
Impact of lymph node dissection on the efficacy of immune checkpoint inhibitors in patients with postoperative recurrence of non-small cell lung cancer
Highlight box
Key findings
• The extent of lymph node dissection influences immune checkpoint inhibitor (ICI) efficacy after lung cancer recurrence.
What is known and what is new?
• ICI can be effective not only for unresectable non-small cell lung cancer but also for postoperative recurrence.
• The effect of lymph node dissection on the efficacy of ICI has been unclear; our study shows that lymph node dissection can reduce ICI efficacy for postoperative recurrent lung cancer.
What is the implication, and what should change now?
• ICI administration may be more effective in the preoperative period than in the perioperative period.
• ICI administration in the perioperative period may be better done preoperatively.
Introduction
Background
Comprehensive surgical resection is a curative approach for non-small cell lung cancer (NSCLC). However, the relapse rate ranges from 30% to 75%, and the prognosis after recurrence is unfavorable (1-3). Patients with postoperative recurrence are typically diagnosed with advanced unresectable lung cancer, and chemotherapy is commonly administered in such cases. The advent of immune checkpoint inhibitor (ICI) targeting programmed death protein 1, programmed death ligand 1 (PD-L1), and cytotoxic T lymphocyte antigen 4 has transformed the management of advanced NSCLC. Several clinical trials using ICI with or without cytotoxic chemotherapy have indicated superior antitumor effects and prolonged survival compared with those obtained under conventional chemotherapy alone (4-7). However, to date, clinical trials of ICI have been conducted primarily in patients initially diagnosed with advanced unresectable lung cancer, and it is unclear whether the same efficacy can be expected in patients with postoperative recurrence.
Rationale and knowledge gap
One of the major differences between patients with NSCLC with postoperative recurrence and those with tumors initially diagnosed at an advanced stage (i.e., when the tumor is unresectable) is the presence or absence of lymph node dissection (LND). Therefore, the presence of the lymph nodes may influence the efficacy of ICIs for advanced NSCLC. ICI exert their effects by activating lymphocytes directed toward tumors (8). The lymph nodes also play a role in tumor surveillance. Tumor-draining lymph nodes (TDLNs) are the primary sites of immune activation in NSCLC, and recent studies have established a correlation between TDLNs and the effectiveness of ICI (8-11). Notably, ICI activity was found to diminish in a syngeneic mouse model of head and neck squamous cell carcinoma with neck dissection (12). Although the standard surgical procedures for early-stage lung cancer are lung resection and systematic mediastinal LND, these reports imply that LND could potentially exert a deleterious effect on the efficacy of ICI therapy.
Objective
The aim of the present study was to examine the effects of ICI therapy in patients with postoperative NSCLC recurrence according to the presence or absence of LND and the extent of LND performed. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1806/rc).
Methods
Patient selection and grouping
We conducted a retrospective analysis of 164 patients with lung cancer who received ICI treatment at Tokyo Medical University Hachioji Medical Center between January 2016 and December 2022 (Figure 1). Thirty patients who received durvalumab followed by curative chemoradiotherapy were excluded, leaving a total of 134 patients included for analysis. All diagnoses were made in accordance with the 8th edition of the tumor, node, metastasis (TNM) Classification for Lung and Pleural Tumors of the Union for International Cancer Control (13).
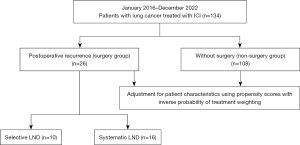
The patients were divided into two groups, one comprising patients who experienced relapse following curative lung resection (surgery group, n=26) and the other consisting of patients who were diagnosed with unresectable lung cancer and, therefore, did not undergo surgery (non-surgery group, n=108). The surgery group was further divided into two groups based on the extent of LND: those who underwent systematic LND (n=16) and those who underwent selective LND (n=10). Systematic LND was defined as LND of the mediastinum, including stations 2R, 4R, and 7 for right upper- and lower-lobe tumors and stations 4L, 5, 6, and 7 for left upper- and lower-lobe tumors. All cases not included in the systematic LND group were defined as selective LND, including cases that did not involve LND, such as those of wedge resection. Per the surgical policy of the institute, selective dissection was performed for cases diagnosed as clinical N0 stage I. If intraoperative findings suggested suspected lymph node metastasis, the decision to perform systematic LND was made. Systematic dissection was performed in patients with confirmed preoperative lymph node metastasis.
