
Complete video-assisted thoracoscopic surgery for pulmonary sequestration
Introduction
Pulmonary sequestration, a rare congenital malformation of lung, is a medical condition in which a part of lung tissue develops away from normal lung tissues. It usually appears over lower lobes, either intralobar or extralobular, warranting surgical operation regardless of symptoms. The conventional approach for pulmonary sequestration is low posterolateral thoracotomy. As the feasibility and safety of complete video assisted thoracoscopic surgery (c-VATS) is confirmed by the development of technology, c-VATS is applied more and more in chest disease, especially in lung cancer. However c-VATS in benign lung disease, especially in pulmonary sequestration, has seldom been reported. Therefore, a retrospective review of 25 cases pulmonary sequestration undergoing c-VATS from January 2009 to May 2012 in our hospital is reported as follows.
Patients and methods
Patients
We retrospectively reviewed 25 patients who underwent c-VATS major lung resection for pulmonary sequestration in the First Affiliated Hospital of Guangzhou Medical College from January 2009 to May 2012 (Table 1). There were 13 males (52%) and 12 females (48%), with a mean age of 34.7 years (range, 16-62 years). Lesions located at left lower lobe were found in 16 cases (64%; posterior basal segment in 12 cases and medial anterior basal segment in 4 cases), located at right lower lobe in 8 cases (32%, posterior basal segment in 6 and medial basal segment in 2), and right middle lobe in 1 case (4%). 9 cases were complicated with pulmonary infection, 2 cases with hemothorax and 1 with spontaneous pneumothorax. Preoperative computed tomography (CT) angiography and three-dimensional reconstruction demonstrated 19 cases were with abnormal blood vessels and confirmed with pulmonary sequestration; 1 case was misdiagnosed as pulmonary cyst syndrome, 4 cases were misdiagnosed as bronchiectasis and one case as benign tumor.
Table 1
| Characteristics | NO. |
|---|---|
| Gender: male/female | 13/12 |
| Age: median (range) | 37.4 [16-62] |
| Complications | |
| None | 13 (52%) |
| Infection | 9 (36%) |
| Hemothorax | 2 (8%) |
| Spontaneous pneumothorax | 1 (4%) |
| Locations | |
| left lower lobe | 16 (64%) |
| Right lower lobe | 8 (32%) |
| Right middle lobe | 1 (4%) |
Thoracoscopic surgery
All cases underwent complete video-assisted thoracoscopic surgery (c-VAST). including lobectomy of left lower lobe in 16 cases, of right lower lobe in 7, of right middle lobe in 1 as well as simple extralobar pulmonary septal pulmonary resection in 1 case. Patients were kept lying on the healthy side after general anesthesia with double-lumen endotracheal intubation. Upper limb was hung on the anesthesia rack for full exposure of axillary fossa. In addition, waist was raised for wider intercostal spaces. Incision about 0.5-1 cm in length was performed in the 7th intercostal space on the anterior axillary line, serving as an observation port. Then a trocar wasinserted and the thoracic cavity was observed with a 30 degree scope. An incision was made in the 5th intercostal space on the anterolateral chest wall as the main port, the length of which was between 3 and 5 cm, depending on the severity of pleural adhesion, the state of fissures and the size of lesion. After separation of adhesion with electrocantery and ultrasound scalpel, thoracoscopic explorations were performed. If necessary, an auxiliary port about 0.5-1 cm in length was made between posterior axillary line and linea scapularis in the same intercostal space of observation port. Then pleura mediastinalis was incised, the preoperatively located aorta and vessels passing through diaphragm was carefully inspected. The abnormal arteries were gently separated from the lower and middle lobe and ligated with endoscopic stapler or by suture ligature. Afterwards, normal veins, arteries and bronchus were successively separated and dissected routinely. Finally, the lung was placed into sample bag and dragged out through operation port.
Results
Intra- and post-operative data are shown in Table 2. Postoperative pathological examination showed pulmonary sequestration without any exception. Abnormal vessels were found in all cases during operation, diameters of which ranged from 0.3 to 1.2 cm. These abnormal vessels originated from thoracic aorta in 20 cases, from aorta abdominalis in 2 and from phrenicartery and intercostal artery in 1 respectively, from thoracic aorta combining with aorta abdominalis in 1. Venous blood returning to pulmonary veins was observed in 19 cases while to azygos veins in 6 cases. In terms of the number of abnormal arteries, 23 cases were observed with 1 to 2 aberrant branches while 2 cases were with more than 3. Mean intraoperative blood loss was 228 mL. For the two cases combining with hemothorax, one was identified with intercostal arterial hemorrhage (of 3,000 mL) during operation, so blood transfusion was performed while no hemorrhagic spots were noted in the other case. Severe errhysis occured during separation in 4 cases with severe pleural adhesions, with intraoperative blood loss ranging from 200 to 500 mL. No patient was transferred to thoracotomy. The mean operation time was 114.2 min, from 78 to 156 mins. The mean postoperative chest intubation time was 3.2 days, ranging from 2 to 7 days. The duration of postoperation hospitalization was 3 to 13 days with the average at 6.6. No perioperative deaths and apparent postoperative complications were found. All patients were discharged from hospital. The mean follow-up took 21.4 months, ranging from 2 to 32 months. No recurrence was noted.
