Prognostic factors of recurrence and disease-free survival in radically resected pulmonary carcinoids: a real-world analysis
Highlight box
Key findings
• Tumor size (cm) and nodal involvement are the most important prognostic factors for recurrence and reduced disease-free survival (DFS).
• Five-year DFS for cases of nodal involvement (pN1/2) was 73.3% and 93.8% without nodal involvement.
• Five-year DFS for pT1, pT2 as well as pT3 was 97.8%, 87.5% and 40%, respectively.
What is known what is new?
• Recurrence is more frequent in atypical (ACs) compared to typical (TCs) carcinoids.
• Tumor size in cm and nodal involvement are correlated with DFS.
What is the implication and what should change now?
• Nodal involvement and tumor size (cm) may be as important for prognosis as histologic subdivision into TCs and ACs.
• Nodal involvement and tumor size (cm) should be highly considered for determining follow-up frequency as well as the diagnostic approach.
Introduction
Pulmonary carcinoids (PCs) are rare malignant tumors deriving from neuroendocrine Kulchitsky cells of the bronchopulmonary mucosa and submucosal glands, reflecting 25% of all carcinoid tumors (1,2). According to the current 2015 World Health Organization (WHO) classification of lung tumors, PCs are categorized into typical carcinoids (TCs) and atypical carcinoids (ACs) due to mitotic count and absence/presence of necrosis (3). Moreover, PCs are rare tumors accounting for less than 2% of all lung tumors (4).
Up to 40% of patients are asymptomatic at time of diagnosis. Accordingly, these tumors are frequently incidental findings on imaging. The most common respiratory symptoms, in descending order, are cough, hemoptysis, poststenotic pneumonia, chest pain and dyspnea. Furthermore, neuroendocrine tumors may synthesize and release different bioactive amines into circulation. Therefore, PCs are rarely diagnosed owing to hormonal hypersecretion causing carcinoid or Cushing syndrome and hardly ever acromegaly (5).
Whenever feasible surgery is the primary treatment of choice for PC with the aim to completely remove the tumor while simultaneously preserving as much functional lung tissue as possible (4).
In general, prognosis of patients diagnosed with PCs is quite favorable. However, chances of cure deteriorate significantly if recurrence occurs. The distinction of distant metastases and locoregional recurrence is of paramount importance, as overall survival is significantly reduced in carcinoids with distant metastases (6).
Hence, the main aim of this retrospective study was to identify risk factors for recurrence and reduced disease-free survival (DFS) in patients diagnosed with PCs who underwent curative intent surgery. Additionally, we aimed to assess the differences between TCs and ACs in terms of epidemiology, tumor characteristics as well as outcomes. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1681/rc).
Methods
We performed a retrospective single-center analysis of all consecutive patients diagnosed with PCs who underwent surgery at the Department of Thoracic Surgery at Clinics Penzing and Floridsdorf in Vienna, Austria, from 2010 to 2019. Notably, before 2019 the Department of Thoracic Surgery was located at the Clinic Penzing (“Otto Wagner Spital”), before it has been completely relocated to the newly built site of Clinic Floridsdorf. The study was conducted according to the guidelines of the Declaration of Helsinki (as revised in 2013). The Ethics Committee of the city of Vienna approved the study (No. EK-14-030-VK) and waived the need for informed consent due to the retrospective design. Neoadjuvant or adjuvant therapy (in addition to primary surgery before a potential recurrence), metastatic stage or R1 situations without an additional curative operation were defined as exclusion criteria for this study. It should be noted that a total of 84 carcinoids among 82 patients were observed. Accordingly, one patient presented with metachronous and one with synchronous PCs. Regarding the survival analysis only one case was added for each patient, in either case the one first operated on. Radical resection as well as lymphadenectomy were performed in all cases, following the recommendations of the European Neuroendocrine Tumor Society (ENETS) (7).
Tumors were classified into TC and AC according to the 2015 WHO classification of lung tumors and staged according to the eighth edition of the lung cancer TNM system (3,8). An area of 2 mm2, corresponding to 10 high-power fields, was examined by the pathologist for mitotic counting. The Ki-67 index was retrospectively extracted from the pathology reports. We used the Ki-67 clone CONFIRM anti-Ki-67 (Jeny30-Jeny9) from Ventana/Roche (Ventana Medical Systems, Inc. 1910 E. Innovation Park Drive, Tucson, Arizona 85755, USA).
Tumors were defined as peripheral if their location was distal to the segmental bronchus and classified as central if diagnosed specifically at that demarcation or proximal by bronchoscopy. Recurrences were evaluated based on the results of biopsies and specific imaging like computed tomography (CT) scans, magnetic resonance imaging (MRI) or somatostatin receptor scintigraphy (SRS). DFS was calculated from the date of resection until tumor recurrence or date of last follow-up/death. Patients’ data were prospectively collected and retrospectively analyzed.