Clinicopathological variables
Clinical characteristics, including complete blood counts and biochemical data, were retrieved from the medical records of patients within 30 days of their first ICI treatment. Patient background and baseline peripheral blood data such as absolute neutrophil, absolute lymphocyte, and total lymphocyte counts were recorded. The neutrophil-to-lymphocyte ratio (NLR) was calculated from these variables as all of these factors have been reported to affect the efficacy of ICI therapy (14). NLR was computed as the absolute neutrophil count divided by the absolute lymphocyte count; patients were dichotomized according to a previously established cut-off value of 5 for the NLR (14). Immunohistochemical expression of PD-L1 was evaluated using the tumor proportion score (TPS) and categorized into three groups: <1%, 1–49%, and ≥50%.
Survival and tumor response to ICI therapy
We examined two survival outcomes: progression-free survival (PFS) with ICI initially used in the treatment sequence (ICI-PFS) and overall survival (OS). ICI-PFS was defined as the time elapsed from the date of the first ICI administration to the date of progression or death, whereas OS was defined as the time elapsed from the date of first-line ICI or chemotherapy administration to the date of death. Tumor progression was diagnosed based on radiological imaging, including chest radiography, chest or abdominal computed tomography (CT), brain magnetic resonance imaging, and positron emission-CT, according to the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 principle, which was performed once every 12 weeks. Furthermore, we evaluated the efficacy of the ICI by calculating the objective response rate (ORR), which is the sum of complete response (CR) and partial response (PR), and the disease control rate (DCR), which is the sum of CR, PR, and stable disease (SD), according to the RECIST v1.1 principle.
Ethical considerations
This study was conducted in accordance with the Declaration of Helsinki (revised in 2013). This study was approved by the Institutional Review Board of Tokyo Medical University Hospital (approval No. T2023-0074). The requirement for informed consent was waived by the Review Board in accordance with the retrospective study protocol.
Statistical analysis
Continuous variables were compared using the Mann-Whitney U test, while categorical variables were compared using the chi-square test. We employed the inverse probability of treatment weighting (IPTW) method to address the potential selection bias due to variations in patient backgrounds between the surgery and non-surgery groups (15). Eight clinicopathological variables were included in the model: sex, age, body mass index (BMI), type of ICI treatment (monotherapy or combination with chemotherapy), treatment line (first-line or later), pack-year smoking history, baseline NLR, and TPS (0 and unknown, 1–49%, 50–100%). Logistic regression analysis was used to calculate the propensity scores. Kaplan-Meier tests were performed to estimate ICI-PFS and OS, and differences between groups were assessed using the log-rank test. Cox proportional hazards models were used in univariate analyses to estimate the associations between survival events (tumor progression or death) and prognostic clinicopathological factors. All P values were two-tailed, and P<0.05 was considered to indicate a statistically significant difference. The SPSS package (version 28.0; SPSS, Inc., Chicago, IL, USA) and Python3 were used for statistical analyses.
Results
Patient characteristics
The patient characteristics are presented in Table 1. As regards ICI treatment, 102 patients (76%) received ICI as first-line therapy and 53 (40%) were treated with ICI monotherapy. The most commonly used ICI was pembrolizumab, administered to 80 patients (60%). In the surgery group, systematic LND was performed in 16 patients (62%), whereas selective LND (including ND0, ND1, and ND2a-1) was performed in 10 patients (38%). No statistically significant differences were observed in the patient characteristics between the surgery and non-surgery groups.
