
The chest X-ray score baseline in predicting continuous oxygen therapy failure in low-risk aged patients after thoracic surgery
Highlight box
Key findings
• Our study reveals that for low-risk patients aged 70 years and above: (I) routine postoperative continuous oxygen therapy (COT) is appropriate, but about 7.85% necessitate escalated oxygen therapy. (II) Transitioning from COT to high-flow nasal cannula (HFNC) yields satisfactory outcomes. (III) The radiographic assessment of lung edema (RALE) score, coupled with surgical details, is a superior predictor of COT failure within 24 h post-surgery compared to the Brixia score.
What is known and what is new?
• Despite the increasing popularity of HFNC for postoperative oxygen therapy, COT continues to be the established standard. According to the guidelines from the European Respiratory Society (ERS), both HFNC and COT are recommended options for low-risk postoperative patients. In settings with limited resources, particularly among individuals aged over 70 years after thoracic surgery, choosing COT might be a more economically viable option.
• However, despite these considerations, there is a notable absence of predictive research on COT failure. This gap in knowledge highlights the need for further investigation into factors influencing the success or failure of COT in postoperative patients, particularly in resource-constrained settings.
What is the implication, and what should change now?
• Given the growing elderly population and prevalent use of COT in postoperative thoracic care, our findings offer new perspectives. The RALE score’s efficacy in early COT failure prediction, combined with convenient bedside chest X-ray scoring, can guide oxygen therapy escalation effectively. This approach holds promise for achieving satisfactory results in resource-limited settings, potentially influencing clinical practices and resource allocation policies.
Introduction
Patients undergoing thoracic surgery, especially elderly individuals undergoing lung resection and esophageal cancer radical surgery, are at a higher risk of experiencing postoperative pulmonary complications (PPCs) (1,2). These PPCs are most prevalent during the initial seven days following surgery and can range from pulmonary atelectasis to acute respiratory distress syndrome (ARDS). One of these PPCs, acute hypoxemic respiratory failure (AHRF), often increases the risk of postoperative failure in continuous oxygen therapy (COT) support. In October 2021, the European Respiratory Society (ERS) guidelines recommended the use of either high-flow nasal cannula (HFNC) or COT for postoperative patients after extubation, particularly those at low risk of pulmonary complications, and favored HFNC over COT in cases of AHRF (3). However, it is important to note that this recommendation is conditional and based on evidence of low certainty due to the lack of relevant research, especially in subgroups of elderly patients and those at low risk. Other guidelines recommend conducting specific trials for these vulnerable populations (4).
With the global aging population, there is a growing number of patients aged 70 years and above undergoing open-chest surgery. This demographic is more prone to developing PPCs, such as AHRF, which increase the risk of surgical mortality and complications (5). While certain preoperative risk factors have been closely associated with AHRF and COT treatment failure (6), there is a lack of studies examining the predictive value of common bedside chest X-rays in these patients. In thoracic surgery, bedside chest X-rays are often taken within 24 h postoperatively to assess thoracic conditions and provide information about postoperative complications. Currently, various scoring systems are used to evaluate chest X-ray images, including the radiographic assessment of lung edema (RALE) score (7), Brixia score (8), Toussie score (9), Al-Smadi score (10), and percentage/area opacification. In 2018, Warren et al. first reported that the RALE score could independently predict the severity of ARDS in terms of oxygenation and outcomes (7), and some study have applied the RALE score to the evaluation and of patients after lung (11), esophagus (12), and heart surgery (13). Subsequently, it has demonstrated valuable applications in predicting the prognosis of coronavirus disease 2019 (COVID-19) patients (14). In 2020, Borghesi et al. conducted a retrospective study of 302 patients with COVID-19 and reported that the Brixia score, a new chest X-ray scoring system, could independently predict the risk of in-hospital mortality in Caucasian patients in Italy (8). Several subsequent studies have reported a close association between the Brixia score and the severity and prognosis of COVID-19, supporting clinical decision-making and the development of predictive models (15,16). However, it is important to note that the Brixia score has only been reported in the context of COVID-19.
COT, the primary postoperative respiratory treatment, is potentially more cost-effective in low-income countries due to resource limitations, despite variable costs between nations and hospitals. Guidelines suggest noninvasive ventilation (NIV) or continuous positive airway pressure (CPAP) for AHRF following COT failure. HFNC may be considered for patients with low tolerance to low oxygen levels (17). Our study, focusing on low-risk thoracic surgery patients aged 70 years and above, administered postoperative COT, with HFNC recommended upon COT failure. Chest X-ray scores within 24 h postoperatively were investigated for predicting COT failure and establishing a link between radiological findings and clinical status.
The aim of this study was to assess the effectiveness of two chest X-ray scores, the RALE score and the Brixia score, in predicting COT treatment failure in patients over 70 years of age after thoracic surgery. We present this article in accordance with the TRIPOD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1786/rc).
Methods
Source of data
This study is a single-center retrospective investigation involving patients aged 70 years and above who underwent surgery at the First Affiliated Hospital of the Army Medical University from January 2019 to December 2021. All postoperative patients received COT upon returning to the hospital ward. A standard bedside chest X-ray was routinely performed within 24 h, and the resulting chest X-ray images were collected and assessed using the RALE score and Brixia score. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval for this retrospective study was obtained from the Ethics Committee of the First Affiliated Hospital of the Army Medical University (ethics ID: KY202262). Given the retrospective nature of the study, the requirement for informed consent was waived.