Statistical analysis
Descriptive and inferential statistical analyses were performed using the statistical package IBM SPSS® version 27 (IBM, Armonk, NY, USA). Within the context of inferential statistics, the significance level, corresponding to the probability of type I error, was set at α=5%, therefore an inferential result P≤0.05 within hypotheses-testing is designated as significant. Student’s t-test and Mann-Whitney’s U test were used comparing the difference between two sample means of a continuous variable. For analyzing the difference of categorical variables between two groups we used Chi-square and Fisher’s exact tests. Kaplan-Meier method was utilized for survival analysis. Independent prognostic factors for DFS and recurrence were determined using multivariable Cox regression and binary logistic regression, respectively. Missing data were treated as unknown. It should be noted that only 60 patients were eligible for Cox regression, as the remaining patients were censored because their follow-up time was shorter than the time to first occurrence of an event.
Results
Sociodemographic characteristics and clinical symptoms
This study included 82 patients, 48 females (58.5%) and 34 males (41.5%) with a mean age of 58.9±14.2 years, representing 84 cases of PCs, 56 TCs (66.7%) and 28 ACs (33.3%). Median follow-up time was 22 months. Symptoms were noted in 53.0% (n=35/66) of the patient collective, cough (n=31; 47.0%) being the most common.
Sociodemographic characteristics and clinical symptoms are displayed in Table 1.
Table 1
| Variables | Total | TC | AC | P value |
|---|---|---|---|---|
| Cases (patients N=82) | 84 (100.0) | 56 (66.7) | 28 (33.3) | – |
| Age (years) | ||||
| Mean ± SD | 58.9±14.2 | 59.3±14.4 | 58.2±14.1 | 0.676 |
| Median (IQR) | 63.4 (49.4; 70.0) | 63.4 (49.5; 70.2) | 63.4 (48.5; 68.9) | – |
| Sex | 0.531 | |||
| Female | 49 (58.3) | 34 (69.4) | 15 (30.6) | |
| Male | 35 (41.7) | 22 (62.9) | 13 (37.1) | |
| Smoking history (N=64) | 35/64 (54.7) | 24/43 (55.8) | 11/21 (52.4) | 0.796 |
| Pack years (N=18) | ||||
| Mean ± SD | 35.0±20.7 | 32.1±22.3 | 45.0±10.0 | 0.198 |
| Median (IQR) | 35 (15; 50) | 30 (10; 50) | 40 (40; 50) | – |
| Symptoms (N=66) | 35/66 (53.0) | 22/44 (50.0) | 13/22 (59.1) | 0.485 |
| Cough | 31 (47.0) | 19 (43.2) | 12 (54.5) | 0.383 |
| Pneumonia | 22 (33.3) | 14 (31.8) | 8 (36.4) | 0.712 |
| Hemoptysis | 10 (15.2) | 5 (11.4) | 5 (22.7) | 0.281 |
| Dyspnea | 6 (9.1) | 5 (11.4) | 1 (4.5) | 0.655 |
| Chest pain | 2 (3.0) | 2 (4.5) | 0 | 0.549 |
| Flush | 1 (1.5) | 1 (2.3) | 0 | >0.999 |
| Pleural effusion | 2 (3.0) | 1 (2.3) | 1 (4.5) | >0.999 |
Data are presented as N (%), unless otherwise stated. N = valid data protocols. TC, typical carcinoid; AC, atypical carcinoid; SD, standard deviation; IQR, interquartile range.
Tumor characteristics
Furthermore, tumor-related parameters at time of resection are displayed in Table 2. 59.5% (n=50) of PCs were peripherally located, without significant difference between TCs and ACs. Additionally, hormone production was only detected in two (2.4%) cases of TCs; serotonin was expressed in one case and adrenocorticotropin (ACTH) in the other.
