculated with Q, I2, and tau statistics. Differences in means were combined across the studies using random-effects models. Heterogeneity was considered statistically significant if Q <0.10 or I2>50%. Dichotomous variables from the included studies were combined to calculate the pooled RR with a 95% CI. We used the funnel plot to evaluate the publication bias and statistically analyzed it using the linear regression test of funnel plot asymmetry and the Egger test (11). All analyses were performed in R version 4.2.2. Statistical significance was considered at P<0.05. The following instructions were used to calculate the mean and standard deviation (SD) by median and interquartile range (IQR) (12):
Results
A total of 3,680 studies were found by searching the databases mentioned. After removing duplicates, 1,548 studies were screened based on title and abstract, 1,473 were removed during the screening, and the remaining 75 studies were assessed based on eligibility criteria. Twenty-six studies met the eligibility criteria (Figure 1). All the studies had a low risk of bias as they were evaluated by the JBI critical appraisal tool (Tables 1,2).

Table 1
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Risk |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stamou et al. (13) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Apostolakis et al. (14) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Benedetto et al. (15) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Montagner et al. (16) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Samanidis et al. (17) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Sinatra et al. (18) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Sugiura et al. (19) | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Low |
| Sun et al. (9) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Tokuda et al. (20) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Wiedemann et al. (21) | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Low |
| Williams et al. (22) | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Unclear | Unclear | Low |
| Usui et al. (23) | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Low |
| Zierer et al. (24) | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Low |
| Tong et al. (25) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Inamura et al. (26) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| O’Hara et al. (27) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Norton et al. (28) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Angleitner et al. (29) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Song et al. (30) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Krüger et al. (4) | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | Low |
| Dong et al. (31) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Liu et al. (32) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Preventza et al. (33) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Jiang et al. (34) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Piperata et al. (35) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
Case-control: Q1. Were the groups comparable other than the presence of disease in cases or the absence of disease in controls? Q2. Were cases and controls matched appropriately? Q3. Were the same criteria used for identification of cases and controls? Q4. Was exposure measured in a standard, valid and reliable way? Q5. Was exposure measured in the same way for cases and controls? Q6. Were confounding factors identified? Q7. Were strategies to deal with confounding factors stated? Q8. Were outcomes assessed in a standard, valid and reliable way for cases and controls? Q9. Was the exposure period of interest long enough to be meaningful? Q10. Was appropriate statistical analysis used?
Table 2
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Risk |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shemirani et al. (36) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Yes | Low |
Cohort: Q1. Were the two groups similar and recruited from the same population? Q2. Were the exposures measured similarly to assign people to both exposed and unexposed groups? Q3. Was the exposure measured in a valid and reliable way? Q4. Were confounding factors identified? Q5. Were strategies to deal with confounding factors stated? Q6. Were the groups/participants free of the outcome at the start of the study (or at the moment of exposure)? Q7. Were the outcomes measured in a valid and reliable way? Q8. Was the follow up time reported and sufficient to be long enough for outcomes to occur? Q9. Was follow up complete, and if not, were the reasons to loss to follow up described and explored? Q10. Were strategies to address incomplete follow up utilized? Q11. Was appropriate statistical analysis used?
Study characteristics
In our study, a total of 8,438 participants were evaluated for possible end-points in comparison between the ACP and RCP groups, and 4,601 in the comparison of the unilateral ACP (uACP) and bilateral ACP (bACP) groups. The total number of participants in the ACP group was 4,947, in the RCP was 3,491, in uACP was 2,003, and in bACP was 2,598. Essential intraoperative parameters and baseline patients’ characteristics are demonstrated in Tables 3,4. Also, the outcome of each study is summarized in Table 5.
Table 3
| Author | Publish year | Country/region | Number of participants (ACP, RCP) | Mean age (years) | CPB time (minutes) | CA time (minutes) | PND | Operative mortality | Core temperature (°C) |
|---|---|---|---|---|---|---|---|---|---|
| Sugiura et al. (19) | 2012 | Japan | 203: ACP: 109; RCP: 94 |
ACP: 67.2±9.3; RCP: 65.6±11.7 |
ACP: 229±72; RCP: 211±51 (P value =0.04) | ACP: 65±15; RCP: 53±16 (P value <0.001) | ACP: 11; RCP: 10 (P value =0.65) | ACP: 2; RCP: 7 (P value =0.18) | ACP: rectal =25.6±1.2; RCP: rectal =23.7±1.1 |
| Sinatra et al. (18) | 2001 | Italy | 41: ACP: 23; RCP: 18 |
ACP: 58.4±13.1; RCP: 61.7±11.2 |
ACP: 249.2±94.1; RCP: 219.4±77.4 (P value = NS) | ACP: 88.8±54.3; RCP: 56±25.2 (P value =0.023) | NM | NM | NM |
| Samanidis et al. (17) | 2021 | Greece | 290: ACP: 117; RCP: 173 |
ACP: 58±12; RCP: 61±14 |
ACP: 226 (95% CI: 197.30–243.5); RCP: 231 (95% CI: 199.5–255.5) (P value <0.001) | ACP: 33 (95% CI: 25.5–47.5); RCP: 25.5 (95% CI: 21.25–32.75) (P value <0.001) | ACP: 12; RCP: 12 (P value = NM) | ACP: 25; RCP: 38 (P value =0.9) | ACP: bladder median =22.4 (21.1–23.2); RCP: bladder =18.4 (17–20.7) |