Selection of participants and definitions
Inclusion criteria: patients aged 70 years and above, post-thoracic surgery, receiving COT in the ward, with outcomes of either successful discharge or oxygen therapy escalation due to COT failure. Exclusion criteria: (I) age <70 years; (II) high-risk patients with preoperative oxygen saturation (SpO2) <92% and preoperative hemoglobin (Hb) ≤10 g/dL, receiving immediate HFNC postoperatively; (III) body mass index (BMI) >24 kg/m2; (IV) history of obstructive sleep apnea, lung transplantation, or other significant medical conditions. Cases with severe record omissions or critical missing information were excluded (Figure 1).
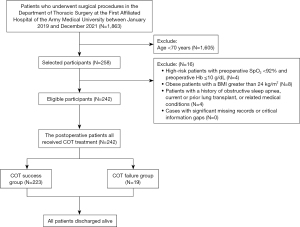
COT failure criteria: patients with AHRF, partial pressure of arterial oxygen (PaO2)/fraction of inspired oxygen (FiO2) 100–300 mmHg, or SpO2 <92%, with increased respiratory rate (RR) (>30 breaths per minute), elevated heart rate, and exclusion of hypoxemia from other complications (e.g., bleeding or heart failure) (18). Patients were categorized into the COT success group or COT failure group based on postoperative outcomes. The COT failure group included patients transitioning to HFNC therapy for unresolved mild to moderate AHRF (3,4).
Reintubation criteria: for severe hypoxemia post-COT or HFNC therapy, persistently unresolved moderate hypoxemia after HFNC therapy, hemodynamic instability, deteriorating neurological status, or meeting at least two of the following criteria: persistent or worsening signs of respiratory failure, RR >40 breaths per minute, copious tracheal secretions, pH <7.35, SpO2 persistently <90% for 5 minutes, and poor response to oxygenation techniques (19).
X-ray scoring systems for severity assessment
After returning to the ward, a bedside chest X-ray was routinely conducted within 24 h of COT treatment, utilizing both anteroposterior (AP) and posteroanterior (PA) projections with a portable digital radiographic device (Technix, Model TMB 400 DR, Grassobbio, Italy). The Brixia scoring system (20) and RLAE system (7) were employed to assess pulmonary abnormalities within 24 h of postoperative COT in the two patient groups.
The Brixia score categorizes the chest X-ray into six regions in AP or PA views, assigning scores based on the characteristics and extent of pulmonary abnormalities: 0 (no abnormality), 1 (interstitial infiltration), 2 (predominantly interstitial), or 3 (predominantly alveolar). The total score ranges from 0 to 18 (Figure 2A).
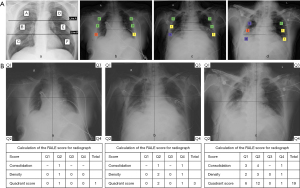
For the RALE score, each radiograph was divided into quadrants, with consolidation scores (0: none, 1: <25%, 2: 25–49%, 3: 50–75%, 4: >75%) representing alveolar opacities’ extent within each quadrant. Additionally, density scores (1= hazy, 2= moderate, 3= dense) evaluated overall opacification density. The final RALE score ranged from 0 (no infiltrates) to 48 (dense consolidation in over 75% of each quadrant) (Figure 2B).
We trained two radiologists with 2 and 3 years of experience, respectively, to apply this scoring system to our chest X-ray images. They independently reviewed and scored all the images, and the average score was used for the analysis. In case of disagreement between the two radiologists, a senior radiologist with more than 10 years of experience and expertise in thoracic imaging reviewed the images and made the final decision.
Data collection
Patient data, including preoperative, intraoperative, and postoperative clinical information, were primarily collected from the electronic medical records system of the First Affiliated Hospital of the Army Medical University. There was no data loss in this study. Preoperative variables included patient age, gender, BMI, smoking index, forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC), surgical location, Hb levels, surgical approach, temperature, heart rate, RR, systolic and diastolic blood pressure. Intraoperative variables comprised surgical duration and intraoperative blood loss. Postoperative variables encompassed Brixia score and RALE score.
Statistical analysis
This study employed SPSS (version 26.0; IBM, NY, USA), R (version 4.3.1; R Foundation, Austria), and Python (version 3.1.5) for statistical analysis, with a two-tailed P value threshold of <0.05. Normality of continuous variables was assessed using the Kolmogorov-Smirnov test. Normally distributed data were presented as mean ± standard deviation; otherwise, median and quartiles were utilized. Categorical variables were expressed as frequencies and percentages.
Initial risk factor screening involved univariate analysis. For normally distributed data, two-sample t-tests were applied, and non-normally distributed data were analyzed using the Mann-Whitney U-test. Categorical variables were compared using Chi-squared or Fisher’s exact test. Variables with P<0.1 or clinical significance in univariate analysis were further assessed in multivariable logistic regression for independent risk factors.