Table 2
| Variables | Total | TC | AC | P value |
|---|---|---|---|---|
| Cases (patients N=82) | 84 (100.0) | 56 (66.7) | 28 (33.3) | – |
| Localization | 0.432 | |||
| Central | 34 (40.5) | 21 (37.5) | 13 (46.4) | |
| Peripheral | 50 (59.5) | 35 (62.5) | 15 (53.6) | |
| Primary tumor site | ||||
| Right | 52 (61.9) | 33 (58.9) | 19 (67.9) | 0.427 |
| Left | 32 (38.1) | 23 (41.1) | 9 (32.1) | |
| Main bronchus | 2 (2.4) | 2 (3.6) | 0 | 0.550 |
| Bronchus intermedius | 3 (3.6) | 3 (5.4) | 0 | 0.547 |
| Upper lobe | 24 (28.6) | 15 (26.8) | 9 (32.1) | 0.608 |
| Middle lobe | 14 (16.7) | 9 (16.1) | 5 (17.9) | >0.999 |
| Lower Lobe | 41 (48.8) | 27 (48.2) | 14 (50.0) | 0.877 |
| Tumorlets | 8 (9.5) | 5 (8.9) | 3 (10.7) | >0.999 |
| Pleural invasion | 1 (1.2) | 1 (1.8) | 0 | >0.999 |
| Mitotic count (per 10 high-power fields) | ||||
| Mean ± SD | 2.06±3.15 | 0.77±0.60 | 4.64±4.41 | <0.001** |
| Median (IQR) | 1.0 (1.0; 2.5) | 1.0 (0; 1.0) | 3.5 (2.5; 5.0) | – |
| Ki-67 index (%) (N=62) | ||||
| Mean ± SD | 4.23±4.48 | 2.34±1.71 | 7.91±5.82 | <0.001** |
| Median (IQR) | 2.0 (1.0; 5.0) | 2.0 (1.0; 2.0) | 7.0 (5.0; 10.0) | – |
| Hormone production | 2 (2.4) | 2 (3.6) | 0 | 0.550 |
| Serotonin | 1 (1.2) | 1 (1.8) | 0 | >0.999 |
| ACTH | 1 (1.2) | 1 (1.8) | 0 | >0.999 |
| Tumor size (cm) | ||||
| Mean ± SD | 2.29±1.23 | 2.05±0.92 | 2.78±1.60 | 0.058 |
| Median (IQR) | 2.0 (1.5; 2.6) | 1.9 (1.5; 2.5) | 2.5 (1.5; 3.6) | – |
| pT | 0.019* | |||
| T1 | 66 (78.6) | 48 (85.7) | 18 (64.3) | |
| T2 | 12 (14.3) | 7 (12.5) | 5 (17.9) | |
| T3 | 6 (7.1) | 1 (1.8) | 5 (17.9) | |
| Nodal involvement | 12 (14.3) | 8 (14.3) | 4 (14.3) | >0.999 |
| pN | >0.999 | |||
| N0 | 72 (85.7) | 48 (85.7) | 24 (85.7) | |
| N1 | 7 (8.3) | 5 (8.9) | 2 (7.1) | |
| N2 | 5 (6.0) | 3 (5.4) | 2 (7.1) | |
Data are presented as N (%), unless otherwise stated. *, P≤0.05; **, P≤0.01. N = valid data protocols. TC, typical carcinoid; AC, atypical carcinoid; SD, standard deviation; IQR, interquartile range; ACTH, adrenocorticotropin.
Surgical approaches and outcome
Surgical management and outcome parameters are displayed in Table 3. Notably, of the six cases with recurrence, three underwent a lobectomy, one a bilobectomy, one a bronchial sleeve lobectomy, and one a lobectomy with a partial vascular sleeve resection. Moreover, all patients underwent also a complete mediastinal lymph node dissection, and all cases were a R0 resection.
Table 3
| Variables | Total | TC | AC | P value |
|---|---|---|---|---|
| Cases (patients N=82) | 84 (100.0) | 56 (66.7) | 28 (33.3) | – |
| Surgical approach | 0.643 | |||
| Thoracotomy | 39 (46.4) | 25 (44.6) | 14 (50.0) | |
| VATS | 45 (53.6) | 31 (55.4) | 14 (50.0) | |
| Surgical resection | 0.701 | |||
| Lobectomy | 60 (71.4) | 39 (69.6) | 21 (75.0) | |
| Bilobectomy | 7 (8.3) | 5 (8.9) | 2 (7.1) | |
| Sleeve lobectomy | 7 (8.3) | 6 (10.7) | 1 (3.6) | |
| Bronchial sleeve resection | 2 (2.4) | 0 | 2 (7.1) | |
| Pneumonectomy | 1 (1.2) | 0 | 1 (3.6) | |
| Wedge resection | 3 (3.6) | 3 (5.4) | 0 | |
| Segmentectomy | 4 (4.8) | 3 (5.4) | 1 (3.6) | |
| Hospital stay (days) | ||||
| Mean ± SD | 9.8±8.4 | 9.5±7.4 | 10.2±10.2 | 0.720 |
| Median (IQR) | 8.0 (6.0; 10.0) | 8.0 (6.0; 10.0) | 8.0 (6.5; 10.0) | – |
| Recurrence | 6 (7.1) | 1 (1.8) | 5 (17.9) | 0.014* |
| Locoregional recurrence | 1 (1.2) | 1 (1.8) | 0 | >0.999 |
| Distant metastasis | 6 (7.1) | 1 (1.8) | 5 (17.9) | 0.014* |
| Liver metastasis | 4 (4.8) | 0 | 4 (14.3) | 0.011* |
| Brain metastasis | 1 (1.2) | 1 (1.8) | 0 | >0.999 |
| Bone metastasis | 3 (3.6) | 0 | 3 (10.7) | 0.034* |