| Sun et al. (9) | 2021 | Taiwan | 223: before matching ACP: 55, RCP: 168; after matching ACP: 54, RCP: 54 |
Before matching ACP: 56.4±10.0, RCP: 58.4±13.5; after matching ACP: 56.2±10.0, RCP: 55.34±12.3 |
Before matching ACP: 268±46, RCP: 275±32 (P value =0.735); after matching ACP: 266±32, RCP: 273±29 (P value =0.760) | Before matching ACP: 89±6, RCP: 62±10 (P value =0.000); after matching ACP: 88±5, RCP: 63±10 (P value =0.000) | Before matching ACP: 10, RCP: 13 (P value =0.027); after matching ACP: 10, RCP: 6 (P value =0.027) | Before matching ACP: 6, RCP: 26 (P value =0.402); after matching ACP: 10, RCP: 6 (P value =0.279) | NM |
| Tokuda et al. (20) | 2014 | Japan | 4,128: before matching ACP: 2,769, RCP: 1,359; after matching ACP: 1,320, RCP: 1,320 |
Before matching ACP: 69.6±11.6, RCP: 68.2±12.1 (P value =0.001); after matching ACP: 68.5±12.0, RCP: 68.6±11.9 (P value =0.880) |
NM | Before matching ACP: 115.9±37.6, RCP: 102.1±38.3 (P value <0.001); after matching ACP: 116±36, RCP: 102±38 (P value <0.001) | NM | NM | ACP: 24.5±2.9; RCP: 22.6±3.0 |
| Wiedemann et al. (21) | 2013 | Austria | 213: ACP: 122; RCP: 91 | ACP: 62 (95% CI: 33–85); RCP: 56 (95% CI: 18–87) |
ACP: 161 (95% CI: 101–303); RCP: 198 (95% CI: 121–404) (P value <0.01) | ACP: 30 (95% CI: 14–92); RCP: 30 (95% CI: 14–88) (P value =0.993) | ACP: 11; RCP: 15 (P value =0.033) | ACP: 12; RCP: 20 (P value =0.047) | NM |
| Apostolakis et al. (14) | 2008 | Greece | 48: ACP: 23; RCP: 25 | ACP: 61±15.6; RCP: 60±17.1 |
ACP: 179±28.65; RCP: 184±33.12 (P value =0.58) | NM | ACP: 1; RCP: 1 (P value =0.48) | NM | ACP: 16–18 °C in 14 patients, 18–20 °C in 9 patients; RCP: 16–18 °C in 13 patients, 18–20 °C in 12 patients |
| Stamou et al. (13) | 2016 | USA | 139: ACP: 84; RCP: 55 | ACP: 58 (95% CI: 29–87); RCP: 62 (95% CI: 23–83) |
ACP: 227 (95% CI: 112–430); RCP: 207 (95% CI: 102–454) (P value =0.023) | ACP: 31 (95% CI: 0–73); RCP: 36 (95% CI: 4–61) (P value <0.001) | NM | NM | ACP: circulatory arrest temperature =19 (8–26); RCP: circulatory arrest temperature =17 (10–20) |
| Usui et al. (23) | 1999 | Japan | 166: ACP: 91; RCP: 75 | ACP: 59.5±12.5; RCP: 61.1±13.2 |
ACP: 297±99; RCP: 269±112 | NM | NM | ACP: 22; RCP: 16 | ACP: nasopharyngeal =21.6±3.1; RCP: nasopharyngeal =18.7±2.1 |
| Zierer et al. (24) | 2005 | Germany | 56: ACP: 38; RCP:18 | ACP: 62±11; RCP: 55±11 |
ACP: 120±50; RCP: 176±34 | NM | ACP: 5; RCP: 3 | ACP: 6; RCP: 1 | ACP: 21±1.3; RCP: 20.8±2.4 |
| Shemirani et al. (36) | 2017 | Iran | 102: ACP: 54; RCP: 48 | NM | NM | NM | NM | ACP: 1; RCP: 36 | NM |
| Williams et al. (22) | 2012 | USA | 37: ACP: 8; RCP: 29 | ACP: 61.5±11.7; RCP: 59.2±13.7 |
ACP: 190±43; RCP: 188±58 | ACP: 34±11; RCP: 34±11 | NM | ACP: 5; RCP: 6 | ACP: 17±4.1; RCP: 19±2.7 |
| O'Hara et al. (27) | 2020 | USA | 4,395: ACP: 2,950; RCP: 1,445 | ACP: 60.0±13.4; RCP: 60.7±14.0 |
NM | ACP: 35.0 (26.0–48.0); RCP: 33.0 (25.0–45.0) | NM | ACP: 466; RCP: 239 | ACP: bladder, nasal, other =22.0 (18.4–25.0); RCP: bladder, nasal, other =17.6 (19–21.9) |
Data are presented as mean ± standard deviation, median (interquartile range), or mean and 95% CI or number. ACP, antegrade cerebral perfusion; RCP, retrograde cerebral perfusion; CPB, cardiopulmonary bypass; CA, circulatory arrest; PND, permanent neurologic dysfunction; NS, not significant; NM, not mentioned; CI, confidence interval.
Table 4
| Author [year] | Country/region | Mean age (years) | Number of participants in each group | CPB time (minutes) | X-clamp time (minutes) | CA time (minutes) | PND | TND | Operative mortality | ICU-stay (days) | Operation time (min) | Core temperature (°C) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wiedemann [2013] (21) | Austria | Total: 62 (33–85) | uACP: 53; bACP: 38 |
uACP: 165 (101–303); bACP: 157 (102–285) |
uACP: 109 (62–166); bACP: 95 (61–179) |
uACP: 32 (15–88); bACP: 29 (12–74) |
uACP: 7; bACP: 4 |
NM | uACP: 10; bACP: 2 |
uACP: 0.2 (0.04–2.58); bACP: 0.16 (0.04–2.29) | NM | NM |
| Tong [2017] (25) | China | uACP: 53±14; bACP: 47±12.75 |
uACP: 82; bACP: 121 |
uACP: 200.0±53.75; bACP: 204±42.5 |
uACP: 103.0±30.25; bACP: 105.0±31.5 |
uACP: 23.0±9.25; bACP: 24.0±8.0 |
uACP: 11; bACP: 9 |
uACP: 6; bACP: 5 |
uACP: 17; bACP: 14 |
uACP: 17±11.5; bACP: 16±17.75 | NM | uACP: rectal =25.9±1.4/nasopharyngeal =23.7±1.1; bACP: rectal =26.0±1.3/nasopharyngeal =23.6±1.1 |
| Angleitner [2020] (29) | Austria | uACP: 60.9±14.3; bACP: 61.0±13.8 |
uACP: 93; bACP: 91 |
uACP: 209 (159–265); bACP: 227 (185–303) |
uACP: 107 (79–152); bACP: 126 (91–172) |
uACP: 38 (30–57); bACP: 30 (25–45) |
uACP: 18; bACP: 17 |
uACP: 9; bACP: 7 |
uACP: 15; bACP: 11 |
NM | uACP: 400 (335–480); bACP: 415 (342–515) |
NM |
| Norton [2020] (28) | USA | uACP: 59.5 (52–69.5); bACP: 57 (48–66) |
uACP: 140; bACP: 167 |
uACP: 224.5 (191.5–280); bACP: 230 (188–285) |
uACP: 144 (103–184.5); bACP: 173 (133–224) |
uACP: 29 (22.5–38); bACP: 45 (38–55) |
NM | NM | uACP: 2/49; bACP: 4/49 |
NM | NM | uACP: 20 (18–24); bACP: 17 (16–18) |
| Song [2022] (30) | South Korea | uACP: 61.8±14.2; bACP: 62.2±14.0 |
uACP: 94; bACP: 94 |
NM | NM | NM | uACP: 8; bACP: 11 |
uACP: 19; bACP: 17 |
uACP: 7; bACP: 14 |
NM | NM | uACP: rectal =27.5 (26.7–28.0)/esophagus =23.9 (22.0–25.2); bACP: rectal =27.7 (27.0–28.1)/esophagus =23.9 (22.1–25.2) |
| Inamura [2006] (26) | Japan | uACP: 60.7±10.8; bACP: 57.8±10.3 |
uACP: 19; bACP: 19 |
uACP: 259±30; bACP: 262±36 |
NM | uACP: 64.2±19.8; bACP: 54.9±14.5 |
uACP: 3; bACP: 1 |
uACP: 4; bACP: 4 |
uACP: 4; bACP: 2 |
NM | uACP: 507.4±107.2; bACP: 485.2±114.9 |
NM |
| Piperata [2022] (35) | Japan | uACP: 66 (59–74); bACP: 65 (57–75) |
uACP: 189; bACP: 189 |
uACP: 170 (148–204); bACP: 195 (162–233) |
uACP: 82 (66–112); bACP: 100 (83–130) |
uACP: 35 (28–44); bACP: 36 (28–44) |
uACP: 8; bACP: 26 |
uACP: 21; bACP: 23 |
uACP: 13; bACP: 19 |
uACP: 4 (2–8); bACP: 4 (2–7) |
NM | uACP: 28 (28–28); bACP: 27.5 (25–28) |
| Krüger [2011] (4) | Germany | uACP: 59.77±13.67; bACP: 60.41±13.81 |
uACP: 628; bACP: 453 |
NM | NM | uACP: 32.2±17.9; bACP: 37.6±23.6 |
uACP: 12.6 (10.0–15.2); bACP: 14.1 (10.9–17.3) |
NM | uACP: 13.9 (11.1–16.6); bACP: 15.9 (12.5–19.3) |
uACP: 8.4±13.9; bACP: 8.9±12.5 |
uACP: 318.3±101.8; bACP: 345.2±111.0 |
NM |
| Preventza [2015] (33) | USA | uACP: 58.2±14.9; bACP: 58.9±14.6 |
