
Compare three diagnostic criteria of progressive pulmonary fibrosis
Highlight box
Key findings
• Both the 1- and 2-year criterion are the reasonable choice to define progressive pulmonary fibrosis (PPF).
What is known and what is new?
• The 1-year criterion is the standard definition of PPF.
• Our study demonstrated that the 2-year criteria had similar diagnostic efficiency with the 1-year criteria.
What is the implication, and what should change now?
• The clinical practitioners should make flexible application with the 1- and 2-year criteria.
• The 0.5-year standard was inefficient to differentiate the PPF from interstitial lung disease, which should be carefully used in future research and clinical practice.
Introduction
Fibrotic interstitial lung disease (ILD) consists a heterogenous group of diseases characterized by varying degree of pulmonary interstitial inflammation and fibrosis (1). Idiopathic pulmonary fibrosis (IPF) is the most prevalent ILD featured with progressive fibrosing interstitial pneumonia with unknown causes. Apart from IPF, about 13–40% percent of patients with non-IPF fibrotic ILD also experience progressive deterioration in interstitial fibrosis and lung function (2,3), which is defined as progressive pulmonary fibrosis (PPF). PPF typically demonstrates worsening respiratory symptoms, lung function decline, continuing fibrosis and decreased quality of life (4). Several studies have explored the natural history of PPF, including the change in pulmonary function, survival and risk factors for mortality (5-7). However, the results varied greatly according to different diagnostic criterion of PPF.
Recently, nintedanib and pirfenidone have been demonstrated to slow the decline of forced vital capacity (FVC) in patients with PPF (8,9). It is therefore important to identify the progressive phenotype for the precise treatment. Till now, researchers have proposed three diagnostic criteria for PPF, which assess the progression in the preceding 6 (8), 12 (10,11) and 24 (5-7,9) months respectively (12). And the international working group of IPF has proposed a standard definition of PPF (13). However, there have been limited data on the comparison of the three criteria using the same cohort data. The goal of this study was to compare the three different diagnostic criteria. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-481/rc).
Methods
Study population
We reviewed the fibrotic ILD cases diagnosed at People’s Hospital of Deyang City, form January, 2016 to December, 2021. Adult patients with diagnosis of ILD, were included in this analysis. Patients with diagnosis of IPF or with antifibrotic therapy more than 6 months were excluded from the cohort. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of People’s Hospital of Deyang City (No. 2023-04-084-K01) and informed consent was taken from all the patients.
Diagnostic criteria
ILD diagnoses were made by multidisciplinary discussion using established diagnostic criteria. The usual interstitial pneumonia (UIP) pattern defines the typical changes of IPF in radiology and pathology. However, it’s not a clinical diagnosis. IPF was diagnosed according to the recent guidelines (13), with probable IPF defined as a diagnosis of IPF by multidisciplinary discussion. Patients with IPF and probable IPF were excluded for the assessment of progressive fibrosing. PPF was diagnosed by three criteria (Table 1): (I) the 0.5-year criterion: in the preceding 6 months, an absolute decline in FVC% of over 5% or worsening respiratory symptoms not due to cardiac, pulmonary, vascular, or other causes (8); (II) the 1-year criterion: PPF was defined with at least two of the three criteria: worsening respiratory symptoms; in the preceding 12 months, an absolute decline in predicted FVC% of over 5% or an absolute decline in predicted percent diffusing capacity of the lungs for carbon monoxide (DLCO%) of 10%; increased fibrosis on high-resolution computed tomography (HRCT) scan (13); (III) the 2-year criterion: in the preceding 24 months, an absolute decline in predicted FVC% of over 10%, or an absolute decline in predicted FVC% of 5–10% with worsening respiratory symptoms or increased fibrosis on HRCT scan, or worsening respiratory symptoms and increased fibrosis on HRCT (9). Connective tissue disease-associated ILD (CTD-ILD) was diagnosed in collaboration with a rheumatologist using diagnostic criteria where available.
Table 1
| Domain | 5-year | 1-year | 2-year |
|---|---|---|---|
| Symptoms | Worsening respiratory symptoms | Worsening respiratory symptoms | Worsening respiratory symptoms |
| Pulmonary function | An absolute decline in FVC% over 5% | An absolute decline in predicted FVC% over 5% or an absolute decline in DLCO% of 10% | An absolute decline in predicted FVC% over 10%, or an absolute decline in predicted FVC% of 5–10% |
| Radiology | – | Increased fibrosis on HRCT | Increased fibrosis on HRCT |
PPF, progressive pulmonary fibrosis; FVC, forced vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide; HRCT, high-resolution computed tomography.