| Pancreatic metastasis | 1 (1.2) | 0 | 1 (3.6) | 0.333 |
| Adrenal metastasis | 1 (1.2) | 0 | 1 (3.6) | 0.333 |
| Mortality | 10 (11.9) | 5 (8.9) | 5 (17.9) | 0.290 |
| Dead for disease | 2 (2.4) | 0 | 2 (7.1) | 0.175 |
| Dead for other causes | 8 (9.5) | 5 (8.9) | 3 (10.7) | |
| Alive with disease | 3 (3.6) | 1 (1.8) | 2 (7.1) | 0.257 |
Data are presented as N (%), unless otherwise stated. *, P≤0.05. N = valid data protocols. TC, typical carcinoid; AC, atypical carcinoid; VATS, video-assisted thoracoscopic surgery; SD, standard deviation; IQR, interquartile range.
A minimally invasive approach [video-assisted thoracoscopic surgery (VATS)] was the preferred procedure (n=45; 53.6%), while thoracotomy was performed in the remaining cases (n=39; 46.4%). Furthermore, in most cases lobectomies (including bilobectomies and sleeve lobectomies) were performed (n=74; 88.1%). Median hospital stay after the procedure was 8 days.
Recurrences occurred in one patient (1.8%) with TCs and five patients (17.9%) with ACs (P=0.014). These appeared primarily as distant metastases (n=6; 7.1%), involving mainly the liver and bones. Only one locoregional recurrence (1.8%) was noted. From the six patients with recurrence, two underwent adjuvant radiotherapy (alone or in combination), four underwent radionuclide therapy, and one underwent complex abdominal metastasectomy.
Throughout the 10-year duration of this study, 10 patients (11.9%) died, only two (2.4%) due to the disease.
Independent prognostic factors influencing DFS and recurrence
Determining prognostic parameters affecting DFS, univariable Cox regression was initially performed. Parameters included in this approach were age, sex, smoking, histology, tumor size (cm), localization, mitotic count, Ki-67 index and nodal involvement. Notably, as almost 80% of our patients had been in stage pT1 (see Table 2), we elected to choose ‘tumor size in cm’ as a more relevant size parameter for subsequent statistical analysis.
Thereafter, multivariable Cox regression was performed including histology, tumor size (cm) and mitotic count. Ultimately, only tumor size (cm) was identified as a statistically significant independent parameter for reduced DFS in radically resected PCs (P=0.018; HR =1.77), shown in Table 4.
Table 4
| Prognostic factors | Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Age (years) | 1.056 | 0.973–1.146 | 0.192 | – | – | – | |
| Male sex | 1.270 | 0.254–6.345 | 0.771 | – | – | – | |
| Smoking | 0.455 | 0.082–2.514 | 0.367 | – | – | – | |
| Atypical carcinoid | 9.429 | 1.097–81.017 | 0.041* | 4.273 | 0.365–49.97 | 0.247 | |
| Tumor size (cm) | 2.042 | 1.340–3.111 | <0.001** | 1.766 | 1.103–2.826 | 0.018* | |
| Peripheral location | 0.322 | 0.059–1.766 | 0.192 | – | – | – | |
| Mitotic count | 1.114 | 1.020–1.217 | 0.016* | 0.985 | 0.878–1.106 | 0.801 | |
| Ki-67 index (%) (N=41) | 1.118 | 1.005–1.243 | 0.041* | – | – | – | |
| Nodal involvement (N1/2) | 4.537 | 0.911–22.601 | 0.065 | – | – | – | |
Data are presented as N (%), unless otherwise stated. *, P≤0.05; **, P≤0.01. N = valid data protocols. HR, hazard ratio; CI, confidence interval.
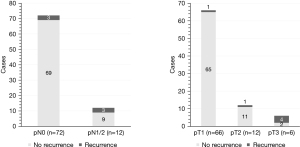
Moreover, binary logistic regression was utilized to reveal prognostic parameters associated with higher risk of recurrence. In addition, we used the same variables as for the Cox regression. Tumor size in cm (P=0.023; OR =11.88) and nodal involvement (P=0.043; OR =2,695.6) emerged as prognostic factors inducing higher risk of recurrence in radically resected PCs (Table 5), which is further illustrated in Figure 1.