uACP: 90; bACP: 63 |
uACP: 126.6±40.1; bACP: 121.7±56.5 |
NM | uACP: 33.7±11.4; bACP: 45.6±26.5 |
uACP: 12; bACP: 7 |
uACP: 10; bACP: 5 |
uACP: 12; bACP: 8 |
uACP: 12.6±13.4; bACP: 11.8±13.7 |
uACP: 366.9±101.3; bACP: 364.8±104.2 |
NM |
| Liu [2020] (32) | China | uACP: 55.38±10.40; bACP: 54.00±9.37 |
uACP: 124; bACP: 197 |
uACP: 260.07±76.79; bACP: 235.79±46.60 |
uACP: 154.53±36.50; bACP: 154.94±33.75 |
uACP: 26.60±6.78; bACP: 25.62±6.31 |
uACP: 22; bACP: 16 |
uACP: 32; bACP: 31 |
uACP: 12; bACP: 10 |
uACP: 18.73±5.67; bACP: 17.10±5.18 | NM | uACP: nasopharngeal =24.92±0.28; bACP: nasopharngeal =27.09±1.22 |
| Dong [2020] (31) | China | uACP: 49.8±11; bACP: 50.6±12 |
uACP: 36; bACP: 25 |
uACP: 218±39; bACP: 174±29.1 |
uACP: 129.3±26.4; bACP: 96.2±20.4 |
NM | uACP: 3; bACP: 0 |
uACP: 5; bACP: 2 |
uACP: 3; bACP: 0 |
uACP: 2.45 (0.9–6.18); bACP: 1.41 (0.77–2.083) | uACP: 468±72; bACP: 420±48 |
uACP: 24.6±0.9; bACP: 29±0.8 |
| Jiang [2023] (34) | China | uACP: 53 (47–60); bACP: 53 (48–59) |
uACP: 276; bACP: 319 |
uACP: 187±19; bACP: 189±23 |
uACP: 90 (79–101); bACP: 89 (79–98) |
uACP: 20±2; bACP: 20±2 |
uACP: 22/276; bACP: 9/319 |
uACP: 27; bACP: 21 |
uACP: 38; bACP:17 |
uACP: 4 (3–5); bACP: 3 (2–3) |
uACP: 401±24; bACP: 404±26 |
uACP: rectal =22.1 (20.3–26.6)/nasopharyngeal =20.2 (18.7–25.3); bACP: rectal =22.3 (20.6–26.2)/nasopharyngeal =20.6 (19.2–24.6) |
| Montagner [2022] (16) | Germany | uACP: 69 (59–77); bACP: 66 (60–75.3) |
uACP: 62; bACP: 62 |
uACP: 202 (166.5–254.5); bACP: 197.5 (152.8–254.5) |
uACP: 92.5 (78.3–123); bACP: 103.5 (82–133) |
uACP: 31.5 (21–41); bACP: 35.5 (29.5–44.3) |
NM | NM | uACP: 11; bACP: 11 |
uACP: 8 (3–20.8); bACP: 10 (4–20) |
uACP: 390 (333–470); bACP: 377.5 (296.8–449) |
uACP: 27.5 (24–28); bACP: 28 (26–28) |
| Benedetto [2021] (15) | UK | uACP: 62.3±15.4; bACP: 62.5±13.6 |
uACP: 117; bACP: 760 |
NM | uACP: 120±53.0; bACP: 141±70.4 |
uACP: 34.7±20.5; bACP: 43.7±36.0 |
NM | NM | uACP: 19; bACP: 135 |
NM | NM | NM |
Data are presented as mean ± standard deviation, median (interquartile range), or mean and 95% CI or number. uACP, unilateral antegrade cerebral perfusion; bACP, bilateral cerebral perfusion; CPB time, cardiopulmonary bypass; CA, circulatory arrest; PND, permanent neurologic dysfunction; TND, transient neurologic deficit; ICU, intensive care unit; NM, not mentioned; CI, confidence interval.
Table 5
| Study | Surgery strategy | Outcome |
|---|---|---|
| Usui 1999 (23) | ACP vs. RCP | RCP is preferred over ACP. Moreover, RCP decreases the chance of brain embolization |
| Zierer 2005 (24) | ACP vs. RCP | The antegrade group had a higher mortality rate. The ACP group had lower CPB time, and ACP was effective in cerebral protection |
| Williams 2012 (22) | ACP vs. RCP | ACP group had a higher mortality rate. The study suffered from selection bias |
| Shemirani 2017 (36) | ACP vs. RCP | ACP had lower mortality in the long term, and ACP was preferred over the RCP method |
| Stamou 2016 (13) | ACP vs. RCP | ACP and RCP both are safe methods for brain protection |
| Apostolakis 2008 (14) | ACP vs. RCP | ACP had a lower incidence rate of TND and shorter ICU-stay time. ACP and RCP both had almost similar mortality rates |
| Wiedemann 2013 (21) | ACP/HCA vs. RCP/HCA | The ACP group had a longer three and five-year survival rate than the RCP |
| uACP/HCA vs. bACP/HCA | Both uACP and bACP groups had similar mortality rates | |
| Tokuda 2014 (20) | ACP/HCA vs. RCP/HCA | ACP and RCP groups had the same mortality rate and neurologic complications |
| Sun 2021 (9) | ACP vs. RCP | The ACP group had a higher PND incidence rate compared to RCP, and the mortality rate between the groups was almost equal |
| Samanidis 2021 (17) | ACP/MHCA vs. RCP/DHCA | Both ACP and RCP had equivalent rates of early mortality, ICU-stay, and incidence of PND |
| Sugiura 2012 (19) | ACP/MHCA vs. RCP/MHCA | ACP group had no advantage over RCP. Both groups had equivalent rates of early mortality and PND |
| Sinatra 2001 (18) | ACP/DHCA vs. RCP/DHCA | Mortality and neurologic complications were not significant between the groups |
| O’Hara 2020 (27) | ACP vs. RCP | Mortality between the groups was not significant |
| Montagner 2022 (16) | uACP vs. bACP vs. RCP | Operation time in the RCP group was longer compared to other groups. uACP, bACP, and RCP methods were safe and equivalent in terms of mortality and neurologic complications |
| Benedetto 2021 (15) | uACP/DHCA vs. bACP/DHCA vs. RCP/DHCA | uACP and bACP were preferred over DHCA alone |
| Tong 2017 (25) | uACP vs. bACP | bACP had no advantage over uACP in terms of mortality and PND incidence |
| Angleitner 2020 (29) | uACP vs. bACP | bACP had better overall survival compared to uACP |
| Norton 2020 (28) | uACP vs. bACP | uACP and bACP had almost the same results in terms of mortality and stroke. However, uACP was a simple method and less complicated compared to bACP |
| Song 2022 (30) | uACP vs. bACP | There were no significant changes between the two groups in mortality, PND and TND |
| Inamura 2006 (26) | uACP vs. bACP | No significant changes between uACP and bACP in PND, TND and mortality (P value >0.05) |
| Piperata 2022 (35) | uACP/MHCA vs. bACP/MHCA | uACP and bACP had no differences in mortality and TND. While bACP had a higher incidence rate of PND. uACP is suggested when considering all limitations |
| Jiang 2023 (34) | uACP vs. bACP | bACP had a lower incidence of PND and mortality compared to uACP |
| Krüger 2011 (4) | uACP vs. bACP | bACP had a higher incidence of PND and mortality than the bACP group |
| Preventza 2015 (33) | uACP vs. bACP | uACP is preferred for its less complicated Technique. Both groups had the same rates of mortality and TND |
| Liu 2020 (32) | uACP vs. bACP | bACP had a lower incidence of PND and TND, while no significant changes were observed in mortality |
| Dong 2020 (31) | uACP/MHCA vs. bACP/MHCA | Both groups had almost same incidence of PND, TND and mortality (P value >0.05) |
ACP, antegrade cerebral perfusion; RCP, retrograde cerebral perfusion; CPB, cardiopulmonary bypass; TND, transient neurologic deficit; ICU, intensive care unit; HCA, hypothermic circulatory arrest; uACP, unilateral antegrade cerebral perfusion; bACP, antegrade bilateral cerebral perfusion; PND, permanent neurologic dysfunction; MHCA, moderate hypothermic circulatory arrest; DHCA, deep hypothermic circulatory arrest.