Propensity score analysis with 1:2 matching was conducted using the nearest neighbor method, employing a caliper width set to 0.1 times the standard deviation of the logit of the propensity score. Results were visualized through forest plots. Model calibration and discrimination were evaluated using the Hosmer-Lemeshow test, calibration curves, receiver operating characteristic (ROC) curves, and area under the curve (AUC). ROC curves for COT failure prediction were generated using independent risk factors before and after matching. Clinical utility was assessed through decision curves and clinical impact curves analysis.
Results
Baseline characteristics
In a group of 1,863 patients undergoing thoracic surgery, 242 met the inclusion criteria and received postoperative COT. Among them, 223 had successful COT, while 19 (7.85%) faced COT failure and were subsequently escalated to HFNC therapy, lasting an average of 6 days (ranging from 2 to 27 days). All patients were discharged alive. Noteworthy preoperative differences were observed, especially in surgical location (P=0.003). Specifically, 20 (9.0%) of the COT group underwent esophageal cancer radical surgery, compared to 7 (36.8%) in the COT failure group. The COT failure group also had a significantly longer average operation time (3.4 vs. 2.8 h, P=0.003) and a higher proportion of thoracotomy (26.3% vs. 9.4%, P=0.004) (Table 1).
Table 1
| Characteristic | COT success (n=223) | COT failure (n=19) | P |
|---|---|---|---|
| Gender, male | 122 (54.7) | 14 (73.7) | 0.149 |
| Age, years | 72 [71–75] | 73 [71–76] | 0.280 |
| BMI, kg/m2 | 23.6 [21.5–25.5] | 21.1 [20.2–26.0] | 0.217 |
| Smoking index | 0 [0–420] | 0 [0–400] | 0.415 |
| FEV1/FVC, % | 79.72 [76.12–85.12] | 80.51 [68.99–87.8] | 0.954 |
| COPD | 27 (12.1) | 3 (15.8) | 0.714 |
| Hypertension | 35 (15.7) | 3 (15.8) | 0.662 |
| Diabetes | 19 (8.5) | 3 (15.8) | 0.394 |
| Location | 0.003 | ||
| Lung | 192 (86.1) | 11 (57.9) | |
| Esophagus | 20 (9.0) | 7 (36.8) | |
| Others | 11 (4.9) | 1 (5.3) | |
| Operation times, h | 2.8 [2.1–3.3] | 3.4 [2.5–4.6] | 0.003 |
| Hb, g/L | 131.6±15.5 | 133.6±12.7 | 0.597 |
| Temperature, ℃ | 36.5 [36.3–36.6] | 36.5 [36.5–36.7] | 0.071 |
| HR, beats/minute | 79 [74–85] | 81 [75–85] | 0.490 |
| RR, bpm | 20 [19–20] | 20 [19–20] | 0.589 |
| SBP, mmHg | 128 [123–139] | 127 [124–139] | 0.808 |
| DBP, mmHg | 76 [71–81] | 76 [73–80] | 0.653 |
| Surgical approach | 0.004 | ||
| Thoracoscopy | 188 (84.3) | 8 (42.1) | |
| Thoracotomy | 21 (9.4) | 5 (26.3) | |
| Thoracoabdominal laparoscopy | 14 (6.3) | 6 (31.6) | |
| Peroperative bleeding, mL | 150 [100–200] | 200 [100–250] | 0.033 |
| Brixia score | 3 [2–5] | 5 [3–8] | 0.003 |
| RALE score | 4 [2–6] | 10 [2–17] | 0.002 |
Data are presented as number (percentage), median [interquartile range] or mean ± standard deviation. COT, continuous oxygen therapy; BMI, body mass index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; COPD, chronic obstructive pulmonary disease; Hb, hemoglobin; HR, heart rate; RR, respiratory rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; RALE, radiographic assessment of lung edema.
Within 24 h postoperatively, patients experiencing COT failure exhibited higher Brixia and RALE scores compared to the success group (P=0.003 and P=0.002, respectively). Propensity score matching (1:2) maintained significant differences in esophageal cancer cases (P=0.034), operation time (P=0.007), and thoracotomy proportion (P=0.008). Following the matching, Brixia and RALE scores remained significantly different between groups (P=0.001 and P=0.012, respectively). However, median scores in both groups increased. The COT success group had a median Brixia score of 3 (range: 2–4) and a median RALE score of 3.5 (range: 2–5), while the COT failure group had a median Brixia score of 5 (range: 3–8) and a median RALE score of 10 (range: 2–17) (Table 2). For continuous variables with a significance level of P<0.05, their data distribution will be visually represented using box plots (Figures 3,4).