Table 5
| Prognostic factors | Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Age (years) | 1.049 | 0.973–1.131 | 0.210 | – | – | – | |
| Male sex | 1.452 | 0.275–7.668 | 0.661 | – | – | – | |
| Smoking | 0.375 | 0.063–2.219 | 0.280 | – | – | – | |
| Atypical carcinoid | 12.273 | 1.355–111.152 | 0.026* | 9.005 | 0.365–49.977 | 0.424 | |
| Tumor size (cm) | 3.426 | 1.648–7.122 | <0.001** | 11.878 | 1.412–99.916 | 0.023* | |
| Peripheral location | 0.326 | 0.056–1.893 | 0.212 | – | – | – | |
| Mitotic count | 1.527 | 1.095–2.128 | 0.012* | 1.842 | 0.938–3.617 | 0.076 | |
| Ki-67 index (%) (N=41) | 1.254 | 1.053–1.494 | 0.011* | – | – | – | |
| Nodal involvement (N1/2) | 7.444 | 1.300–42.628 | 0.024* | 2,695.6 | 1.296–∞ | 0.043* | |
Data are presented as [N (%)], unless otherwise stated. *, P≤0.05; **, P≤0.01. N = valid data protocols. OR, odds ratio; CI, confidence interval.
Survival analysis
First, we used the Kaplan-Meier approach for comparing the overall and DFS between TC (n=55) and AC (n=27) (Figure 2). Mantel-Cox’s log-rank test revealed no significant difference (P=0.543) in terms of overall survival. Five-year overall survival was 87.5% and 84.7% for TCs and ACs, respectively.
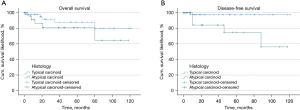
Five-year DFS was 97.5% and 74.9% (P=0.012) for TCs and ACs, respectively.
We then examined the influence of nodal involvement as well as the pT category on DFS. We found that nodal involvement (P=0.041) and the pT category (P<0.001) significantly influenced DFS (Figure 3).
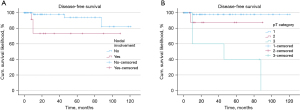
Five-year DFS for cases of nodal involvement (pN1/2, n=12) was 73.3% and 93.8% without nodal involvement (n=70). Furthermore, 5-year DFS for pT1 (n=64), pT2 (n=12) as well as pT3 (n=6) was 97.8%, 87.5% and 40%, respectively.
Discussion
PCs are rare neuroendocrine tumors of the lung, accounting for approximately 2% of all lung malignancies (1,4). Compared to other lung malignancies like small cell lung cancer (SCLC), adenocarcinoma or squamous cell carcinoma, these tumors behave less aggressively and exhibit better overall survival. However, if recurrence occurs, their otherwise favorable prognosis is significantly worsened (6). Therefore, the main aim of this study was to identify parameters influencing DFS and recurrence rate in patients who underwent radical resection for TCs and ACs.
Patient demographics and clinical presentation
In this study we present a male:female ratio of 0.71, which is within the range of other studies describing a slight predominance in female patients (2). Moreover, our patient collective consisted of a reasonably high proportion of ACs (66.7%), considering that ratios of 8–10:1 between TCs and ACs are commonly described in the literature (4,5).
We did not find any difference between the two histologic subtypes regarding age. In contrast, Caplin et al. described that the mean age for TCs is 45 years, as ACs generally occur one decade later (4).
Symptoms were recognized in 53.0% of the cohort, cough (47.0%) being the most frequent. These findings are consistent with previous reports claiming that up to 40–50% of patients are asymptomatic at time of diagnosis (5).
Controversy surrounds the question whether a history of smoking, considered one of the strongest risk factors for SCLC or large cell neuroendocrine carcinoma (LCNEC) (9), may also be associated with the development of PCs. In our cohort, 54.7% of patients did smoke at time of diagnosis or had a smoking history. Nevertheless, no conclusions concerning the risk potential of tobacco consumption can be drawn, since the number of cases is far too small and a comparison with a healthy cohort was not established.
Tumor characteristics
In general, PCs have been reported to be centrally localized, Detterbeck et al. indicated a percentage of 70% (10). However, in more recent studies, the ratios have converged or even shown a higher proportion of peripherally located PCs (11,12). These discrepancies may be explained by the fact that no uniform definition has been established for “central” and “peripheral”. This study reveals a predominance of peripheral tumors (59.5%). Although no statistically significant difference was evident between TCs, a tendency of TCs toward peripheral localization is notable. Additionally, the majority of our cases occurred in the right lung, which is well described in preceding studies (13,14).