Data collection
Baseline timepoint for PPF patients was defined as the time when the patients started the assessment of progressive fibrosing. Demographic data, including age and sex, and smoking history were recorded at the first clinic visit. Longitudinal data included pulmonary function test (PFT, including FVC% and DLCO%), six minutes walking distance (6MWD), HRCT and University of California, San Diego Shortness of Breath Questionnaire (UCSD-SOBQ), were measured in the follow-up visits. PFT and 6MWD were conducted out according to the relevant guidelines. There might be bias because of the losing follow up. So, we compared the baseline characteristics of patients with 6, 12, 24 months follow up to patients without 6, 12, 24 months follow up, respectively. UIP pattern on HRCT was defined as basal and subpleural predominant honeycombing opacities associated with traction bronchiectasis (13). HRCT was evaluated by two experienced radiologists separately. The standard of assessment is shown in Figure S1. The controversial cases were discussed, until reaching a consensus.
UCSD-SOBQ is a domain-specific patient-reported measurement. It consists 24 questions assessing the severity of breathlessness in daily activities. Each question requests an answer scored from 0 to 5, representing ‘no dyspnea’ to ‘maximally’. The sum of the scores indicates the severity of dyspnea (14), with higher scores representing worse condition of dyspnea. We used 8 points as the minimal important difference of UCSD-SOBQ, which was derived from patients with IPF (15).
Statistical analysis
Quantitative data were presented as mean ± standard deviation or median (interquartile range); the enumeration data were presented as number (percent); For normally distributed data, one-way ANOVA was used for multi-group comparisons, and then Bonferroni’s correction was adopted for the ex-post test. An independent sample t-test was used for two-group comparisons. P<0.05 was used to indicate statistical significance. All analyses were performed using Stata version 16 (StataCorp LLC, TX, USA).
Survival analysis
Survival time was defined from the date of baseline to the date of death or lung transplantation. Patients were censored at the time of last clinic visit, or initiation of antifibrotic treatment. Kaplan-Meier was used to display the survival time in patients with PPF. Log-rank test was used to compare the survival time in sub-group analysis. Cox regression was performed to determine risk factors for mortality. First, univariate Cox analyses were performed, followed by the multivariate Cox analysis using the significant risk factors (P<0.05) in the univariate analysis. The cutoff value were selected out according to the references (5,16) and the median value of the cohort.
Estimation of FVC% changes
The changes in FVC% was estimated by the mixed-effect model, in which the fixed effects included the time interval for the PFT measurement, three groups of different diagnostic criteria, age, sex, smoking history, baseline FVC% and DLCO%. Then the predicted values of FVC% change were fitted by linear regression, and we also calculated the average change of FVC% in 1 year. For the sensitive analysis, we selected out the patients meeting to 0.5-year criteria but not the 1-year criteria, patients meeting to 2-year criteria but not the 1-year criteria for analysis of survival and PFT measurement.
Results
Baseline data of PPF by the three diagnostic criteria
A total of 2,476 patients diagnosed with non-IPF fibrotic ILD were included (Table S1). We identified 246 PPF patients by 0.5-year standard, 154 patients by 1-year standard and 281 patients by 2-year standard (Figure 1). Among them, 95% patients (n=147) in 1-year group were also included in 2-year group. In contrast, there were only 123 patients both in 0.5-year (50%) and 2-year (44%) group, and 83 patients both in 0.5-year (34%) and 1-year (54%) group. There were no significant differences of baseline characteristics between patients with and without 6-month PFT data (Table S2), with and without 12-month PFT data (Table S3), with and without 24-month PFT data (Table S4).
Then we compared clinical diagnosis, demographic and PFT data of the three groups of patients (Table 2). Specifically, 0.5-year standard included more patients with CTD-ILD [other autoimmune-ILD, 0.5-year (33%) vs. 2-year (21%), P<0.05]. As a result, fewer patients in 0.5-year group had UIP pattern on HRCT (26% vs. 47–49%, P<0.05). The interval time between diagnosis of ILD and diagnosis of PPF were 11, 19, 12 months for 0.5-, 1-, and 2-year groups, respectively. These results indicated that 2- and 1-year standard identified patients with similar clinical characteristics, which were different from the patients in 0.5-year group.