Synthesis of results
In our meta-analysis, we analyzed seven possible end-points: CPB time, PND, TND, CA time, CCT, ICU-stay duration, and operative mortality. The estimated mean age in the uACP group was 59.18±5.36 years and in bACP was 58.11±5.66 years.
CPB time
The difference in means for the CPB time in the ACP-RCP comparison is demonstrated in Figure 2A. Ten studies reported data on CPB time. Due to the evidence of high heterogeneity of the intervention effects on CPB time among studies, we used the random effects. The overall MD for the time between ACP and RCP was not statistically significant in the random effects model [MD =−5.5945, 95% CI: (−23.1052, 11.9162), P value =0.5312], and the calculated z-score was −0.63. The heterogeneity test showed significant differences between individual studies [I2=75.3% (53.9%, 86.7%); tau2 =548.5713 (144.0957, 2,525.9154); tau =23.4216 (12.0040, 50.2585); P value <0.01]. The funnel plot was symmetrical (Figure 2B).

In the uACP-bACP comparison, eleven studies reported data on CPB time. Additionally, the uACP-bACP comparison suffered from a high level of heterogeneity; therefore, we used the random effects (Figure 3A). The overall MD for CPB time between ACP and RCP was also not statistically significant [MD =2.0235, 95% CI: (−10.2206, 14.2676), P value =0.7460]. The calculated z-score was 0.32. The heterogeneity test revealed significant differences between individual studies in the uACP-bACP comparison [I2=84.2% (73.2%, 90.6%); tau2 =331.7600 (114.4925, 1,112.2125); tau =18.2143 (10.7001, 33.3498); P value <0.01]. The funnel plot was symmetrical (Figure 3B).
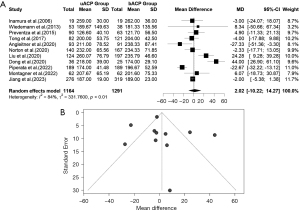
CA time
The difference in means for CA time in the ACP-RCP comparison is demonstrated in Figure 4A. We extracted data from nine studies for CA time. There was evidence of high heterogeneity of the intervention effects on CA time among the studies. The overall difference in means for CA time between ACP and RCP was statistically significant in the random effects model [MD =10.1338, 95% CI: (3.7946, 16.4731), P value =0.0017]. The pooled z-score for the overall effect was 3.13. Due to the high heterogeneity, we used a random effects model [I2=97.0% (95.8%, 97.9%); tau2 =73.3361 (26.5063, 418.6038); tau =8.5636 (5.1484, 20.4598); P value <0.01]. CA time was higher in the ACP group. The funnel plot was symmetrical (Figure 4B).

In the uACP-bACP comparison, 12 studies reported data on CA time. Additionally, the uACP-bACP comparison suffered from heterogeneity, thus we used the random effects model (Figure 5A). The overall MD for the CA time in ACP and RCP was not statistically significant [MD =−2.7784, 95% CI: (−7.0227, 1.4660), P value =0.1995]. The calculated z-score was −1.28. The heterogeneity test revealed significant differences between individual studies in the uACP-bACP comparison [I2=94% (92.0%, 96.2%); tau2 =47.9470 (20.6292, 164.7087); tau =6.9244 (4.5419, 12.8339); P value <0.01]. The funnel plot was symmetrical (Figure 5B).

CCT
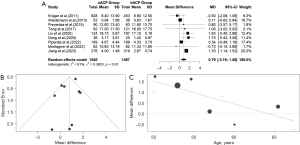
The difference in means for CCT in the ACP-RCP comparison is demonstrated in Figure 6A. We extracted data from four studies for CCT. There was evidence of low heterogeneity of the intervention effects on CCT among the studies. The overall difference in means for CCT between ACP and RCP was not statistically significant in the common effect model [MD =−1.3130, 95% CI: (−6.5450, 3.9189), P value =0.6228]. The pooled z-score for the overall effect was −0.49. Heterogeneity was low; therefore, we used a common effect model [I2=0.0% (0.0%, 84.7%); tau2 =0 (0.0000, >100.0000); tau =0 (0.0000, >10.0000); P value =0.5278]. The funnel plot was symmetrical (Figure 6B). In the meta-regression analysis of CCT and age, the estimated difference between the groups was 0.9754, and the P value was 0.3443. Additional information is presented in Figure 6C.

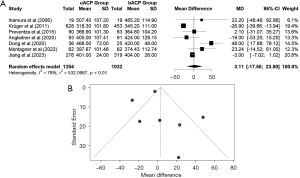
The difference in means for the CCT in the uACP-bACP comparison was demonstrated in Figure 7A. We extracted data from 10 studies for the CCT. There was evidence of high heterogeneity of the intervention effects on the CCT among the studies. The overall difference in means for the CCT between uACP and bACP was not statistically significant in the random effects model [MD =−6.4517, 95% CI: (−17.7371, 4.8336), P value =0.2625]. The pooled z-score for the overall effect was −1.12. Due to the high heterogeneity, we used a random effects model [I2=90% (84.6%, 94.1%); tau2 =285.8772 (111.7485, 1,023.7113); tau =16.9079 (10.5711, 31.9955); P value <0.01]. CCT was almost similar between the uACP and bACP. The funnel plot was symmetrical (Figure 7B). In the meta-regression analysis of CCT and age, the estimated difference between the groups was −1.7886, and the P value was 0.0223 (Figure 7C).

ICU-stay time
The difference in means for ICU-stay time in the ACP-RCP comparison is demonstrated in Figure 8A. We extracted data from five studies for ICU-stay time. There was evidence of high heterogeneity of the intervention effects on CCT among the studies. The overall difference in means for ICU-stay time between ACP and RCP was not statistically significant in the random effects model [MD =−0.5891, 95% CI: (−1.6257, 0.4475), P value =0.2654]. The pooled z-score for the overall effect was −1.11. Heterogeneity was high; therefore, we used a random effects model [I2=59% (0.0%, 84.5%); tau2 =0.8293 (0.0000, 11.8364); tau =0.9107 (0.0000, 3.4404); P value =0.047]. The funnel plot was symmetrical (Figure 8B). In the meta-regression analysis of ICU-stay time and age, the estimated difference between the groups was 0.1480, and the P value was 0.4622 (Figure 8C).