Our findings regarding Ki-67 (Table 2) are in accordance with the current 2015 WHO guidelines, which indicate a Ki-67 index of 0–5% and 6–20% for TCs and ACs, respectively (3).
Previous studies have shown that ACs compared to TCs present larger tumors as well as higher prevalence of nodal metastases (15,16). Concerning tumor size (cm), a statistically significant difference in pT stage for TCs and ACs was observed, whereas tumor size in cm was tending in the same direction, however not statistically significant. Impressively, 17.9% of ACs were resected at stage pT3 contrasting to only 1.8% of TCs. Regarding nodal involvement we report the same percentage of TCs and ACs (14.3%) already developed lymph node metastases at time of resection. Thus, no difference was ascertained in our study, which is in contrast to most results in the literature suggesting that lymph node metastasis is significantly more frequent in ACs compared to TCs (12,17). It may be contended, though, that our results are quite in line with what can be expected, namely because tumor size and lymph node involvement are established prognostic factors. However, as almost 80% of our cases had pT1 tumors, and 85% were in a pN0 situation, focusing on T and N factors alone would therefore not have fully captured the strong prognostic relevance of tumor size and lymph node involvement. Thus, for PCs the adoption of ‘tumor size in cm’ seems to be a more relevant prognostic parameter than T stage. We believe, this insight is a new knowledge.
Treatment
Furthermore, there is an ongoing debate regarding the best surgical approach in the literature. In recent studies, VATS has been associated with advantages over thoracotomy regarding pain and postoperative complications (18). We report a predominance of patients being treated by using VATS over thoracotomy, which was primarily utilized for large as well as centrally located tumors. In our study, the procedure of choice was a lobectomy. Based on the literature, it is unclear whether sublobar resection achieves the same outcome for PCs as lobectomy. Several previous papers declared no difference (19), whereas others reported the superiority of lobectomy (6,17). Xu et al. demonstrated no statistically significant difference for overall survival in PCs ≤3 cm in a large-scale study comparing lobectomy and sublobar resection (20). Similarly, Ernani et al. did not observe improved overall survival for lobectomy compared with sublobar resection in non-metastatic ACs (21).
Outcome and prognostic factors
Recurrences occurred significantly more frequent in ACs compared to TCs. In accordance, Filosso et al. described recurrence rates of 5% for TCs and 20% for ACs after radical resection (22). Metastases primarily appeared as distant metastases, mainly involving the liver and bones. Locoregional recurrence was noted only once.
We observed a 5-year overall survival of 87.5% and 84.7% for TCs and ACs, respectively. According to the 5- and 10-year overall survival rates reported in the literature, the long-term survival for PCs after primary resection in our cohort is reasonably good. Indeed, in the literature the 5- and 10-year overall survival for TCs has been found to be 86% to 93% and 76% to 88% (12,23,24), respectively, compared with a 5-year overall survival of 80% to 87% and a 10-year overall survival of 43% to 69% for ACs (6,15,24,25). Cañizares et al. presented that 5-year overall survival of ACs is dramatically shortened to only 39% following distant metastasis. Furthermore, they stated that median survival is reduced to 38 months in ACs developing distant metastasis, compared with 180 months in disease-free patients (6). Consequently, focusing on prognostic factors influencing DFS to find patients at risk of recurrence is necessary to further improve the outcome. Our survival analysis revealed significantly reduced DFS for ACs compared to TCs, 5-year DFS was 97.5% for TCs and 74.9% for ACs, which is consistent with preceding studies indicating 5-year DFS of 82% to 99% and 72% to 81% for TCs and ACs, respectively (26-28).
Only tumor size (cm) proved to be an independent statistically significant factor associated with reduced DFS in the performed multivariable Cox regression. Tumor size (cm) and nodal involvement were independently and statistically significantly associated with higher risk of recurrence in radically resected PCs, as determined by multivariable logistic regression. In recent studies, which also identified advanced age and male sex as prognostic factors, competing risk model nomograms (29) and scores (30) were developed to evaluate long-term survival. When using these models, we highly recommend including lymph node involvement and tumor size (cm) as prognostic factors for the calculation of long-term survival, regardless of histologic type.
