
The usefulness of the open surgical technique of sternocostal elevation for asymmetric pectus excavatum: a retrospective study
Highlight box
Key findings
• Surgical procedure for asymmetric pectus excavatum (PE) is usually difficult and troublesome. Sternocostal elevation (SCE) is a potentially useful procedure for asymmetric cases because it requires no additional process compared with procedure for symmetric cases.
What is known and what is new?
• SCE has been reported to be highly safe procedure, and we reveal its versatility for asymmetric PE.
• There has been no good indicator for the success of operation for asymmetric PE. We suggest a new numerical index as an indicator of asymmetry improvement and to be used for more standard thorax morphology as a postoperative result evaluator.
What is the implication, and what should change now?
• The Nuss procedure is the mainstream surgical treatment for PE; however, SCE is compatible with various deformities without major complications. The procedure may gain attention.
Introduction
Background
Pectus excavatum (PE) is the most common congenital chest wall deformity that occurs in about one out of every 300 to 1,000 live births (1). Generally, it is defined as depression of the sternum and anterior chest wall into the thoracic cavity (2). Patients with severe deformities experience physical complaints from childhood, such as frequent respiratory infections and impaired weight gain. Furthermore, patients with mild deformities often experience chest pain, palpitation, and psychological complaints.
Rationale and knowledge gap
To date, the Nuss procedure has been the mainstream for surgical repair of PE because of its emergence (3). In contrast, some studies reported several complications associated with the Nuss procedure (4-6); therefore, open surgery for PE is performed at few institutions. In particular, surgical repair of asymmetric cases is more difficult and challenging than that of symmetric cases. We performed sternocostal elevation (SCE), a procedure that does not involve exogenous implants and wherein a section of the costal cartilage is resected and all of the cartilage stumps are reconstructed to the sternum. SCE is the name of surgical procedure; it was developed by Wada et al. in 1981 (7), and Iida (one of our co-authors) has made improvements to make it less invasive. Iida et al. have also reported its usefulness and safety (8,9). We examined the extent of resection of the costal cartilage while comparing the left and right balance during surgery. This procedure is considered to be adaptable to asymmetric PE because the same process can be performed in symmetric types.
Objective
This study aimed to assess the feasibility of SCE for asymmetric PE. In addition to the correction of the morphology by SCE, the safety of the surgery and postoperative quality of life were also investigated. SCE has been considered superior to other surgical techniques because it can handle various thoracic deformities with the same surgical technique. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1824/rc).
Methods
Patients
Of the 256 patients who underwent SCE at Shonan Kamakura General Hospital between July 2014 and July 2022, 58 (22.7%) with asymmetric PE were retrospectively examined. A difference >10 mm was observed between the hemi-thoraxes near the midclavicular line in the horizontal computed tomography (CT) section. This criterion regarding asymmetry is at our discretion. Patients with advanced scoliosis having a Cobb angle of 25° or higher were excluded as severe scoliosis generally influences asymmetric PE. Finally, 51 (19.9%) patients were analyzed. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Shonan Kamakura General Hospital (TGE01913-024). Informed consent was obtained from all participants included in the study.
Surgical procedure for SCE
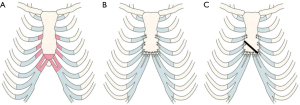
Iida et al. improved the procedures for SCE, and we performed SCEIII as a standard procedure in this study (8,10). In SCEIII, we resected a part of the third or fourth to seventh costal cartilages, as well as the lower part of the sternum below the sixth cartilage junction. Furthermore, adequate lengths of cartilage were resected, and all cartilage stumps were reconstructed to the sternum; specifically, the sixth and seventh rib cartilages were sutured to the lower cutting face of the sternum. We used #1 or #3 braided polyester sutures with a round needle, which could withstand strong tension and was less likely to cause damage to the ribs and sternum, such as cartilage cutting. The procedure is illustrated in Figure 1A,1B. In this procedure, the sternum is pulled laterally via shortened and resutured costal cartilages, and the resultant force raises the sternum ventrally. Furthermore, the sternum pulls the ribs and simultaneously corrects their inclination and the protrusion of the costal arches. In cases of asymmetric deformity, different numbers and lengths of the cartilage were resected on each side, and the procedure was the same as in the symmetry cases. SCEIII may be insufficient to correct the deformity in rare cases of asymmetric deformity with strong sternal torsion. In such cases, SCEIV is performed, wherein an oblique slit is usually added to the sternum by using an air tome, and the sternum is folded along the slit to correct sternal torsion (Figure 1C).