The difference in means for ICU-stay time in the uACP-bACP comparison is demonstrated in Figure 9A. We extracted data from nine studies for ICU-stay time. There was evidence of high heterogeneity in the intervention effects on ICU-stay time among the studies. The overall difference in means (95% CI) for ICU-stay time between uACP and bACP was statistically significant in the random effects model [MD =0.7928, 95% CI: (0.1904, 1.3952), P value =0.0099]. The pooled z-score for the overall effect was 2.58. Due to the high heterogeneity, we used a random effects model [I2=61.4% (20.2%, 81.4%); tau2 =0.3653 (0.0150, 1.8705); tau =0.6044 (0.1226, 1.3677); P value <0.01]. The uACP group had longer ICU-stay times compared to bACP. The funnel plot was symmetrical (Figure 9B). In the meta-regression analysis of ICU-stay time and age, the estimated difference between the groups was 0.7928, and the P value was 0.0099 (Figure 9C).
TND
The forest plot for TND in the ACP and RCP comparison is demonstrated in Figure 10A. Data was extracted from four studies regarding TND. There was evidence of low heterogeneity in the intervention effects of TND among the studies. The pooled RR (95% CI) for TND between uACP and bACP was RR =0.8768, 95% CI: (0.6508, 1.1814), P value =0.3875. The pooled z-score for the overall effect was −0.86. Due to the low heterogeneity, the common effect model was used [I2=0.0% (0.0%, 84.7%); tau2 =0 (0.0000, 2.4121); tau =0 (0.0000, 1.5531)]. TND did not differ between the groups. The funnel plot was symmetrical (Figure 10B).

The forest plot for TND in the uACP and bACP comparison is demonstrated in Figure 11A. Data was extracted from nine studies regarding TND. There was evidence of low heterogeneity in the intervention effects of TND among the studies. The pooled RR for TND between uACP and bACP was RR =1.32, 95% CI: (1.05, 1.67), P value =0.0199. The pooled z-score for the overall effect was 2.33. Due to the low heterogeneity, the common effect model was used [I2=0% (0.0%, 64.8%); tau2 =0 (0.0000, 0.0604); tau =0 (0.0000, 0.2458), P value =0.8835]. The risk of TND occurring in bACP is higher than in uACP. The funnel plot was symmetrical (Figure 11B).

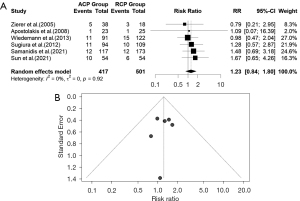
PND
The forest plot for PND between ACP and RCP is demonstrated in Figure 12A. We extracted data from six studies for PND. There was evidence of low heterogeneity of the intervention effects on PND among studies, therefore we used the common effect model. The pooled RR (95% CI) for PND between ACP and RCP was 1.23 RR, 95% CI: (0.84, 1.80) (P value= 0.2662). The calculated z-score for the overall effect was 1.11. We used the common effect model due to low heterogeneity [I2=0% (0.0%, 74.6%); tau2 =0 (0.0000, 0.1674); tau =0 (0.0000, 0.4091); P value =0.9201]. As a result, the chance of PND in ACP and RCP is similar. The funnel plot was symmetrical (Figure 12B). In the uACP-bACP comparison, ten studies reported data on PND. However, this suffered from high heterogeneity therefore we used the random effects (Figure 13A). The pooled RR for the PND between uACP and bACP was RR =1.2786, 95% CI: (0.7931, 2.0615) (P value =0.3132). The estimated z-score was 1.01. The heterogeneity test revealed significant differences between individual studies in uACP-bACP comparison [I2=63.5% (27.9%, 81.5%), tau2 =0.3390 (0.0456, 1.5754); tau =0.5822 (0.2135, 1.2552); P value =0.0034]. No difference was seen between uACP and bACP in terms of PND. The funnel plot was symmetrical (Figure 13B).

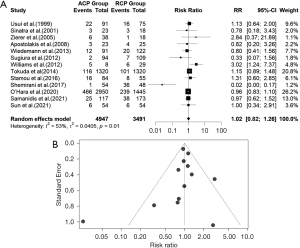
Operative mortality
The forest plot for operative mortality in the ACP-RCP comparison is shown in Figure 14A. Data were extracted from thirteen studies on operative mortality. There was evidence of high heterogeneity in the intervention effects on operative mortality among the studies. The pooled RR (95% CI) for operative mortality in the ACP and RCP comparison was RR =1.02, 95% CI: (0.82, 1.26), P value =0.9368. The calculated z-score for the overall effect was 0.08. We used a random effects model due to the high heterogeneity [I2=53% (11.7%, 74.9%); tau2 =0.0405 (0.0229, 2.9795); tau =0.2012 (0.1512, 1.7261); P value =0.0126]. The funnel plot was symmetrical (Figure 14B). In the uACP-bACP comparison, thirteen studies reported data on operative mortality. Additionally, the uACP-bACP comparison suffered from high heterogeneity, therefore, we used a random effects model (Figure 15A). The RR (95% CI) for operative mortality between uACP-bACP groups was RR =1.2117, 95% CI: (0.8797, 1.6691), P value =0.2398. The estimated z-score was 1.18. The heterogeneity test showed significant differences between individual studies in uACP-bACP comparison [I2=52.0% (9.6%, 74.5%), tau2 =0.1619 (0.0085, 0.8291); tau =0.4024 (0.0921, 0.9106); P value =0.0149]. uACP has no advantage over bACP in terms of operative mortality. Similarly, ACP and RCP operative mortalities are equivalent. The funnel plot was symmetrical (Figure 15B).

Core temperature
The difference in means for the core temperature in the ACP-RCP comparison is demonstrated in Figure 16A. Eight studies reported data on core temperature. Due to the evidence of high heterogeneity of the intervention effects on core temperature among studies, we used the random effects. The overall MD for the core temperature between ACP and RCP was statistically significant in the random effects model [MD =1.8563, 95% CI: (0.9263, 2.7863), P value =0.0244]; the core temperature was higher for the ACP group compared to the RCP, and the calculated z-score was 3.91. The heterogeneity test showed significant differences between individual studies [I2=88.4% (79.5%, 93.5%), tau2 =1.4050 (0.4572, 11.4114); tau =1.1853 (0.6762, 3.3781); P value <0.01]. The included studies did not have significant publication bias (P value =0.96). The funnel plot was symmetrical (Figure 16B).
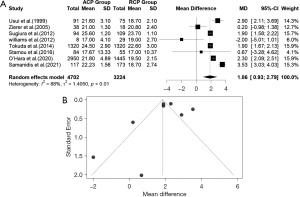
Also, the difference in means for the core temperature in the uACP-bACP comparison is depicted in Figure 17A. Eight studies reported data on core temperature comparing the uACP and bACP methods. Due to the evidence of high heterogeneity of the intervention effects on core temperature among studies, we used the random effects. The heterogeneity test demonstrated significant differences between individual studies [I2=99.1% (98.8%, 99.3%), tau2 =5.4484 (2.3337, 22.8482); tau =2.3342 (1.5276, 4.7800); P value <0.01]. Unlike the core temperature in ACP-RCP comparison, the overall MD (95% CI) for the core temperature between uACP and bACP was not statistically significant in the random effects model [MD =−0.3310, 95% CI: (−1.9611, 1.2992), P value =0.6907], and the calculated z-score was −0.40. The core temperature was almost the same between the uACP and bACP. The funnel plot was symmetrical (Figure 17B).