We report 5-year DFS, independently of the histologic subtype, for pT1, pT2 and pT3 being 97.8%, 87.5% and 40%, respectively, which clearly illustrates the influence of tumor size (cm). Studies describing the impact of tumor size (cm) or pT stage on 5-year DFS are scarce to nonexistent in the literature. Lee et al. reported 5-year DFS of 90.6% and 81.2% for pT1 and pT2–4, respectively, which is in accordance with our findings (26). Hence, PCs presenting at pT1/2 are considered having a very good prognosis. Further growth of the tumor indicates significantly worsened prognostic outcome for stage pT3. Furthermore, we determined 5-year DFS of 93.8% for PCs staged pN0 compared to 73.3% for those displaying nodal involvement (pN1/2). Similar results have been obtained in previous studies. Cusumano et al. described 5-year DFS of 99%, 88%, and 60% for pN0, pN1, and pN2, respectively (27). Additionally, Lee et al., who divided their cohort for statistical analysis into groups characterized by pN1/2 and pN0, described 5-year DFS as 89% for pN0 and 78% for pN1/2 (26).
Limitations
The most important limitations for the present work arise from the fact that this is a single center study with a comparatively small number of cases. However, since this is a single center study from the largest thoracic surgical center in Austria, with all consecutive patients included, this is only a relative limitation. Moreover, the published references with more included patients are either multicentric studies, or are based on large population databases, like Surveillance, Epidemiology, and End Results (SEER), National Cancer Database or similar. Due to the relatively small number of cases, only few recurrences could be detected, which complicates the identification of prognostic factors. Data of approximately 10 years were conducted in this retrospective study. Therefore, we were unable to report all parameters for each patient and had to deal with the difficulties of missing data.
Conclusions
In conclusion, PCs represent an infrequent tumor entity characterized by fairly good prognosis. However, if recurrence occurs, especially in case of distant metastasis, the overall outcome is remarkably reduced. Hence, the main aim to continuously improve the outcome of patients diagnosed with TCs or ACs is to identify parameters influencing DFS and recurrence rate after radical resection.
Recurrence after complete resection is relatively rare, but more frequent in ACs compared to TCs. Thus, DFS is evidently reduced in ACs. Nodal involvement and tumor size (cm) appear to be the most important prognostic factors associated with recurrence of resected PCs. Regarding the frequency of nodal involvement, no difference between TCs and ACs was observed. Conversely, a tendency toward higher pT stages for ACs became apparent.
Therefore, we suggest that these parameters should be highly considered for determining follow-up frequency as well as the diagnostic approach. To confirm and strengthen our results, further comparable studies with large sample sizes are required.
Acknowledgments
This manuscript has been presented at the Annual ESTS Meeting 2022 in The Hague, Netherlands, 19.06.2022 as well as at the 63rd Annual Meeting of the Austrian Society of Surgery in Graz, Austria, 17.06.2022. Furthermore, parts of this project were approved as a diploma thesis at the Medical University of Vienna.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1681/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1681/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1681/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1681/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of the city of Vienna approved the study (No. EK-14-030-VK) and waived the need for informed consent due to the retrospective design.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Travis WD. Pathology and diagnosis of neuroendocrine tumors: lung neuroendocrine. Thorac Surg Clin 2014;24:257-66. [Crossref] [PubMed]
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003;97:934-59. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. Introduction to The 2015 World Health Organization Classification of Tumors of the Lung, Pleura, Thymus, and Heart. J Thorac Oncol 2015;10:1240-2. [Crossref] [PubMed]
- Caplin ME, Baudin E, Ferolla P, et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol 2015;26:1604-20. [Crossref] [PubMed]
- Gustafsson BI, Kidd M, Chan A, et al. Bronchopulmonary neuroendocrine tumors. Cancer 2008;113:5-21. [Crossref] [PubMed]
- Cañizares MA, Matilla JM, Cueto A, et al. Atypical carcinoid tumours of the lung: prognostic factors and patterns of recurrence. Thorax 2014;69:648-53. [Crossref] [PubMed]
- Singh S, Bergsland EK, Card CM, et al. Commonwealth Neuroendocrine Tumour Research Collaboration and the North American Neuroendocrine Tumor Society Guidelines for the Diagnosis and Management of Patients With Lung Neuroendocrine Tumors: An International Collaborative Endorsement and Update of the 2015 European Neuroendocrine Tumor Society Expert Consensus Guidelines. J Thorac Oncol 2020;15:1577-98. [Crossref] [PubMed]
- Rami-Porta R, Asamura H, Travis WD, et al. Lung cancer - major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:138-55.