Table 2
| Characteristic | COT success (n=34) | COT failure (n=19) | P |
|---|---|---|---|
| Gender, male | 22 (64.7) | 14 (73.7) | 0.555 |
| Age, years | 72 [71–74] | 73 [71–76] | 0.349 |
| BMI, kg/m2 | 22.04±3.10 | 22.54±3.43 | 0.591 |
| Smoking index | 0 [0–525] | 0 [0–400] | 0.604 |
| FEV1/FVC, % | 77.50±7.75 | 79.32±9.77 | 0.460 |
| COPD | 6 (17.6) | 3 (15.8) | 0.836 |
| Hypertension | 8 (23.5) | 4 (21.1) | 0.863 |
| Diabetes | 5 (14.7) | 3 (15.8) | 0.916 |
| Location | 0.034 | ||
| Lung | 29 (85.3) | 11 (57.9) | |
| Esophagus | 3 (8.8) | 7 (36.8) | |
| Others | 2 (5.9) | 1 (5.3) | |
| Operation time, h | 2.7±1.26 | 3.74±1.39 | 0.007 |
| Hb, g/L | 130.62±11.10 | 133.58±12.69 | 0.381 |
| Temperature, ℃ | 36.5 [36.3–36.6] | 36.5 [36.5–36.7] | 0.071 |
| HR, beats/minute | 76.68±9.33 | 80.26±8.56 | 0.173 |
| RR, bpm | 20 [19–20] | 20 [19–20] | 0.589 |
| SBP, mmHg | 128.41±11.8 | 128.32±12.39 | 0.978 |
| DBP, mmHg | 77.5±8.23 | 77.58±9.05 | 0.974 |
| Surgical approach | 0.008 | ||
| Thoracoscopy | 28 (82.4) | 8 (42.1) | |
| Thoracotomy | 3 (8.8) | 5 (26.3) | |
| Thoracoabdominal laparoscopy | 3 (8.8) | 6 (31.6) | |
| Peroperative bleeding, mL | 150 [87.5–200] | 200 [100–250] | 0.080 |
| Brixia score | 3 [2–4] | 5 [3–8] | 0.001 |
| RALE score | 3.5 [2–5] | 10 [2–17] | 0.012 |
Data are presented as number (percentage), median [interquartile range] or mean ± standard deviation. COT, continuous oxygen therapy; BMI, body mass index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; COPD, chronic obstructive pulmonary disease; Hb, hemoglobin; HR, heart rate; RR, respiratory rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; RALE, radiographic assessment of lung edema.


Construction of the prediction model
As shown in Table 3, the results of univariate logistic regression analysis revealed significant associations (P<0.1) between COT failure and surgical location, duration of surgery, surgical approach, RALE score, Brixia score, and preoperative body temperature.
Table 3
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age | 1.07 (0.92–1.24) | 0.374 | |||
| Sex | |||||
| Female | Ref | ||||
| Male | 0.43 (0.15–1.24) | 0.118 | |||
| BMI | 0.91 (0.78–1.05) | 0.200 | |||
| Smoking index | 1.00 (1.00–1.00) | 0.891 | |||
| Location | |||||
| Lung | Ref | ||||
| Esophagus | 6.11 (2.13–17.52) | 0.001 | |||
| Others | 1.59 (0.19–13.42) | 0.672 | |||
| FEV1/FVC | 0.99 (0.94–1.04) | 0.779 | |||
| COPD | |||||
| No | Ref | ||||
| Yes | 1.36 (0.37–4.98) | 0.641 | |||
| Hypertension | |||||
| No | Ref | ||||
| Yes | 1.01 (0.28–3.64) | 0.991 | |||
| Diabetes | |||||
| No | Ref | ||||
| Yes | 2.01 (0.54–7.53) | 0.299 | |||
| Hb | 1.01 (0.98–1.04) | 0.596 | |||
| Operation time | 1.69 (1.21–2.37) | 0.002 | |||
| Surgical approach | |||||
| Thoracoscopy | Ref | ||||
| Thoracotomy | 5.60 (1.68–18.67) | 0.005 | 3.19 (0.85–11.91) | 0.084 | |
| Thoracoabdominal laparoscopy | 10.07 (3.07–33.09) | <0.001 | 8.96 (2.43–32.98) | 0.001 | |
| Peroperative bleeding | 1.00 (1.00–1.00) | 0.639 | |||
| Brixia score | 1.38 (1.14–1.67) | 0.001 | |||
| RALE score | 1.19 (1.11–1.29) | <0.001 | 1.18 (1.08–1.28) | <0.001 | |
| Temperature | 10.35 (0.96–112.02) | 0.054 | |||
| HR | 1.02 (0.96–1.08) | 0.505 | |||
| RR | 1.15 (0.65–2.04) | 0.623 | |||
| SBP | 0.99 (0.96–1.03) | 0.712 | |||
| DBP | 1.02 (0.96–1.08) | 0.480 | |||
COT, continuous oxygen therapy; OR, odds ratio; CI, confidence interval; BMI, body mass index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; COPD, chronic obstructive pulmonary disease; Hb, hemoglobin; RALE, radiographic assessment of lung edema; HR, heart rate; RR, respiratory rate; SBP, systolic blood pressure; DBP, diastolic blood pressure.
In the multivariate logistic regression analysis, two independent risk factors for COT failure were identified: the surgical approach thoracotomy [odds ratio (OR) =3.19, 95% CI: 0.85–11.91, P=0.084] and thoracoabdominal laparoscopy (OR =8.96, 95% CI: 2.43–32.98, P=0.001), and the RALE score (OR =1.18, 95% CI: 1.08–1.28, P<0.001). A forest plot was generated to illustrate the impact and confidence intervals of each independent variable (Figure S1).