Operation time
The difference in means for operation time in the uACP-bACP comparison is demonstrated in Figure 18A. We extracted data from seven studies for operation time. There was evidence of high heterogeneity in the intervention effects on ICU-stay time among the studies. The overall difference in means for ICU-stay time between uACP and bACP was not statistically significant in the random effects model [MD =3.1062, 95% CI: (−17.5845, 23.7970), P value =0.7686]. The pooled z-score for the overall effect was 0.29. Due to the high heterogeneity, we used a random effects model [I2=78% (54.0%, 89.3%); tau2 =532.0987 (97.2462, 3,086.4432); tau =23.0673 (9.8613, 55.5558); P value <0.01]. Operation time was almost the same in the uACP and bACP groups. The funnel plot was symmetrical (Figure 18B).
Publication bias
The funnel plot analysis, as shown in the figures, did not reveal any significant asymmetry concerning the treatment effect for any of the examined outcomes in the study. As a result, there is no publication bias related to the outcomes.
Discussion
This meta-analysis showed no significant difference between the two main cerebral perfusion methods in terms of mortality and PND; while TND risk in the bACP group was higher than the uACP group, ICU-stay time was longer in the uACP group compared to bACP, CA time during ACP was longer than during RCP, and core temperature was higher in ACP. There were no significant differences in CPB, and operative mortality endpoints, whereas TND in uACP was higher compared to bACP. In meta-regression analysis an increase in age is associated with longer ICU stay time and higher CCT in uACP compared to bACP.
ACP requires a more complicated setting during operation and approximates the physiological brain perfusion through the cannula (37). In ACP, the CA time is prolonged given the safe operation time duration for surgery. However, it may increase the risk of embolic events and neurologic deficits (38). On the other hand, RCP decreases the risk of emboli formation, but RCP cannot allocate blood flow in the brain the same as ACP and cannot provide normal brain perfusion (39). The heterogeneity of the studies strongly influenced the endpoints. In our study, the risk of mortality and PND were similar between the comparator groups. TND risk did not differ between ACP and RCP, however, the pooled RR for TND demonstrated that uACP is superior to bACP in preventing TND in ATAAD surgery.
For the last decade, ACP and RCP methods have been used for brain protection in the aortic arch, ATAAD, and aortic aneurysm surgery. Both methods can maintain cerebral perfusion in ATAAD surgeries. However, these methods have advantages and disadvantages. Presumably, ACP may be associated with a greater risk of cerebral embolism, while RCP may be associated with a greater risk of cerebral hypoperfusion (40). There remains controversy over which technique is preferable. A general agreement on which technique, ACP or RCP, provides superior clinical cerebral protection efficiency is needed. Considering ACP has no advantage over RCP, further research on this topic is essential, particularly in institutions using advancing minimally invasive procedures.
To resolve this debate, we assessed operative mortality, PND, CA time, and CPB time in both groups. Several studies consistently report a close association between embolic events and PND after aortic surgery, which is not correlated with the method used for brain protection (25,41,42). However, there are conflicting results regarding operative mortality. Montagner et al. evaluated three cerebral perfusion methods in a retrospective, matched study. The authors reported that there was no difference in early mortality between the groups (P value =0.86). They also found that unilateral, bACP and RCP are safe, and techniques that can be performed based on the patient’s condition, availability of clinical center, and surgeon expertise. However, Montagner et al. study had a small number of patients after propensity score matching which may lead to statistical mistakes. Also, the study was limited because of its retrospective design and single-center setting (16). In addition, Sun et al. investigated 223 patients who underwent ATAAD surgery with either ACP or RCP strategies. Both strategies had almost equal early mortality rates (10.9% vs. 15.4% before matching, P value =0.402). The small number of patients and retrospective data were the deficiencies of this study (9). However, Shemirani et al. found that the ACP method, during hypothermic circulatory arrest (HCA), had lower mortality than RCP in terms of long-term mortality (P<0.001) (36).
Some other studies reported the mortality rate in ACP during moderate hypothermic circulatory arrest (MHCA/ACP) and RCP during deep hypothermic circulatory arrest (DHCA/RCP). Samanidis et al. reported that the 30-day mortality rate in MHCA/ACP (early mortality rate =22.0%) and in DHCA/RCP (early mortality rate =21.4%) were almost equal. The authors also evaluated the PND rates; PND was 2.3% with RCP vs. 3.4% with ACP, and no difference was seen between the two groups. The small number of ATAAD patients was the limitation of this study (17). However, in a propensity-matched analysis of 290 patients who underwent aortic arch surgery, the MHCA/ACP method had lower 30-day mortality compared with DHCA/RCP (MHCA/ACP mortality rate =7.5% vs. DHCA/RCP mortality rate =22.5%) (43). In the latter study, aortic aneurysm data was also analyzed. In this meta-analysis, we only included the ATAAD patients and excluded other types of aortic surgery. More data is required to evaluate the mortality risk ratio in MHCA/ACP vs. DHCA/RCP. In addition, in a meta-analysis of 5,060 patients who underwent aortic arch surgery with DHCA + ACP or DHCA + RCP, there was no difference in mortality and PND; the early mortality incidence was 5.184% for the ACP and 5.175% for the RCP group [RR =1.12, 95% CI: (0.84, 1.49), P=0.432], and the PND incidence was 6% for ACP and 4.7% for RCP group [RR =1.02, 95% CI: (0.75, 1.37), P=0.911] (44). In another meta-analysis of 7,023 patients who underwent aortic surgery, both DHCA plus ACP and DHCA plus RCP groups had similar outcomes in terms of mortality rate [RR =1.182, 95% CI: (0.957, 1.459)] and PND [RR =0.991, 95% CI: (0.747, 1.316)] (45). Similarly, according to our analysis, both ACP and RCP methods in ATAAD surgery have similar mortality rates. Our analysis showed that the PND rate was the same between the groups. Theoretically, RCP decreases the risk of emboli, but a further multicenter study with a larger sample size is required to evaluate the incidence of PND in ACP and RCP groups. Thus, the choice of procedure can be determined based on clinic resources and individual patient conditions. Moreover, our analysis showed that CA time is longer in ACP compared to RCP.
Also, some studies concluded that CA time is longer in the ACP group. For instance, Tokuda et al. demonstrated that CA time was 116±36 min for ACP and 102±38 min for RCP (P value <0.001) (20). Also, in evaluating a total of 1,929 patients who underwent ATAAD surgery, Benedetto and colleagues showed that CA time was shorter in the RCP group (RCP 31.2±18.5 vs. uACP 34.7±20.5) (15). However, some other studies by Wiedemann et al. and Stamou et al. found no significant difference between ACP and RCP groups. The limitations of these studies include their retrospective multicenter evaluations and small sample sizes. Since different surgeons from different centers performed the surgeries, the analysis was probably biased. The results may be affected by selection bias, and the outcomes regarding each neuroprotection approach may reflect the results of the surgeon who performed the procedure (13,21). Overall, CA time is longer ACP. Two studies (16,29) reported data for uACP and bACP (15,16). In both of the studies, CA time was significantly higher in the bACP group. However, there was an inconsistency in CPB time between the two studies. Including 967 uACP patients and 879 bACP patients, a meta-analysis study reported similar results in the HCA and cerebral perfusion times, mortality, PND, TND, acute kidney injury, and reoperation for bleeding between uACP and bACP groups (46). Furthermore, we observed that the core temperature was notably elevated in the ACP group, resulting in reduced durations for cooling and rewarming, as well as the operation and CPB times (31). Nonetheless, we did not identify any significant difference in the CPB durations between the groups.