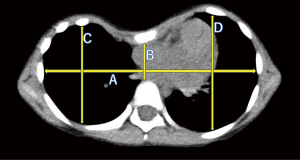
Parameters from the CT
All patients underwent CT examination pre- and postoperatively, and the two indices and other measurements were compared. Postoperative CT was performed 1 month after surgery. We obtained the following measurements of the intrathoracic area (Figure 2):
- A: maximum transverse internal thoracic diameter;
- B: minimum anteroposterior diameter perpendicular to diameter A;
- C: maximum anteroposterior diameter of the right hemithorax, measured perpendicular to diameter A;
- D: maximum anteroposterior diameter of the left hemithorax, measured perpendicular to diameter A.
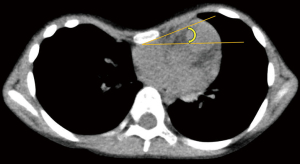
The pre- and postoperative Haller indices (B/A) and differences between the left and right thoraxes (|C − D|) were first measured and then compared. The new index was also calculated. A previous study described an asymmetry index that directly compares the anteroposterior diameters of the left and right rib cages (C/D) (11); however, in cases with large anteroposterior diameters, even if there is a left-right difference of rib cages, it is judged as underestimation. In the present study, was set as an index of correction for asymmetric thorax, which has never been used in any literature. Furthermore, the pre- and postoperative sternal torsion angles, defined as the angle of the axis of the mediolateral to the horizontal length, were compared (Figure 3).
Based on the international consensus for minimizing radiation exposure, magnetic resonance imaging (MRI) is preferred over CT. However, we performed low-dose helical CT twice. Our CT evaluation may be acceptable when considering the difficulty of performing MRI for small children.
Statistical analysis
Continuous variables were presented as medians and ranges and compared using the t-test. Statistical significance was set at a P value <0.05. Statistical analysis was conducted using the EZR software (Easy R ver.3.6.1, Saitama Medical Center, Jichi Medical University, Saitama, Japan) (12).
Results
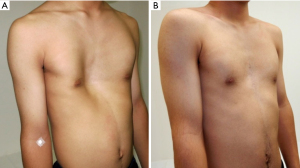
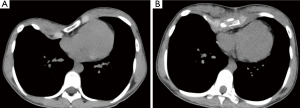
Table 1 depicts the clinical characteristics and measured values. The median age of the patients was 15 [4–46] years; 37 patients (72.5%) were men. The pre- and postoperative median Haller indices, differences between the left and right thoraxes, newly set indices, and sternal torsion angle significantly improved. We showed a representative chest pre- and postoperatively (Figure 4). In this study, only 5 (9.8%) patients required SCEIV. The median operation time was 181 [112–274] min, and the median blood loss was 80 [10–360] mg; none of the patients required blood transfusion. The length of postoperative hospital stay was 5 days, which extended up to 7 days for patients requiring flights to distant regions. All patients improved; however, 15 (29.4%) patients experienced symptoms such as chest pain (six patients), dyspnea on exertion (five patients), palpitation (one patient), arrhythmia (one patient), postprandial vomiting (one patient), and difficulty breathing (one patient) preoperatively. Nonsteroidal anti-inflammatory drugs (NSAIDs) were used to manage postoperative pain in all patients during hospital stay, and epidural anesthesia was used in adults. However, none of the patients required regular oral medication for >2 weeks after discharge. No patient complained about the postoperative thoracic form, and no complications occurred. All patients did not require reoperations for any reason. They were able to walk to the bathroom on the first postoperative day, return to their preoperative daily life within 1 week postoperatively, and return to school or work within 4 weeks. In addition, they resumed all types of activities, including contact sports, within 3 months after surgery.