Furthermore, after conducting 1:2 propensity score matching, similar results were observed. The univariate analysis revealed significant associations (P<0.1) between COT failure and surgical location, surgical procedure, Brixia score, RALE score, and preoperative body temperature. Two independent risk factors for the failure of COT, identified through multivariate analysis, include the duration of surgery (OR =2.14, 95% CI: 1.16–3.95, P=0.015) and RALE score (OR =1.27, 95% CI: 1.09–1.47, P=0.002) (Table 4). For relevant forest plots, please refer to Figure S2.
Table 4
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age | 1.08 (0.91–1.28) | 0.391 | |||
| Sex | |||||
| Female | Ref | ||||
| Male | 0.74 (0.23–2.40) | 0.612 | |||
| BMI | 1.00 (0.85–1.18) | 0.985 | |||
| Smoking index | 1.00 (1.00–1.00) | 0.672 | |||
| Location | |||||
| Lung | Ref | ||||
| Esophagus | 6.68 (1.65–27.00) | 0.008 | |||
| Others | 1.27 (0.12–13.46) | 0.841 | |||
| FEV1/FVC | 1.03 (1.00–1.10) | 0.453 | |||
| COPD | |||||
| No | Ref | ||||
| Yes | 1.34 (0.30–6.02) | 0.699 | |||
| Hypertension | |||||
| No | Ref | ||||
| Yes | 1.10 (0.360–3.31) | 0.991 | |||
| Diabetes | |||||
| No | Ref | ||||
| Yes | 1.09 (0.23–5.16) | 0.916 | |||
| Hb | 1.01 (0.97–1.06) | 0.616 | |||
| Operation time | 1.94 (1.21–3.12) | 0.006 | 2.14 (1.16–3.95) | 0.015 | |
| Surgical approach | |||||
| Thoracoscopy | Ref | ||||
| Thoracotomy | 4.06 (0.99–16.63) | 0.051 | |||
| Thoracoabdominal laparoscopy | 7.31 (1.67–32.00) | 0.008 | |||
| Peroperative bleeding | 1.00 (1.01–1.00) | 0.104 | |||
| Brixia score | 1.59 (1.20–2.11) | 0.001 | |||
| RALE score | 1.25 (1.1–1.41) | <0.001 | 1.27 (1.09–1.47) | 0.002 | |
| Temperature | 20.53 (0.87–485.75) | 0.061 | |||
| HR | 1.04 (0.97–1.11) | 0.293 | |||
| RR | 1.15 (0.64–2.09) | 0.639 | |||
| SBP | 1.00 (0.95–1.05) | 0.977 | |||
| DBP | 1.00 (0.94–1.07) | 0.974 | |||
COT, continuous oxygen therapy; OR, odds ratio; CI, confidence interval; BMI, body mass index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; COPD, chronic obstructive pulmonary disease; Hb, hemoglobin; RALE, radiographic assessment of lung edema; HR, heart rate; RR, respiratory rate; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Validation of the prediction model
The calibration curve and Hosmer-Lemeshow test confirmed the model’s good fit (Figure S3, Hosmer-Lemeshow χ2: 11.858, P=0.245). The ROC curve demonstrated promising discriminatory ability (AUC: 0.768, 95% CI: 0.715–0.821). Surgical approach has an AUC of 0.718 (95% CI: 0.661–0.774), Brixia score has an AUC of 0.703 (95% CI: 0.646–0.761, cutoff: 2.187), and RALE score has an AUC of 0.712 (95% CI: 0.655–0.769, cutoff: 3.997) (Figure 5, Table 5).
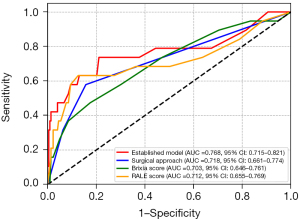
Table 5
| Model set | AUC (95% CI) | Accuracy | Sensitivity | Specificity | PPV | NPV | Brixa cut-off points | RALE cut-off points |
|---|---|---|---|---|---|---|---|---|
| Established model | 0.768 (0.715–0.821) | 0.785 | 0.737 | 0.789 | 0.230 | 0.972 | 1.338 | 2.676 |
| Surgical approach | 0.718 (0.661–0.774) | 0.822 | 0.579 | 0.843 | 0.239 | 0.959 | – | – |
| Brixia score | 0.703 (0.646–0.761) | 0.798 | 0.474 | 0.825 | 0.188 | 0.948 | 2.187 | – |
| RALE score | 0.712 (0.655–0.769) | 0. 855 | 0.632 | 0.874 | 0.300 | 0.965 | – | 3.977 |
RALE, radiographic assessment of lung edema; AUC, area under the curve; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value.
For the Established Model, the accuracy was 0.785, sensitivity was 0.737, specificity was 0.789, positive predictive value (PPV) was 0.230, and negative predictive value (NPV) was 0.972. Surgical approach showed an accuracy of 0.822, sensitivity of 0.579, specificity of 0.843, PPV of 0.239, and NPV of 0.959. The Brixia score demonstrated an accuracy of 0.798, sensitivity of 0.474, specificity of 0.825, PPV of 0.188, and NPV of 0.948. The RALE score has an accuracy of 0.855, sensitivity of 0.632, specificity of 0.874, PPV of 0.300, and NPV of 0.965 (Table 5). The decision curve (Figure S4) and clinical impact curve (Figure S5) illustrated that, in comparison to “no treatment” and “full treatment” strategies, the use of this model results in increased clinical net benefit across nearly all probability thresholds, indicating strong clinical utility.