Table 2
| Variable | 0.5-year | 1-year | 2-year |
|---|---|---|---|
| Sample size | 246 | 154 | 281 |
| Unclassifiable ILD | 45 (18%) | 37 (24%) | 58 (21%) |
| HP | 25 (10%) | 18 (12%) | 41 (15%) |
| Other ILD | 34 (14%) | 22 (14%) | 45 (16%) |
| CTD-ILD | 142 (58%) | 77 (50%) | 137 (49%) |
| Rheumatoid arthritis-ILD | 34 (14%) | 19 (12%) | 36 (13%) |
| Scleroderma-ILD | 28 (11%) | 21 (14%) | 43 (15%) |
| Other autoimmune-ILD | 80 (33%) | 37 (24%) | 58 (21%)# |
| Age, years | 62±11 | 64±12 | 63±12 |
| Male sex | 107 (43%) | 66 (43%) | 112 (40%) |
| Ever-smoker | 148 (60%) | 104 (68%) | 184 (65%) |
| Smoking pack-years | 17 [5–36] | 15 [7–29] | 17 [7–35] |
| FVC, %-predicted | 73±20 | 76±19 | 75±19 |
| DLCO, %-predicted | 55±18 | 59±20 | 58±19 |
| 6MWD, meters | 430±130 | 412±119 | 429±113 |
| UCSD-SOBQ total score | 39±25 | 42±25 | 39±24 |
| UIP pattern on HRCT | 65 (26%) | 73 (47%)* | 158 (49%)# |
| Begin with diagnosis† | 113 (46%) | 75 (49%) | 165 (59%)# |
| Time from diagnosis (months)‡ | 11 [7–19] | 19 [8–30] | 12 [7–24] |
| Time interval of evaluation (months)§ | 4.3±1.1 | 7.9±2.6 | 14.7±6.2 |
Data are shown as number (%), mean ± standard deviation, or median (interquartile range). *, P<0.05 the group of 1-year compared with the group of 0.5-year; #, P<0.05 the group of 2-year compared with the group of 0.5-year; †, the number of patients who begin the assessment of progression at the time of clinical diagnosis; ‡, mean time (months) from the time of clinical diagnosis to the time of starting the assessment of progression; §, mean time (months) between the two measurements for assessing progression. PPF, progressive pulmonary fibrosis; ILD, interstitial lung disease; HP, hypersensitivity pneumonitis; CTD-ILD, connective tissue disease-associated ILD; FVC, forced vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide; 6MWD, 6-minute walk distance; UCSD-SOBQ, University of California, San Diego Shortness of Breath Questionnaire; UIP, usual interstitial pneumonia; HRCT, high-resolution computed tomography.
Estimated changes in FVC%
We used mixed effect model to evaluate the factors affecting lung function decline. In the model, we evaluated influences of fixed effects on changes of FVC%, which included time interval for the PFT measurement, age, sex, smoking history, baseline FVC% and DLCO%. Only time interval for PFT measurement was correlated with FVC% change (Figure 2, P<0.001). The average annual change in FVC% was −1.0% (95% CI: −0.4%, −1.6%) in 0.5-year group, −2.7% (95% CI: −1.8%, −3.5%) in 1-year group, and −4.1% (95% CI: −3.5%, −4.5%) in 2-year group.
Survival of PPF
The survival time of the three groups is displayed in Figure 2. All three groups of patients didn’t reach the medium survival time in the 4-year follow up. The 2-, 3-, and 4-year survival rates after diagnosis of progressive fibrosing were 83%, 72%, and 62% in 2-year group, 74%, 66%, and 66% in 1-year group, 85%, 76%, and 74% in 0.5-year group.