Table 2
| Mean duration of operation (min) | 114.2±31.2 |
| Mean blood loss (mL) | 228±96.5 |
| Mean duration of chest drainage (days) | 3.2±1.4 |
| Mean length of hospital stay (days) | 6.6±3.1 |
| Complications | 0 |
| Recurrence | 0 |
Discussion
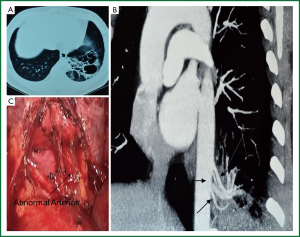
Pulmonary sequestration is a congenital malformation characterized by a mass of non-functioning lung tissue separated from the normal bronchopulmonary tree and vascularised by an aberrant systemic artery (1). It is a rare developmental abnormality accounting for 0.15-6.4% of all congenital lung anomalies (2). It is more common in lower lobes, ether intralobar (75%) or extralobar (25%) (3) and often develops long in patients. The initial symptoms often appear in youth, including recurrent cough, sputum, fever, chest pain, hemoptysis, etc. Digital subtraction arteriography (DSA) was the gold standard for preoperative diagnosis. Previous studies showed that pulmonary sequestration was often misdiagnosed as congenital pulmonary cyst or bronchiectasis complicated by infection, with a preoperative misdiagnosis rate of 71% (4). In this study, 19 cases were diagnosed as pulmonary sequestration, 1 case was misdiagnosed as pulmonary cyst syndrome, 4 cases as bronchiectasis and 1 case as benign tumor. As imaging advances, non-invasive multi-slice spiral CT scan may show the location and morphology of the lesion perspicuously; preoperative CT angiography and three-dimensional reconstruction may clearly display the aberrant arteries from the aorta with blood flow into the lesion (Figure 1 A,B,C), which increases the diagnosis rate of pulmonary sequestration at 80% (5) and contributes to the accurate positioning of aberrant feeding artery during the operation. Patients suspected with pulmonary sequestration in this study underwent preoperative CT angiography and 3D reconstruction, 19 out of whom (76%) were noted with aberrant feeding artery. Before operation, pulmonary sequestration should be differentiated with pulmonary cyst, bronchiectasis, chronic pulmonary abscess, benign lung tumor, diaphragmatic hernia and lung cancer. In our opinion, pulmonary sequestration is likely to occur to those with recurrent lung infection and showed with lower lung field shadow by X-ray. The awareness and experience of CT radiologist and chest physician on pulmonary sequestration play a crucial role in the diagnosis process. CT scan and three-dimensional reconstruction benefits the recognition of the abnormal arteries and increases the preoperative diagnosis rate of pulmonary sequestration for patients with lower pulmonary shadow and recurrent infections, especially for those with pulmonary cyst and infection.
The definitive treatment is surgical excision. Once a patient is diagnosed with pulmonary sequestration, resection is suggested regardless of symptoms. In the early days, low posterolateral thoracotomy was the main approach for pulmonary sequestration because of the hidden aberrant arteries, pleural adhesions and other reasons, nevertheless leading to greater trauma and slower recovery (6). In recent years, as VATS develops and skills of thoracic surgeons improve, there have been reports on VATS in pulmonary sequestration. In 1994, Watine et al. firstly reported extralobar pulmonary sequestration treated with VATS (7). Subsequently, Tsang et al. found patients undergoing VATS had shorter operation time and postoperative hospital stay, less intraoperative blood loss and postoperative pain than those undergoing conventional thoracotomy (8). Kestenholz et al. reported VATS in treating 14 cases of intralobar pulmonary sequestration, suggesting VATS as an option for pulmonary sequestration in experienced hospitals (9). There has been domestic report on VATS lobectomy for intralobar sequestration as well. However, literatures on VATS in pulmonary sequestration remain scanty as the thoracoscopic technique starts up late with a long learning process. It is generally accepted that main factors hindering thoracoscopy in pulmonary sequestration include difficulty in handling aberrant arteries and pleural adhesion during operation.