After 1:2 propensity score matching, our calibration curve affirmed a well-fitted model (Figure S6). The Hosmer-Lemeshow goodness-of-fit test supported this, yielding a χ2 value of 8.410 with P of 0.395. The established model attained an AUC of 0.839 (95% CI: 0.740–0.938). Operation time, for predicting COT failure, has an AUC of 0.715 (95% CI: 0.594–0.837). Brixia score exhibited an AUC of 0.764 (95% CI: 0.650–0.878, cutoff: 6.027), and RALE score has an AUC of 0.710 (95% CI: 0.588–0.832, cutoff: 17.314) (Figure 6, Table 6).
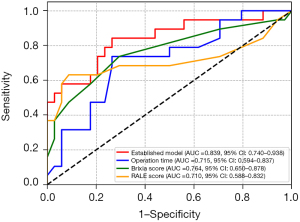
Table 6
| Model set | AUC (95% CI) | Accuracy | Sensitivity | Specificity | PPV | NPV | Brixa cut-off points | RALE cut-off points |
|---|---|---|---|---|---|---|---|---|
| Established model | 0.839 (0.740–0.938) | 0.774 | 0.842 | 0.735 | 0.640 | 0.893 | 4.635 | 9.271 |
| Operation time | 0.715 (0.594–0.837) | 0.736 | 0.737 | 0.735 | 0.609 | 0.833 | – | – |
| Brixia score | 0.764 (0.650–0.878) | 0.717 | 0.737 | 0.706 | 0.583 | 0.828 | 6.027 | – |
| RALE score | 0.710 (0.588–0.832) | 0.811 | 0.632 | 0.912 | 0.800 | 0.816 | – | 17.314 |
RALE, radiographic assessment of lung edema; AUC, area under the curve; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value.
Following matching, the established model’s accuracy was 0.774, sensitivity was 0.842, specificity was 0.735, PPV was 0.640, and NPV is 0.893. The accuracy for operation time was 0.736, with sensitivity at 0.737, specificity at 0.735, PPV at 0.609, and NPV at 0.833. Brixia score’s accuracy was 0.717, with sensitivity at 0.737, specificity at 0.706, PPV at 0.583, and NPV at 0.828. For RALE score, the accuracy was 0.811, sensitivity was 0.632, specificity was 0.912, PPV was 0.800, and NPV was 0.816 (Table 6). The decision curve (Figure S7) and clinical impact curve (Figure S8) continued to highlight the model’s strong clinical utility, surpassing “no treatment” and “full treatment” strategies across a wide range of probability thresholds.
Discussion
Our scoring system has clinical implications for identifying and managing patients who are at risk of developing postoperative respiratory failure after thoracic surgery. By combining the RALE score, which reflects the extent and severity of lung injury, and the surgical approach, which indicates the degree of surgical trauma, our scoring system can provide a simple and convenient way to stratify patients according to their risk of COT failure.
Currently, there is a scarcity of research data on the effectiveness of COT and HFNC in subgroups of elderly individuals and those with poor lung reserve (4). In the context of these vulnerable populations, this study discovered that in patients aged 70 years and above who are at low risk of respiratory complications: (I) postoperatively, COT can be administered routinely, but a subset, amounting to 7.85%, requires oxygen therapy escalation. (II) Regarding the escalation of oxygen therapy from COT, guidelines recommend NIV and CPAP, with HFNC considered for less tolerant patients. In this study, upgrading to HFNC yielded satisfactory treatment outcomes. (III) Within 24 h postsurgery, the routine bedside chest X-ray score, when combined with the surgical approach, RALE score was better at predicting COT failure than the Brixia score. The surgical approach constitutes a vital predictive parameter in the model established in this study. We categorized surgical approach into thoracoscopy, thoracotomy (OR =5.60, 95% CI: 1.68–18.67, P=0.005), and thoracoabdominal laparoscopy (OR =10.07, 95% CI: 3.07–33.09, P<0.001). With the introduction of minimally invasive surgical concepts and the continual refinement of laparoscopic techniques, thoracoscopic surgery has gained widespread recognition among both physicians and patients for its safety, reliability, and long-term effectiveness. Conversely, open-chest surgery imposes several disadvantages for patients, including significant surgical trauma, long-term postoperative chronic pain, higher postoperative complications, extended hospitalization periods, and prolonged recovery times (21,22). Currently, open-chest procedures are primarily reserved for challenging surgeries or complex anatomical structures. Thoracoabdominal laparoscopy is predominantly used in esophageal cancer patients, where radical surgery remains the most crucial treatment modality for resectable esophageal cancer. In pursuit of surgical efficacy, this inevitably results in extensive surgery and a higher rate of postoperative complications (23). Furthermore, patients must recover from the trauma inflicted during surgery, such as the extended surgery duration and the wide-ranging traumatized areas experienced by esophageal cancer patients, often involving the neck, chest, and abdomen. Our findings reveal that when the surgical approach is thoracotomy or thoracoabdominal laparoscopy, patients postoperatively are more prone to requiring an upgrade to HFNC therapy compared to those undergoing thoracoscopy, aligning with our expectations.