- Hassan MM, Phan A, Li D, et al. Risk factors associated with neuroendocrine tumors: A U.S.-based case-control study. Int J Cancer 2008;123:867-73. [Crossref] [PubMed]
- Detterbeck FC. Clinical presentation and evaluation of neuroendocrine tumors of the lung. Thorac Surg Clin 2014;24:267-76. [Crossref] [PubMed]
- Ferolla P, Daddi N, Urbani M, et al. Tumorlets, multicentric carcinoids, lymph-nodal metastases, and long-term behavior in bronchial carcinoids. J Thorac Oncol 2009;4:383-7. [Crossref] [PubMed]
- Soldath P, Binderup T, Kjær A, et al. Long-term survival and recurrence after resection of bronchopulmonary carcinoids: A single-center cohort study of 236 patients. Lung Cancer 2021;156:109-16. [Crossref] [PubMed]
- Detterbeck FC. Management of carcinoid tumors. Ann Thorac Surg 2010;89:998-1005. [Crossref] [PubMed]
- Cao C, Yan TD, Kennedy C, et al. Bronchopulmonary carcinoid tumors: long-term outcomes after resection. Ann Thorac Surg 2011;91:339-43. [Crossref] [PubMed]
- Filosso PL, Oliaro A, Ruffini E, et al. Outcome and prognostic factors in bronchial carcinoids: a single-center experience. J Thorac Oncol 2013;8:1282-8. [Crossref] [PubMed]
- Bertino EM, Confer PD, Colonna JE, et al. Pulmonary neuroendocrine/carcinoid tumors: a review article. Cancer 2009;115:4434-41. [Crossref] [PubMed]
- Thakur S, Florisson D, Telianidis S, et al. Pulmonary carcinoid tumours: A multi-centre analysis of survival and predictors of outcome following sublobar, lobar, and extended pulmonary resections. Asian Cardiovasc Thorac Ann 2021;29:532-40. [Crossref] [PubMed]
- Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602-9. [Crossref] [PubMed]
- Fox M, Van Berkel V, Bousamra M 2nd, et al. Surgical management of pulmonary carcinoid tumors: sublobar resection versus lobectomy. Am J Surg 2013;205:200-8. [Crossref] [PubMed]
- Xu S, Li X, Ren F, et al. Sublobar Resection Versus Lobectomy for Early-Stage Pulmonary Carcinoid Tumors ≤3 cm in Size: A SEER Population-Based Study. Ann Surg 2022;276:e991-9. [Crossref] [PubMed]
- Ernani V, Appiah AK, Rodriguez D, et al. Lobar versus sublobar resection for atypical lung carcinoid: An analysis from the National Cancer Database. Cancer 2023;129:860-6. [Crossref] [PubMed]
- Filosso PL, Rena O, Donati G, et al. Bronchial carcinoid tumors: surgical management and long-term outcome. J Thorac Cardiovasc Surg 2002;123:303-9. [Crossref] [PubMed]
- Filosso PL, Guerrera F, Evangelista A, et al. Prognostic model of survival for typical bronchial carcinoid tumours: analysis of 1109 patients on behalf of the European Association of Thoracic Surgeons (ESTS) Neuroendocrine Tumours Working Group. Eur J Cardiothorac Surg 2015;48:441-7; discussion 447. [Crossref] [PubMed]
- Okereke IC, Taber AM, Griffith RC, et al. Outcomes after surgical resection of pulmonary carcinoid tumors. J Cardiothorac Surg 2016;11:35. [Crossref] [PubMed]
- Maurizi G, Ibrahim M, Andreetti C, et al. Long-term results after resection of bronchial carcinoid tumour: evaluation of survival and prognostic factors. Interact Cardiovasc Thorac Surg 2014;19:239-44. [Crossref] [PubMed]
- Lee PC, Osakwe NC, Narula N, et al. Predictors of Disease-free Survival and Recurrence in Patients with Resected Bronchial Carcinoid Tumors. Thorac Cardiovasc Surg 2016;64:159-65. [Crossref] [PubMed]
- Cusumano G, Fournel L, Strano S, et al. Surgical Resection for Pulmonary Carcinoid: Long-Term Results of Multicentric Study-The Importance of Pathological N Status, More Than We Thought. Lung 2017;195:789-98. [Crossref] [PubMed]
- Machuca TN, Cardoso PF, Camargo SM, et al. Surgical treatment of bronchial carcinoid tumors: a single-center experience. Lung Cancer 2010;70:158-62. [Crossref] [PubMed]
- Wang T, Zhou J, Zheng Q, et al. A Competing Risk Model Nomogram to Predict the Long-Term Prognosis of Lung Carcinoid. Ann Surg Oncol 2023;30:5830-9. [Crossref] [PubMed]
- Chiappetta M, Sperduti I, Ciavarella LP, et al. Prognostic score for survival with pulmonary carcinoids: the importance of associating clinical with pathological characteristics. Interact Cardiovasc Thorac Surg 2020;31:315-23. [Crossref] [PubMed]