Table 1
| Variables | Values (n=51) | P value |
|---|---|---|
| Age (years) | 15 [4–46] | – |
| Sex | – | |
| Male | 37 (72.5) | |
| Female | 14 (27.5) | |
| Procedure | – | |
| SCEIII | 46 (90.2) | |
| SCEIV | 5 (9.8) | |
| Measured values | ||
| Haller index | <0.05 | |
| Preoperative | 4.92 [2.28–8.90] | |
| Postoperative | 3.75 [2.26–6.43] | |
| Left-right difference (mm) | <0.05 | |
| Preoperative | 14 [10–41] | |
| Postoperative | 7 [0–22] | |
| Left-right difference (mm)/average (mm) | <0.05 | |
| Preoperative | 0.108 [0.066–0.34] | |
| Postoperative | 0.048 [0–0.179] | |
| Sternal torsion angle (°) | <0.05 | |
| Preoperative | 16.5 [1.0–45.2] | |
| Postoperative | 15.1 [0–38.0] | |
Values are expressed as median [range] or n (%). SCE, sternocostal elevation.
Discussion
Key findings
This study showed satisfactory results in the CT evaluation before and after the SCE procedure for asymmetric PE, and SCE is the same for symmetric and asymmetric PE. Even if sternum torsion slightly remained, the body surface line between the left and right sides was often improved to near symmetry. We conducted cross-sectional imaging on CT pre- and postoperatively (Figure 5). The median value of the postoperative Haller index was 3.75; values ≥3.25 were evaluated as severe and an indication for surgery. However, many patients with asymmetric PE in this study had more severe values preoperatively; however, they all showed improvement. Furthermore, the Haller index is a preoperative indicator of severity and not specified as a standard postoperative value. Moreover, we recognize that the most important aspects of correction evaluation are pre- and postoperative comparisons and patient satisfaction. Asymmetric PE is difficult to be treated and more prevalent in adults than in young patients (13). Yoshida et al. described how chest wall morphologically changes from childhood to adulthood (14). They concluded that chest depression progressed only on the right side. Although the chest wall has continuous inspiratory negative pressure, the heart prevents further depression on the left side (15). In addition, the sternum twists toward the right side. We have experienced a rare case of asymmetric PE with strong depression on the left side, and its exact cause remains unknown. Torsion correction is generally difficult in adults, regardless of whether the sternum is tilted to the left or right.
Capunay et al. described the relationship between sternal torsion and cardiac compression (16) and used cardiac MRI to assess cardiac compression indexes to define surgical candidacy. Surgery for asymmetric PE aimed to release cardiac compression and correct the thoracic form, which is an interesting idea. Surgery in patients with asymmetric PE usually requires careful preoperative planning and surgical ingenuity, regardless of the surgical technique. We consider SCE valuable because it remains the same regardless of whether PE is symmetric or asymmetric.
Strengths and limitations
Because our procedure involves step-by-step reconstruction starting from the costal cartilage of the cranial side, the balance of the left and right rib cages could be adjusted each time. These characteristics make a more precise correction of the rib cage possible. SCE is a highly safe procedure, and no complications occurred in the participants in this study. Several patients with PE are young individuals maintaining an acceptable quality of life preoperatively, so any events interfering with daily life may have more negative impacts than chest deformity, such as the experience of residual pain or unscheduled reoperation that can occur when using a metal bar.
Our study has several limitations. First, this was a single-center, retrospective study with a relatively small sample size. Additionally, SCE was rarely performed outside our institution. This may be because of fine-tuning of chest wall reconstruction in SCE has a strong art aspect and requires a high level of proficiency to achieve patient satisfaction. Examination of the learning curve is also an issue for future research. Second, this was not a comparative study with other surgical procedures. Third, long-term follow-up data are unavailable because the outpatient follow-up period is after 1 year postoperatively, and several patients lived a great distance away from the hospital. Contact via e-mail is always possible; however, we did not receive any communication of recurrence or complaints.