We have been performing VATS in chest disease since 1994, and have gained rich experience. The main advantage of VATS in pulmonary sequestration lies on a wider perspective provided by the wide-angle 30° lenses, which helps a lot in exploring and treating the diaphragm surface and pulmonary ligament. At the same time, a high resolution operation view with clearer details may be obtained by adjusting focus of the lens. In our study, 25 pulmonary sequestration patients underwent c-VATS with no significant postoperative complications in all cases. Our experience is summarized as follows: (I) Operation time: if patient suffers from acute or subacute inflammation, operation is performed after fully anti-inflammation aberrant feeding arteries lie in lower pulmonary ligament or lesions, adhering to chest wall and diaphragm, as they are difficult to be found. (II) During operation, cautious dissection of arteries and veins are essential, especially for the abdominal arteries of systemic circulation. Besides, it’s important to find out abnormal arteries in case of multiple branches lesion. Aberrant vessel is easy rupturing and bleeding due to deficiency of muscle layers and local infection. Therefore, isolation is performed cautiously. (III) Meanwhile, attention is paid to prevent serious consequences caused by aberrant vascular retracting back to the mediastinum or inferior diaphragm. In our series, we mainly used endoscopic stapler and double ligation or suture to dissect aberrant feeding artery. The main difficulty in artery dissection with stapler is how to go through the interspace adjacent to the artery, and we solved it under the guidance of a small urinary catheter. Ligating the proximal end of vessels with suture is suggested to prevent uncontrollable bleeding in case of cutter misprocessing (Figure 1D). (IV) Pulmonary vessels: for large vessels, we mainly use endoscopic stapler. By tangential clamp, we go around the blood vessels and then titanium clips may be used. CT angiography and three-dimensional reconstruction are advised in patients preoperatively suspected with pulmonary sequestration to predict the number and position of aberrant feeding arteries. The prediction is very important for exploring abnormal blood vessels, reducing and avoiding bleeding during operation. For those suspected with aberrant feeding artery from the abdominal aorta, routine CT scan till the layer under diaphragm is suggested for detecting aberrant arteries. In our series, 2 cases were noted with aberrant feeding arteries from the abdominal aorta, by enhanced CT scan and three-dimensional reconstruction preoperatively. (V) Accidentally, one case was combined with hemothorax because of sudden intercostal artery bleeding, bleeding volume in which reached to 3,000 mL, so special attention should be paid in the future operation. (VI) Extralobar pulmonary sequestration may be interlinked with esophagus. Therefore, in case of esophageal fistula, injury to esophagus during operation should be avoided. (VII) c-VATS should be converted to video-assisted small incision surgery or even thoracotomy if there are severe adhesions or accidental vascular injuries.
In conclusion, as thoracic surgeons are more and more experienced in VATS lobectomy and endoscopic equipments develop, pleural adhesion is currently a relative rather than absolute contraindication. It is quick and safe to separate most pleural adhesions under thoracoscopy as long as the right approach is taken into the thoracic cavity. Moreover, thoracoscopy helps to find the aberrant feeding vessels, providing much clearer surgical field and thus enhancing operation safety. Still, VATS lobectomy for pulmonary sequestration will be further confirmed for its safety and effectiveness.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Clements BS, Warner JO. Pulmonary sequestration and related congenital bronchopulmonary-vascular malformations: nomenclature and classification based on anatomical and embryological considerations. Thorax 1987;42:401-8. [PubMed]
- Savic B, Birtel FJ, Tholen W, et al. Lung sequestration: report of seven cases and review of 540 published cases. Thorax 1979;34:96-101. [PubMed]
- Sersar Sameh I, El Diasty M, Ibrahim Hammad R, et al. Lower lobe segments and pulmonary sequestrations. J Thorac Cardiovasc Surg 2004;127:898-9. [PubMed]
- Ahmed M, Jacobi V, Vogl TJ. Multislice CT and CT angiography for non-invasive evaluation of bronchopulmonary sequestration. Eur Radiol 2004;14:2141-3. [PubMed]
- La Quanglia MP. Congenital anomalies of the lung. In: Pearson GF, Cooper JD, Deslauriers J, et al. eds. Thoracic Surgery. 2nd ed. Philadelphia: Churchill Livingstone, 2002:509-11.
- Watine O, Mensier E, Delecluse P, et al. Pulmonary sequestration treated by video-assisted thoracoscopic resection. Eur J Cardiothorac Surg 1994;8:155-6. [PubMed]
- Tsang FH, Chung SS, Sihoe AD. Video-assisted thoracic surgery for bronchopulmonary sequestration. Interact Cardiovasc Thorac Surg 2006;5:424-6. [PubMed]
- Kestenholz PB, Schneiter D, Hillinger S, et al. Thoracoscopic treatment of pulmonary sequestration. Eur J Cardiothorac Surg 2006;29:815-8. [PubMed]
- Li SB, He JX, Yang YY, et al. Intralobar pulmonary sequestration, diagnosis and surgical treatment. Chin J Thorac Cardiovasc Surg 2006;8:271-2.