Currently, for chest X-ray scoring, the lungs are divided into six zones according to the Brixia score, Toussie score, and Al-Smadi score, while the RALE score divides the lungs into four quadrants. Therefore, we selected two representative scoring methods with different divisions for this study. Additionally, considering our focus on patients at low risk of respiratory complications, it is preferable to employ scoring tools with a broader score range. Consequently, we chose the Brixia score and the RALE score. Up to this point, the Brixia score has demonstrated its utility as a clear and straightforward tool, primarily for COVID-19 (24). To the best of our knowledge, our study is the first to evaluate the Brixia score as a predictive indicator for diseases other than COVID-19. The RALE score has been reported to have utility in risk stratification and prognosis in conditions such as pulmonary edema (25), ARDS (26,27) and COVID-19 (28). Our study also revealed the roles of both scores in predicting postoperative COT failure in elderly, low-risk thoracic surgery patients. The RALE score outperformed the Brixia score, especially in the logistic multivariate regression analysis, where the RALE score emerged as an independent risk factor, while the Brixia score did not. The possible explanation for this distinction is that these elderly, low-risk patients showed minimal chest X-ray changes within 24 h, resulting in lower overall scores. In contrast to the narrower score range of the Brixia score (ranging from 0 to 18), the broader range of the RALE score (ranging from 0 to 48) was better equipped to reveal these differences. Our results show that the cut-off value of the RALE score for the established model after matching is 9.271, which means that when a patient’s postoperative chest radiograph RALE score is higher than this value, HFNC should be considered instead of COT. We prefer to use the cut-off value after matching because the matching process reduced the imbalance of covariates between the two groups, resulting in a more homogeneous and comparable sample. This suggests that the RALE score might be more sensitive, especially in patients with low scoring levels. For the treatment decision between COT or HFNC after thoracic surgery, the conclusions from previous studies have been inconsistent, with a low level of evidence (29,30). Therefore, we were unable to identify any previously published studies comparing the predictive value of the RALE score for COT failure with our study results.
This study has several limitations. First, it is a single-center retrospective study with a small sample size and a limited patient population. The retrospective data collection and assessment may introduce some unavoidable biases. Therefore, our findings should be further validated in a large sample randomized controlled trial. Second, the COT failure group had relatively few cases. While this reflects the real proportion of COT failure in elderly, low-risk patients, it might lead to insufficient evidence, even though matching statistical corrections had been performed. Third, our model development and validation were based on the same data set, which may lead to overfitting and optimistic estimates of model performance. Although we used bootstrapping to correct for overfitting and to obtain unbiased estimates of model performance, external validation in an independent data set is needed to confirm the validity and reliability of our model and we plan to conduct a prospective multicenter study in the future to validate our findings.
Conclusions
In summary, regarding postoperative bedside chest X-ray information in elderly, low-risk patients undergoing thoracic surgery, the combination of the RALE score with surgical approach may be more effective in predicting the risk of postoperative COT failure transitioning to HFNC compared to the Brixia score. This routine, cost-effective, and noninvasive approach aids in assessing the severity of postoperative lung lesions, monitoring treatment response, and providing early warning signs for oxygen therapy escalation. However, further prospective research is needed to validate the effectiveness and applicability of this predictive model in different settings and populations.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1786/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1786/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1786/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1786/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval for this retrospective study was obtained from the Ethics Committee of the First Affiliated Hospital of the Army Medical University (ethics ID: KY202262). Given the retrospective nature of the study, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stéphan F, Boucheseiche S, Hollande J, et al. Pulmonary complications following lung resection: a comprehensive analysis of incidence and possible risk factors. Chest 2000;118:1263-70. [Crossref] [PubMed]
- Harpole DH, Liptay MJ, DeCamp MM Jr, et al. Prospective analysis of pneumonectomy: risk factors for major morbidity and cardiac dysrhythmias. Ann Thorac Surg 1996;61:977-82. [Crossref] [PubMed]