Comparison with similar researches
The Nuss procedure has been widely used since its emergence (3). However, it is reportedly challenging for asymmetric cases; thus, various methods have been proposed. Kuyama et al. reported the efficacy of chondrotomy and sternotomy combined with the Nuss procedure (17). In addition, multiple-bar replacement or a combination of the Nuss and Ravitch procedures was suggested (18,19). Moreover, the Nuss procedure was reported to be associated with a relatively high incidence of complications, including life-threatening events. Bar dislocation or infection may result in reoperation, and residual pain may interfere with daily activities of patients (4-6). We should consider morbidity rate as a more important factor in providing surgical invasion than the part or length of surgical incision or operation time. Thus, we consider SCE superior to the Nuss method.
Explanations of the findings
Although there are no clear criteria indicating the success or failure of PE surgery, we believe that improvement in anterior thoracic depression and costal arch protrusion provides good satisfaction. The Haller index is widely used as a morphological indicator; however, an accurate numerical index to assess the degree of asymmetry is lacking. The correction index is well known as an accurate assessment of pectus severity and a reflection of the potential degree of surgical repair. However, we judged this index was difficult to use in assessing the improvement of asymmetric PE, and it was not measured in this study. Thus, we set a new numerical index as an indicator of asymmetry improvement to be used for more standard thoracic morphology. To date, a reasonable assessment method for asymmetric PE remains unknown. We suggest that the improvement of asymmetry and thickening of the anteroposterior diameter will result in the ideal chest wall morphology. Thus, we judged that this index sufficiently contained that element and so we decided to adopt it; however, no data support this validity. Recently Bellía-Munzón et al. proposed a novel index, the titanic index, to assess the cephalocaudal extent of the excavation of PE (20), and various geometric indicators will be proposed for the three-dimensional evaluation of thoracic deformity in the future. In this study, we revealed that the objective indicators obtained on CT scans were all improved by SCE, and all patients were satisfied.
Implications and actions needed
We think the methods for surgical repair of asymmetric PE remain controversial; however, SCE is compatible with various chest deformities (pectus carinatum) without major complications in the same procedure, even in case of asymmetric PE. Although there have been no reports about SCE other than our reports, the procedure may gain attention. Thus, a more detailed study of SCE may be necessary, such as a prospective quantitative assessment of postoperative pain or satisfaction. In addition, the evaluation of cardiopulmonary function pre- and postoperatively is also interesting. A previous study showed that SCE improved cardiac function (21). Concerning pulmonary function, improvement postoperatively may be expected only in cases of severe reduction of lung function in a patient with PE (21,22). Although SCE reduces the rib cage volume, the procedure does not adversely affect respiratory function. Moreover, normal chest morphology is more important for respiratory function than rib cage volume. Because surgical methods have significantly improved over the years, re-evaluation may be necessary. In this study, some patients had mild electrocardiogram abnormalities preoperatively. However, cardiac function showed no obvious abnormalities; therefore, echocardiogram was not routinely performed postoperatively. In addition, we included several cases during the coronavirus disease 2019 pandemic, and respiratory function tests that generate aerosols have been suspended in our institution during that period and cannot be performed because of droplet precaution. This is an issue to be considered in the future.
Conclusions
Although left-right thorax difference may remain in the case of severe deformity when using SCE, this surgical method is considered to be highly useful for asymmetric PE.
Acknowledgments
We would like to thank all the individuals for their advice on preparing this manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1824/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1824/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1824/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1824/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Shonan Kamakura General Hospital (TGE01913-024). Informed consent was obtained from all participants included in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kelly RE Jr, Shamberger RC, Mellins RB, et al. Prospective multicenter study of surgical correction of pectus excavatum: design, perioperative complications, pain, and baseline pulmonary function facilitated by internet-based data collection. J Am Coll Surg 2007;205:205-16. [Crossref] [PubMed]
- Kelly RE Jr. Pectus excavatum: historical background, clinical picture, preoperative evaluation and criteria for operation. Semin Pediatr Surg 2008;17:181-93.
- Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg 1998;33:545-52.
- Park HJ, Lee SY, Lee CS. Complications associated with the Nuss procedure: analysis of risk factors and suggested measures for prevention of complications. J Pediatr Surg 2004;39:391-5; discussion 391-5. [Crossref] [PubMed]
- Watanabe A, Watanabe T, Obama T, et al. The use of a lateral stabilizer increases the incidence of wound trouble following the Nuss procedure. Ann Thorac Surg 2004;77:296-300. [Crossref] [PubMed]
- Akhtar M, Razick DI, Saeed A, et al. Complications and Outcomes of the Nuss Procedure in Adult Patients: A Systematic Review. Cureus 2023;15:e35204.
- Wada J. Chest deformity. Tokyo: Bunkodo; 1987:96-105.
- Iida H, Sunazawa T, Ishida K, et al. Surgical repair of pectus excavatum not requiring exogenous implants in 113 patients. Eur J Cardiothorac Surg 2010;37:316-21. [Crossref] [PubMed]
- Iida H. Surgical repair of pectus excavatum. Gen Thorac Cardiovasc Surg 2010;58:55-61. [Crossref] [PubMed]
- Iida H, Sudo Y, Yamada Y, et al. Nonprosthetic surgical repair of pectus excavatum. Ann Thorac Surg 2006;82:451-6. [Crossref] [PubMed]
- İşcan M, Kılıç B, Turna A, et al. The effect of minimally invasive pectus excavatum repair on thoracic scoliosis. Eur J Cardiothorac Surg 2020;ezaa328. [Crossref] [PubMed]
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452-8. [Crossref] [PubMed]
- Park HJ, Sung SW, Park JK, et al. How early can we repair pectus excavatum: the earlier the better? Eur J Cardiothorac Surg 2012;42:667-72. [Crossref] [PubMed]
- Yoshida A, Uemura S, Yamamoto M, et al. Correlation of asymmetric chest wall deformity and growth in patients with pectus excavatum. J Pediatr Surg 2013;48:771-5. [Crossref] [PubMed]
- Kandel J, Haller A. Chest wall and breast. Philadelphia: Lippincott-Raven Publishers; 1997.
- Capunay C, Martinez-Ferro M, Carrascosa P, et al. Sternal torsion in pectus excavatum is related to cardiac compression and chest malformation indexes. J Pediatr Surg 2020;55:619-24. [Crossref] [PubMed]
- Kuyama H, Uemura S, Yoshida A. Chondrotomy and sternotomy combined with the Nuss procedure for severe asymmetric pectus excavatum: how to do it. Surg Today 2021;51:1237-40. [Crossref] [PubMed]
- Ben XS, Deng C, Tian D, et al. Multiple-bar Nuss operation: an individualized treatment scheme for patients with significantly asymmetric pectus excavatum. J Thorac Dis 2020;12:949-55. [Crossref] [PubMed]
- Pawlak K, Gąsiorowski Ł, Dyszkiewicz W. Complex corrective procedure in surgical treatment of asymmetrical pectus excavatum. Kardiochir Torakochirurgia Pol 2017;14:110-4.
- Bellía-Munzón G, Sanjurjo D, Toselli L, et al. Novel index to estimate the cephalocaudal extent of the excavation in pectus excavatum: The Titanic index. J Pediatr Surg 2023;58:605-7. [Crossref] [PubMed]
- Kowalewski J, Barcikowski S, Brocki M. Cardiorespiratory function before and after operation for pectus excavatum: medium-term results. Eur J Cardiothorac Surg 1998;13:275-9. [Crossref] [PubMed]
- Kaguraoka H, Ohnuki T, Itaoka T, et al. Degree of severity of pectus excavatum and pulmonary function in preoperative and postoperative periods. J Thorac Cardiovasc Surg 1992;104:1483-8.