For further comparison, we selected out the patients meeting to 0.5-year criteria but not the 1-year criteria (n=154), and compared these patients with group of 1-year (Figure 3). Patients in 0.5-year group had higher survival rate (P=0.004), and milder decrease of FVC% (P<0.001) compared with 1-year group. Then the similar analyses were conducted between 2-year and 1-year group. Patients in group of 2-year had higher survival rate (P=0.02), and milder decrease of FVC% (P=0.002) compared with 1-year group. However, at time of the 4th year, there was similar survival rate and FVC% changes in group of 2-year and 1-year. Besides, compared with group of 1-year, group of 2-year had 1.59% less decline of FVC%. But, the decline of FVC% was 6.53% less in group of 0.5-year, compared with 1-year group. In conclusion, the 1- and 2-year standard included the patients with the similar survival rates and similar FVC descent speed, which were worse than the 0.5-year standard.

Risk factors for mortality of PPF
We performed the univariate Cox regression in patients with PPF (Table 3), and found four risk factors correlated with mortality, including baseline FVC% (HR 3.4, 95% CI: 1.9–6.1, P<0.001), baseline DLCO% (HR 7.3, 95% CI: 2.8–18.7, P<0.001), change of UCSD-SOBQ scores (HR 5.3, 95% CI: 1.2–22.6, P=0.02) and smoking history (HR 2.1, 95% CI: 1.1–3.8, P=0.02). In multivariate analysis, only baseline DLCO% <50% was correlated with mortality, with a hazard ratio of 3.4 (95% CI: 1.1–10.6, P=0.03).
Table 3
| Variable | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Baseline FVC% <60% | 3.4 (1.9–6.1) | <0.001 | 1.6 (0.6–4.2) | 0.30 | |
| Baseline DLCO% <50% | 7.3 (2.8–18.7) | <0.001 | 3.4 (1.1–10.6) | 0.03 | |
| UIP pattern | 1.3 (0.7–2.4) | 0.52 | – | – | |
| Increase of UCSD-SOBQ >8 | 5.3 (1.2–22.6) | 0.02 | 3.2 (0.7–13.8) | 0.13 | |
| Non-CTD vs. CTD | 1.0 (0.6–1.6) | 0.92 | – | – | |
| Smoked | 2.1 (1.1–3.8) | 0.02 | 1.8 (0.6–5.1) | 0.26 | |
| Age† | 1.0 (0.8–1.3) | 0.89 | – | – | |
| GERD | 0.7 (0.4–1.3) | 0.26 | – | – | |
†, age was stratified by 10 years old. FVC, forced vital capacity; DLCO, diffusing capacity of the lung for carbon monoxide; UIP, usual interstitial pneumonia; UCSD-SOBQ, University of California, San Diego Shortness of Breath Questionnaire; CTD, connective tissue disease; GERD, gastroesophageal reflux disease.
Survival analysis of subgroups
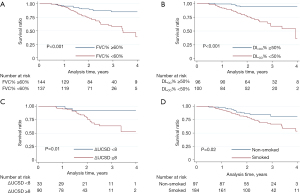
We further compared the survival of subgroups divided by the pre-selected risk factors (Figure 4). The 4-year survival was worse for patients with baseline FVC%<60% (P<0.001) and baseline DLCO% <50% (P<0.001) compared to patients with higher baseline PFT. And patients with changed UCSD-SOBQ <8 (P=0.01) had higher survival rate than those with greater changed UCSD-SOBQ scores. When analyzed by smoking history, the survival at 4-year was higher for patients without smoking history (P=0.02) compared with patients with smoking histories. There was no difference in survival rate between CTD and non-CTD patients (Figure S2).
Discussion
It is vital to distinguish PPF from fibrotic ILDs, given the beneficial effect of antifibrotic therapy for PPF (8,9). However, there are limited data on the comparison of different diagnostic criteria for PPF. We demonstrated in a large and diverse population of fibrotic ILD that the 1- and 2-year standard were superior than the 0.5-year standard to identify PPF in patients with non-IPF fibrotic ILD, and baseline DLCO% is the independent predictor of mortality for PPF. These findings suggest that both the 1- and 2-year criterion are appropriate to use in future clinical trials, and also has potential utility in routine clinical practice to guide the use of antifibrotic agents.
The 2-year criterion was used most widely (5-7,17) since it was proposed in the INBUILD trial (9). Compared with 2-year criterion, the 0.5-year criterion sets a lower threshold (5%) of FVC% change. It may include patients with temporary deterioration on PFT, particularly for those who experience the onsets of autoimmune inflammation in patients with CTD-ILD or IPAF. These patients are not traditionally-defined progressive fibrosing. In this study, the 0.5-year standard included 58% patents with CTD-ILD, which was more than the other two groups. Besides, the 1- and 2-year criteria integrated the symptom, PFT and radiology, which gave a comprehensive evaluation of disease progression. Above all, both the 1- and 2-year criterion are appropriate to distinguish the progressive phenotype from fibrotic ILD.