The primary focus of our meta-analysis was ATAAD surgery outcomes, while the previous meta-analysis conducted by Hu et al. (44), Guo et al. (45), and Hameed et al. (47) included aortic arch surgeries, we only included acute type and emergent settings presenting with aortic dissection and excluded elective procedures such as aortic aneurysms. The previous meta-analysis also showed that both RCP and ACP groups in aortic arch surgeries were safe and had an acceptable early mortality rate. These meta-analyses found that ACP and RCP techniques have the same cerebral protection during HCA (44,45). Moreover, our work distinguishes itself from recently reported meta-analyses by adding papers published in recent years, minimizing historical bias, and including a larger sample size than the study by Guo et al. (45) and Hu et al. (44). The study by Hameed et al. included the DHCA method as well as ACP and RCP, and conducted a network meta-analysis (47). The analysis revealed no differences between ACP and RCP for all outcomes. Our data confirm the advantage of both brain protection techniques in survival after CA. Also, bACP is a superior method for preventing TND. These results are in line with the narrative review of 24 original articles published by Pitts et al., in which the authors conclude that the use of ACP is favored for surgeries under moderate hypothermia compared to RCP and deep hypothermia. While bACP is suggested for longer CA durations, uACP is safe for shorter durations. There is no definitive time threshold established, but 30–50 min has been proposed (48). Besides, RCP is the less complicated method and can be performed in more centers and by most surgeons, as well as with minimal procedures, without an increase in CPB, CA, or hospital stay time.
Finally, ATAAD surgery is a complex and evolving field with various surgical methods, perfusion techniques, CA time, and treatments. Identifying predictive factors of mortality in ATAAD surgery remains challenging, indicating the need for further research in cerebral perfusion during aortic surgery. However, if a surgeon is faced with a scenario where the risk of TND is a significant concern (such as poor preoperative mental status, diabetes, and manifest peripheral arterial disease) and they have the expertise and resources to manage ACP, they might opt for uACP (49).
Limitations
This study has some limitations. First, most of the included studies were retrospective observational studies. Retrospective studies have the possibility of selection bias. Second, ATAAD surgery is performed in acute settings, causing various confounding factors such as the patient’s baseline status, operation procedure, anesthesia treatment, different surgeons, and operation time, which can lead to bias. Third, the small sample size of some studies may cause bias and heterogeneity. Fourth, aggregated data from the studies were used for pooled analysis instead of individual patient-level data. We did not include the operation time endpoint for ACP-RCP comparison due to inadequate statistical evidence of the included articles. Further evaluation of operation time is required between the ACP and RCP.
Conclusions
In conclusion, this meta-analysis demonstrated that the ACP and RCP are both safe and preferable techniques to use in emergent settings. There was no significant difference between the two methods in terms of operative mortality. ACP and RCP both were safe for PND and TND, also uACP and bACP are equivalent in terms of PND. However, the uACP technique is preferred due to the lower risk of TND compared to the bACP. Both strategies can be used in operations, and the approach depends on the patient’s conditions and considering clinical centers or surgeons’ experience and preferences. Future studies are required to evaluate which strategy has more benefits for patients undergoing aortic dissection surgery, such as randomized clinical trials to evaluate the mortality and risk of neurologic deficits in each group.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1039/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1039/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1039/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Malaisrie SC, Szeto WY, Halas M, et al. 2021 The American Association for Thoracic Surgery expert consensus document: Surgical treatment of acute type A aortic dissection. J Thorac Cardiovasc Surg 2021;162:735-758.e2. [Crossref] [PubMed]
- Gawinecka J, Schönrath F, von Eckardstein A. Acute aortic dissection: pathogenesis, risk factors and diagnosis. Swiss Med Wkly 2017;147:w14489. [Crossref] [PubMed]
- Giambuzzi I, Mastroiacovo G, Roberto M, et al. Preoperative neurological dysfunctions: what is their meaning in patients presenting with acute type A aortic dissection? Minerva Cardioangiol 2020;68:511-7. [Crossref] [PubMed]
- Krüger T, Weigang E, Hoffmann I, et al. Cerebral protection during surgery for acute aortic dissection type A: results of the German Registry for Acute Aortic Dissection Type A (GERAADA). Circulation 2011;124:434-43. [Crossref] [PubMed]
- Dumfarth J, Kofler M, Stastny L, et al. Stroke after emergent surgery for acute type A aortic dissection: predictors, outcome and neurological recovery. Eur J Cardiothorac Surg 2018;53:1013-20. [Crossref] [PubMed]
- Pitts L, Kofler M, Montagner M, et al. The impact of malperfusion patterns in elderly patients undergoing surgery for acute type A aortic dissection. Eur J Cardiothorac Surg 2023;64:ezad288. [Crossref] [PubMed]
- Bassano C, Nardi P, Colella DF, et al. Neurologic Dysfunction after Aortic Dissection Surgery: Different Cerebral Hypothermic Antegrade Perfusion Techniques. J Anesth Clin Res 2018;9:813.
- Weisberg AB, Nemeh H, Kabbani L, et al. Operative strokes after repair of acute type A dissections: predisposing factors and implications. J Cardiovasc Surg (Torino) 2020;61:220-5. [Crossref] [PubMed]
- Sun S, Chien CY, Fan YF, et al. Retrograde cerebral perfusion for surgery of type A aortic dissection. Asian J Surg 2021;44:1529-34. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [Crossref] [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [Crossref] [PubMed]
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [Crossref] [PubMed]
- Stamou SC, Rausch LA, Kouchoukos NT, et al. Comparison between antegrade and retrograde cerebral perfusion or profound hypothermia as brain protection strategies during repair of type A aortic dissection. Ann Cardiothorac Surg 2016;5:328-35. [Crossref] [PubMed]
- Apostolakis E, Koletsis EN, Dedeilias P, et al. Antegrade versus retrograde cerebral perfusion in relation to postoperative complications following aortic arch surgery for acute aortic dissection type A. J Card Surg 2008;23:480-7. [Crossref] [PubMed]
- Benedetto U, Dimagli A, Cooper G, et al. Neuroprotective strategies in acute aortic dissection: an analysis of the UK National Adult Cardiac Surgical Audit. Eur J Cardiothorac Surg 2021;60:1437-44. [Crossref] [PubMed]
- Montagner M, Kofler M, Pitts L, et al. Matched comparison of 3 cerebral perfusion strategies in open zone-0 anastomosis for acute type A aortic dissection. Eur J Cardiothorac Surg 2022;62:ezac214. [Crossref] [PubMed]
- Samanidis G, Kanakis M, Khoury M, et al. Antegrade and Retrograde Cerebral Perfusion During Acute Type A Aortic Dissection Repair in 290 Patients. Heart Lung Circ 2021;30:1075-83. [Crossref] [PubMed]
- Sinatra R, Melina G, Pulitani I, et al. Emergency operation for acute type A aortic dissection: neurologic complications and early mortality. Ann Thorac Surg 2001;71:33-8. [Crossref] [PubMed]
- Sugiura T, Imoto K, Uchida K, et al. Comparative study of brain protection in ascending aorta replacement for acute type A aortic dissection: retrograde cerebral perfusion versus selective antegrade cerebral perfusion. Gen Thorac Cardiovasc Surg 2012;60:645-8. [Crossref] [PubMed]
- Tokuda Y, Miyata H, Motomura N, et al. Brain protection during ascending aortic repair for Stanford type A acute aortic dissection surgery. Nationwide analysis in Japan. Circ J 2014;78:2431-8. [Crossref] [PubMed]
- Wiedemann D, Kocher A, Dorfmeister M, et al. Effect of cerebral protection strategy on outcome of patients with Stanford type A aortic dissection. J Thorac Cardiovasc Surg 2013;146:647-55.e1. [Crossref] [PubMed]
- Williams ML, Ganzel BL, Slater AD, et al. Antegrade versus retrograde cerebral protection in repair of acute ascending aortic dissection. Am Surg 2012;78:349-51.