- Oczkowski S, Ergan B, Bos L, et al. ERS clinical practice guidelines: high-flow nasal cannula in acute respiratory failure. Eur Respir J 2022;59:2101574. [Crossref] [PubMed]
- Rochwerg B, Einav S, Chaudhuri D, et al. The role for high flow nasal cannula as a respiratory support strategy in adults: a clinical practice guideline. Intensive Care Med 2020;46:2226-37. [Crossref] [PubMed]
- Arozullah AM, Daley J, Henderson WG, et al. Multifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery. The National Veterans Administration Surgical Quality Improvement Program. Ann Surg 2000;232:242-53. [Crossref] [PubMed]
- Yu Y, Qian X, Liu C, et al. Effect of High-Flow Nasal Cannula versus Conventional Oxygen Therapy for Patients with Thoracoscopic Lobectomy after Extubation. Can Respir J 2017;2017:7894631. [Crossref] [PubMed]
- Warren MA, Zhao Z, Koyama T, et al. Severity scoring of lung oedema on the chest radiograph is associated with clinical outcomes in ARDS. Thorax 2018;73:840-6. [Crossref] [PubMed]
- Borghesi A, Zigliani A, Golemi S, et al. Chest X-ray severity index as a predictor of in-hospital mortality in coronavirus disease 2019: A study of 302 patients from Italy. Int J Infect Dis 2020;96:291-3. [Crossref] [PubMed]
- Toussie D, Voutsinas N, Finkelstein M, et al. Clinical and Chest Radiography Features Determine Patient Outcomes in Young and Middle-aged Adults with COVID-19. Radiology 2020;297:E197-206. [Crossref] [PubMed]
- Al-Smadi AS, Bhatnagar A, Ali R, et al. Correlation of chest radiography findings with the severity and progression of COVID-19 pneumonia. Clin Imaging 2021;71:17-23. [Crossref] [PubMed]
- Xuan L, Wang Y, Zheng Y, et al. Delayed lung injury on the nonsurgical side increases mortality in patients after lung cancer surgery: a retrospective cohort study. J Thorac Dis 2023;15:5574-84. [Crossref] [PubMed]
- Leng X, Onaitis MW, Zhao Y, et al. Risk of Acute Lung Injury after Esophagectomy. Semin Thorac Cardiovasc Surg 2022;34:737-46. [Crossref] [PubMed]
- Mostafa K, Wolf C, Seehafer S, et al. Redefining Unilateral Pulmonary Edema after Mitral Valve Surgery on Chest X-ray Imaging Using the RALE Scoring System. J Clin Med 2023;12:6043. [Crossref] [PubMed]
- Ciceri F, Castagna A, Rovere-Querini P, et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin Immunol 2020;217:108509. [Crossref] [PubMed]
- Maroldi R, Rondi P, Agazzi GM, et al. Which role for chest x-ray score in predicting the outcome in COVID-19 pneumonia? Eur Radiol 2021;31:4016-22. [Crossref] [PubMed]
- Au-Yong I, Higashi Y, Giannotti E, et al. Chest Radiograph Scoring Alone or Combined with Other Risk Scores for Predicting Outcomes in COVID-19. Radiology 2022;302:E11. [Crossref] [PubMed]
- Leone M, Einav S, Chiumello D, et al. Noninvasive respiratory support in the hypoxaemic peri-operative/periprocedural patient: a joint ESA/ESICM guideline. Intensive Care Med 2020;46:697-713. [Crossref] [PubMed]
- Xia M, Li W, Yao J, et al. A postoperative comparison of high-flow nasal cannula therapy and conventional oxygen therapy for esophageal cancer patients. Ann Palliat Med 2021;10:2530-9. [Crossref] [PubMed]
- Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015;372:2185-96. [Crossref] [PubMed]
- Borghesi A, Maroldi R. COVID-19 outbreak in Italy: experimental chest X-ray scoring system for quantifying and monitoring disease progression. Radiol Med 2020;125:509-13. [Crossref] [PubMed]
- von Meyenfeldt EM, Marres GMH, van Thiel E, et al. Variation in length of hospital stay after lung cancer surgery in the Netherlands. Eur J Cardiothorac Surg 2018;54:560-4. [Crossref] [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Mariette C, Markar SR, Dabakuyo-Yonli TS, et al. Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer. N Engl J Med 2019;380:152-62. [Crossref] [PubMed]
- Rubin GD, Ryerson CJ, Haramati LB, et al. The Role of Chest Imaging in Patient Management during the COVID-19 Pandemic: A Multinational Consensus Statement from the Fleischner Society. Radiology 2020;296:172-80. [Crossref] [PubMed]
- Kotok D, Yang L, Evankovich JW, et al. The evolution of radiographic edema in ARDS and its association with clinical outcomes: A prospective cohort study in adult patients. J Crit Care 2020;56:222-8. [Crossref] [PubMed]
- Zimatore C, Pisani L, Lippolis V, et al. Accuracy of the Radiographic Assessment of Lung Edema Score for the Diagnosis of ARDS. Front Physiol 2021;12:672823. [Crossref] [PubMed]
- Jabaudon M, Audard J, Pereira B, et al. Early Changes Over Time in the Radiographic Assessment of Lung Edema Score Are Associated With Survival in ARDS. Chest 2020;158:2394-403. [Crossref] [PubMed]
- Hohmann F, Wedekind L, Grundeis F, et al. Early spontaneous breathing for acute respiratory distress syndrome in individuals with COVID-19. Cochrane Database Syst Rev 2022;6:CD015077. [Crossref] [PubMed]
- Ansari BM, Hogan MP, Collier TJ, et al. A Randomized Controlled Trial of High-Flow Nasal Oxygen (Optiflow) as Part of an Enhanced Recovery Program After Lung Resection Surgery. Ann Thorac Surg 2016;101:459-64. [Crossref] [PubMed]
- Brainard J, Scott BK, Sullivan BL, et al. Heated humidified high-flow nasal cannula oxygen after thoracic surgery - A randomized prospective clinical pilot trial. J Crit Care 2017;40:225-8. [Crossref] [PubMed]