From our results, patients in 1- and 2-year group had the similar trajectory of PFT decline and survival. And, almost all patients (95%) identified by 1-year standard were also in 2-year group. However, there were 47.7% of patients in 2-year group who were not diagnosed by 1-year criteria. Compared with the 1-year standard, the 2-year standard prolongs the time of assessment, it raises the opportunity to recruit more patients, as some patients took the PFT test with a time interval of over 1 year, which made some patients with disease progression missing the diagnosis of PPF. Therefore, the 1-year standard included fewer patients than the 2-year criteria. As the 1-year criteria has been proposed as the standard diagnostic criteria to identify PPF, the clinical practitioners should make flexible application with the 1- and 2-year criteria, especially for those meeting the 2-year standard, but not the 1-year standard, who take a certain portion of ILD patients in the real-world situation of China.
There are also disadvantages for the current diagnostic criteria. There is usually a long time between the timepoint when the patients start decline of in the performance PFT and the timepoint when they are diagnosed with progressive fibrosis. In our cohort the mean time for the assessment was 7.9 months for 1-year criterion, and 14.7 months for 2-year criterion, which indicates that the treatment is usually initiated after the course when FVC decline has happened, and the pathological damage has already occurred. In order to make earlier and more precise identification of PPF, there are two suggestions for the future research. First, 0.5-year standard has the potential to make earlier diagnosis, and baseline DLCO% <50% can be set as one restricted condition to enable 0.5-year standard to identify progressive phenotype more precisely. However, additional studies would be needed to validate any changes to the current criterion. Second, there is urgent need to develop new markers for progressive fibrosing. With the application of artificial intelligence in radiology, some studies have already shown that imaging parameters might be able to recognize progressive tendencies earlier than the traditional parameters (18,19). It would be a promising direction to develop radiological marker by artificial intelligence or biological marker from broncho alveolar lavage fluid.
Baseline demographic characteristics of our cohort are generally similar to the patients from the placebo arm of INBUILD study (9). However, compared with INBUILD, we included more patients with CTD-ILD (49% vs. 27%). Accordingly, our cohort had fewer patients with UIP pattern (49% vs. 62%), and our 2-year group had higher FVC% (71% vs. 69%) and DLCO% (58% vs. 48%) than INBUILD placebo group. The annual decline in FVC% (4%) of our cohort was comparable with that in IPF patients (−2.1% in 24 weeks) (20), but smaller than the raw data (7%) without adjustment from a precious PPF cohort (21).
The 4-year survival rate in our cohort was 62%, which was lower than results from two previous studies: 80% for 3-year survival rate (21) and 77.1% for 4-year survival rate (5). Our cohort, together with other two PPF cohorts, did not reach the medium survival time with 4-, 5- and 7-year follow up respectively. In contrast, the medium survival time of IPF is 3–5 years according to previous reports (22,23). These data suggested that the change in FVC% was similar in patients with IPF and patients of PPF, but the overall survival of PPF was better than that of IPF.
Robust predictive risk factors are vital for early identification of progressive fibrosing. For PPF, the previous study has demonstrated that FVC% decline is correlated with mortality in the one year after diagnosis (6). Our study demonstrates that baseline FVC% and DLCO% are correlated with post-diagnosis mortality for PPF. Among them, the baseline DLCO% is an independent predictor for mortality. Consistent with our results, the FVC% and DLCO% have been demonstrated as important predictors for mortality of other types of ILDs, as evidenced by other studies spanning IPF (24-27), systemic sclerosis-associated ILD (28-30), unclassifiable ILD (31,32) and HP (33,34). These data indicate that regular PFT measurement is important to monitor the disease progression in patients with fibrotic ILD.