- Usui A, Yasuura K, Watanabe T, et al. Comparative clinical study between retrograde cerebral perfusion and selective cerebral perfusion in surgery for acute type A aortic dissection. Eur J Cardiothorac Surg 1999;15:571-8. [Crossref] [PubMed]
- Zierer A, Aybek T, Risteski P, et al. Moderate hypothermia (30 degrees C) for surgery of acute type A aortic dissection. Thorac Cardiovasc Surg 2005;53:74-9. [Crossref] [PubMed]
- Tong G, Zhang B, Zhou X, et al. Bilateral versus unilateral antegrade cerebral perfusion in total arch replacement for type A aortic dissection. J Thorac Cardiovasc Surg 2017;154:767-75. [Crossref] [PubMed]
- Inamura S, Furuya H, Yagi K, et al. Recent surgical outcomes of acute type-A aortic dissection. Tokai J Exp Clin Med 2006;31:109-12.
- O’Hara D, McLarty A, Sun E, et al. Type-A Aortic Dissection and Cerebral Perfusion: The Society of Thoracic Surgeons Database Analysis. Ann Thorac Surg 2020;110:1461-7. [Crossref] [PubMed]
- Norton EL, Wu X, Kim KM, et al. Unilateral is comparable to bilateral antegrade cerebral perfusion in acute type A aortic dissection repair. J Thorac Cardiovasc Surg 2020;160:617-625.e5. [Crossref] [PubMed]
- Angleitner P, Stelzmueller ME, Mahr S, et al. Bilateral or unilateral antegrade cerebral perfusion during surgery for acute type A dissection. J Thorac Cardiovasc Surg 2020;159:2159-2167.e2. [Crossref] [PubMed]
- Song SJ, Kim WK, Kim TH, et al. Unilateral versus bilateral antegrade cerebral perfusion during surgical repair for patients with acute type A aortic dissection. JTCVS Open 2022;11:37-48. [Crossref] [PubMed]
- Dong SB, Xiong JX, Zhang K, et al. Different hypothermic and cerebral perfusion strategies in extended arch replacement for acute type a aortic dissection: a retrospective comparative study. J Cardiothorac Surg 2020;15:236. [Crossref] [PubMed]
- Liu Z, Wang C, Zhang X, et al. Effect of different types of cerebral perfusion for acute type A aortic dissection undergoing aortic arch procedure, unilateral versus bilateral. BMC Surg 2020;20:286. [Crossref] [PubMed]
- Preventza O, Simpson KH, Cooley DA, et al. Unilateral versus bilateral cerebral perfusion for acute type A aortic dissection. Ann Thorac Surg 2015;99:80-7. [Crossref] [PubMed]
- Jiang Q, Huang K, Wang D, et al. A comparison of bilateral and unilateral cerebral perfusion for total arch replacement surgery for non-marfan, type A aortic dissection. Perfusion 2023; Epub ahead of print. [Crossref]
- Piperata A, Watanabe M, Pernot M, et al. Unilateral versus bilateral cerebral perfusion during aortic surgery for acute type A aortic dissection: a multicentre study. Eur J Cardiothorac Surg 2022;61:828-35. [Crossref] [PubMed]
- Shemirani H, Mirmohamadsadeghi A, Mahaki B, et al. Evaluation of Acute Aortic Dissection Type a Factors and Comparison the Postoperative Clinical Outcomes between Two Surgical Methods. Adv Biomed Res 2017;6:85. [Crossref] [PubMed]
- Usui A, Miyata H, Ueda Y, et al. Risk-adjusted and case-matched comparative study between antegrade and retrograde cerebral perfusion during aortic arch surgery: based on the Japan Adult Cardiovascular Surgery Database: the Japan Cardiovascular Surgery Database Organization. Gen Thorac Cardiovasc Surg 2012;60:132-9. [Crossref] [PubMed]
- Svyatets M, Tolani K, Zhang M, et al. Perioperative management of deep hypothermic circulatory arrest. J Cardiothorac Vasc Anesth 2010;24:644-55. [Crossref] [PubMed]
- Elmistekawy EM, Rubens FD. Deep hypothermic circulatory arrest: alternative strategies for cerebral perfusion. A review article. Perfusion 2011;26:27-34. [Crossref] [PubMed]
- Maas C, Kok R, Segers P, et al. Intermittent antegrade/selective cerebral perfusion during circulatory arrest for repair of the aortic arch. Perfusion 1997;12:127-32. [Crossref] [PubMed]
- Hagl C, Ergin MA, Galla JD, et al. Neurologic outcome after ascending aorta-aortic arch operations: effect of brain protection technique in high-risk patients. J Thorac Cardiovasc Surg 2001;121:1107-21. [Crossref] [PubMed]
- Gomibuchi T, Seto T, Naito K, et al. Strategies to improve outcomes for acute type A aortic dissection with cerebral malperfusion. Eur J Cardiothorac Surg 2021;59:666-73. [Crossref] [PubMed]
- Perreas K, Samanidis G, Thanopoulos A, et al. Antegrade or Retrograde Cerebral Perfusion in Ascending Aorta and Hemiarch Surgery? A Propensity-Matched Analysis. Ann Thorac Surg 2016;101:146-52. [Crossref] [PubMed]
- Hu Z, Wang Z, Ren Z, et al. Similar cerebral protective effectiveness of antegrade and retrograde cerebral perfusion combined with deep hypothermia circulatory arrest in aortic arch surgery: a meta-analysis and systematic review of 5060 patients. J Thorac Cardiovasc Surg 2014;148:544-60. [Crossref] [PubMed]
- Guo S, Sun Y, Ji B, et al. Similar cerebral protective effectiveness of antegrade and retrograde cerebral perfusion during deep hypothermic circulatory arrest in aortic surgery: a meta-analysis of 7023 patients. Artif Organs 2015;39:300-8. [Crossref] [PubMed]
- Tian DH, Wilson-Smith A, Koo SK, et al. Unilateral Versus Bilateral Antegrade Cerebral Perfusion: A Meta-Analysis of Comparative Studies. Heart Lung Circ 2019;28:844-9. [Crossref] [PubMed]
- Hameed I, Rahouma M, Khan FM, et al. Cerebral protection strategies in aortic arch surgery: A network meta-analysis. J Thorac Cardiovasc Surg 2020;159:18-31. [Crossref] [PubMed]
- Pitts L, Kofler M, Montagner M, et al. Cerebral Protection Strategies and Stroke in Surgery for Acute Type A Aortic Dissection. J Clin Med 2023;12:2271. [Crossref] [PubMed]
- Haldenwang PL, Wahlers T, Himmels A, et al. Evaluation of risk factors for transient neurological dysfunction and adverse outcome after repair of acute type A aortic dissection in 122 consecutive patients. Eur J Cardiothorac Surg 2012;42:e115-20. [Crossref] [PubMed]