Our study has the following limitations related to its retrospective and monocentric design. First, patients were enrolled from a single center, and confirmation of these findings with multicenter data and other populations would be beneficial. Second, the longitudinal data were not collected with fixed time interval, the criteria for disease progression could only be assessed against available data. However, there were no missing survival data, still allowing the robust survival analysis. Third, we included the patients with antifibrotic therapy within 6 months, which might impact on analysis of survival and pulmonary function. However, we reviewed the raw data of our cohort and found that only 11 patients (nintedanib 9; pirfenidone 2) received the antifibrotic therapy. Among them, 6 patients were included in the PPF group (all three criteria). The average time of the antifibrotic treatment was 2.6 (±1.0) months. Besides, these patients initiated the antifibrotic therapy after they were evaluated by the criteria of PPF, and they had no recheck data of pulmonary function during the time of antifibrotic therapy. As a result, the antifibrotic therapy had very small impact on our results. Finally, our data lack detailed information on treatment of immunosuppressants, which may influence the prognosis of PPF. Although, there were limited number of patients (15%) taking immunosuppressive agents.
Conclusions
In conclusion, this study compared the three diagnostic criteria for PPF using a large and heterogeneous population of patients with fibrotic ILD, and demonstrated the DLCO% at baseline is an independent predictor of mortality in patients with PPF. In the current situation, both the 1- and 2-year criterion are the reasonable choices for diagnosis in research and clinical practice.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-481/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-481/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-481/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-481/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of People’s Hospital of Deyang City (No. 2023-04-084-K01) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [Crossref] [PubMed]
- George PM, Spagnolo P, Kreuter M, et al. Progressive fibrosing interstitial lung disease: clinical uncertainties, consensus recommendations, and research priorities. Lancet Respir Med 2020;8:925-34. [Crossref] [PubMed]
- Olson A, Hartmann N, Patnaik P, et al. Estimation of the Prevalence of Progressive Fibrosing Interstitial Lung Diseases: Systematic Literature Review and Data from a Physician Survey. Adv Ther 2021;38:854-67. [Crossref] [PubMed]
- Spagnolo P, Distler O, Ryerson CJ, et al. Mechanisms of progressive fibrosis in connective tissue disease (CTD)-associated interstitial lung diseases (ILDs). Ann Rheum Dis 2021;80:143-50. [Crossref] [PubMed]
- Nasser M, Larrieu S, Si-Mohamed S, et al. Progressive fibrosing interstitial lung disease: a clinical cohort (the PROGRESS study). Eur Respir J 2021;57:2002718. [Crossref] [PubMed]
- Brown KK, Martinez FJ, Walsh SLF, et al. The natural history of progressive fibrosing interstitial lung diseases. Eur Respir J 2020;55:2000085. [Crossref] [PubMed]
- Wuyts WA, Papiris S, Manali E, et al. The Burden of Progressive Fibrosing Interstitial Lung Disease: A DELPHI Approach. Adv Ther 2020;37:3246-64. [Crossref] [PubMed]
- Maher TM, Corte TJ, Fischer A, et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med 2020;8:147-57. [Crossref] [PubMed]
- Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N Engl J Med 2019;381:1718-27. [Crossref] [PubMed]
- Solomon JJ, Danoff SK, Goldberg HJ, et al. The Design and Rationale of the Trail1 Trial: A Randomized Double-Blind Phase 2 Clinical Trial of Pirfenidone in Rheumatoid Arthritis-Associated Interstitial Lung Disease. Adv Ther 2019;36:3279-87. [Crossref] [PubMed]
- Hoffmann-Vold AM, Allanore Y, Alves M, et al. Progressive interstitial lung disease in patients with systemic sclerosis-associated interstitial lung disease in the EUSTAR database. Ann Rheum Dis 2021;80:219-27. [Crossref] [PubMed]
- Wong AW, Ryerson CJ, Guler SA. Progression of fibrosing interstitial lung disease. Respir Res 2020;21:32. [Crossref] [PubMed]
- Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2022;205:e18-47. [Crossref] [PubMed]
- Eakin EG, Resnikoff PM, Prewitt LM, et al. Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire. University of California, San Diego. Chest 1998;113:619-24. [Crossref] [PubMed]
- Swigris JJ, Han M, Vij R, et al. The UCSD shortness of breath questionnaire has longitudinal construct validity in idiopathic pulmonary fibrosis. Respir Med 2012;106:1447-55. [Crossref] [PubMed]
- Nasser M, Larrieu S, Boussel L, et al. Estimates of epidemiology, mortality and disease burden associated with progressive fibrosing interstitial lung disease in France (the PROGRESS study). Respir Res 2021;22:162. [Crossref] [PubMed]
- Hoffmann-Vold AM, Weigt SS, Saggar R, et al. Endotype-phenotyping may predict a treatment response in progressive fibrosing interstitial lung disease. EBioMedicine 2019;50:379-86. [Crossref] [PubMed]
- Wu X, Yin C, Chen X, et al. Idiopathic Pulmonary Fibrosis Mortality Risk Prediction Based on Artificial Intelligence: The CTPF Model. Front Pharmacol 2022;13:878764. [Crossref] [PubMed]
- Yang P, Luo Q, Wang X, et al. Comprehensive Analysis of Fibroblast Activation Protein Expression in Interstitial Lung Diseases. Am J Respir Crit Care Med 2023;207:160-72. [Crossref] [PubMed]
- Ley B, Bradford WZ, Vittinghoff E, et al. Predictors of Mortality Poorly Predict Common Measures of Disease Progression in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 2016;194:711-8. [Crossref] [PubMed]
- Faverio P, Piluso M, De Giacomi F, et al. Progressive Fibrosing Interstitial Lung Diseases: Prevalence and Characterization in Two Italian Referral Centers. Respiration 2020;99:838-45. [Crossref] [PubMed]
- Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:e44-68. [Crossref] [PubMed]
- Krauss E, Tello S, Wilhelm J, et al. Assessing the Effectiveness of Pirfenidone in Idiopathic Pulmonary Fibrosis: Long-Term, Real-World Data from European IPF Registry (eurIPFreg). J Clin Med 2020;9:3763. [Crossref] [PubMed]
- Paterniti MO, Bi Y, Rekić D, et al. Acute Exacerbation and Decline in Forced Vital Capacity Are Associated with Increased Mortality in Idiopathic Pulmonary Fibrosis. Ann Am Thorac Soc 2017;14:1395-402. [Crossref] [PubMed]
- Jo HE, Glaspole I, Grainge C, et al. Baseline characteristics of idiopathic pulmonary fibrosis: analysis from the Australian Idiopathic Pulmonary Fibrosis Registry. Eur Respir J 2017;49:1601592. [Crossref] [PubMed]
- Snyder L, Neely ML, Hellkamp AS, et al. Predictors of death or lung transplant after a diagnosis of idiopathic pulmonary fibrosis: insights from the IPF-PRO Registry. Respir Res 2019;20:105. [Crossref] [PubMed]
- Doubková M, Švancara J, Svoboda M, et al. EMPIRE Registry, Czech Part: Impact of demographics, pulmonary function and HRCT on survival and clinical course in idiopathic pulmonary fibrosis. Clin Respir J 2018;12:1526-35. [Crossref] [PubMed]
- Goh NS, Hoyles RK, Denton CP, et al. Short-Term Pulmonary Function Trends Are Predictive of Mortality in Interstitial Lung Disease Associated With Systemic Sclerosis. Arthritis Rheumatol 2017;69:1670-8. [Crossref] [PubMed]
- Sánchez-Cano D, Ortego-Centeno N, Callejas JL, et al. Interstitial lung disease in systemic sclerosis: data from the spanish scleroderma study group. Rheumatol Int 2018;38:363-74. [Crossref] [PubMed]
- Tyndall AJ, Bannert B, Vonk M, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010;69:1809-15. [Crossref] [PubMed]
- Ryerson CJ, Urbania TH, Richeldi L, et al. Prevalence and prognosis of unclassifiable interstitial lung disease. Eur Respir J 2013;42:750-7. [Crossref] [PubMed]
- Krauss E, El-Guelai M, Pons-Kuehnemann J, et al. Clinical and Functional Characteristics of Patients with Unclassifiable Interstitial Lung Disease (uILD): Long-Term Follow-Up Data from European IPF Registry (eurIPFreg). J Clin Med 2020;9:2499. [Crossref] [PubMed]
- Mooney JJ, Elicker BM, Urbania TH, et al. Radiographic fibrosis score predicts survival in hypersensitivity pneumonitis. Chest 2013;144:586-92. [Crossref] [PubMed]
- Gimenez A, Storrer K, Kuranishi L, et al. Change in FVC and survival in chronic fibrotic hypersensitivity pneumonitis. Thorax 2018;73:391-2. [Crossref] [PubMed]