Table 1
| Variable | Total (n=134) | Surgery group (n=26) | Non-surgery group (n=108) | P value |
|---|---|---|---|---|
| Sex, n [%] | 0.359 | |||
| Male | 102 [76] | 18 [69] | 84 [78] | |
| Female | 32 [24] | 8 [31] | 24 [22] | |
| Age (years), median [range] | 73 [45–85] | 73 [57–85] | 73 [45–85] | |
| Age group (years), n [%] | 0.716 | |||
| <75 | 94 [70] | 19 [73] | 75 [69] | |
| ≥75 | 40 [30] | 7 [27] | 33 [31] | |
| Smoking pack years, n [%] | 0.527 | |||
| <200 | 40 [30] | 9 [35] | 31 [29] | |
| ≥200 | 94 [70] | 17 [65] | 77 [71] | |
| BMI (kg/m2), mean ± SD [range] | 21.8±3.46 [14.7–36.6] | 20.2±2.94 [16.7–26.0] | 22.7±3.52 [14.7–36.6] | |
| Clinical stage, n [%] | ||||
| I | 19 [14] | 15 [58] | 4 [4] | |
| II | 6 [4] | 4 [15] | 2 [2] | |
| III | 22 [16] | 5 [19] | 17 [16] | |
| IV | 87 [65] | 2 [8] | 85 [79] | |
| TPS, n [%] | 0.702 | |||
| <1%, unknown | 59 [44] | 11 [42] | 48 [44] | |
| 1–49% | 38 [28] | 9 [35] | 29 [27] | |
| ≥50% | 37 [28] | 6 [23] | 31 [29] | |
| NLR, n [%] | 0.141 | |||
| <5 | 98 [73] | 22 [85] | 76 [70] | |
| ≥5 | 36 [27] | 4 [15] | 32 [30] | |
| Line of ICI treatment, n [%] | 0.685 | |||
| 1st | 102 [76] | 19 [73] | 83 [77] | |
| ≥2nd | 32 [24] | 7 [27] | 25 [23] | |
| ICI treatment, n [%] | 0.725 | |||
| Chemo + ICI | 81 [60] | 15 [58] | 66 [61] | |
| ICI monotherapy | 53 [40] | 11 [42] | 42 [39] | |
| ICI drug, n [%] | ||||
| Pembrolizumab | 80 [60] | 15 [58] | 65 [60] | |
| Nivolumab | 16 [12] | 4 [15] | 12 [11] | |
| Nivolumab + ipilimumab | 12 [9] | 1 [4] | 11 [10] | |
| Atezolizumab | 26 [19] | 6 [23] | 20 [19] | |
| Histology, n [%] | 0.220 | |||
| Adenocarcinoma | 68 [51] | 16 [62] | 52 [48] | |
| Non-adenocarcinoma | 66 [49] | 10 [38] | 56 [52] | |
| Initial recurrence site, n [%] | – | |||
| Locoregional | – | 7 [27] | – | |
| Distant | – | 19 [73] | – | |
| Surgical procedure, n [%] | – | |||
| Lobectomy | – | 23 [88] | – | |
| Sublober resection | – | 3 [12] | – | |
| LND extent, n [%] | – | |||
| Systematic | – | 16 [62] | – | |
| Selective | – | 10 [38] | – | |
| Number of dissected lymph nodes, median [range] | – | 14 [0–40] | – |
BMI, body mass index; SD, standard deviation; TPS, tumor proportion score; NLR, neutrophil-to-lymphocyte ratio; Chemo, chemotherapy; ICI, immune checkpoint inhibitor; LND, lymph node dissection.
Efficacy of ICI and survival outcomes
In the non-surgery group, all 108 patients (100%) presented with measurable lesions. In contrast, in the surgery group, only 17 of 26 patients (65.4%) had measurable lesions (P<0.001). Table 2 shows the objective response of the tumors to ICI, demonstrating no significant differences between the surgery and non-surgery groups among patients with measurable lesions. The median follow-up time was 17.1 months.
Table 2
| Variables | Surgery group (n=26), n (%) | Non-surgery group (n=108), n (%) | P value |
|---|---|---|---|
| Measurable lesions | <0.001 | ||
| Yes | 17 (65.4) | 108 (100.0) | |
| No | 9 (34.6) | 0 | |
| Objective response in patients with measurable lesions (n=125) | 0.445 | ||
| Complete response | 1 (5.9) | 1 (0.9) | |
| Partial response | 8 (47.1) | 59 (54.6) | |
| Stable disease | 3 (17.6) | 14 (13.0) | |
| Progression disease | 5 (29.4) | 28 (25.9) | |
| Not evaluable | 0 | 6 (5.6) | |
| ORR | 9 (52.9) | 60 (55.5) | 0.840 |
| DCR | 12 (70.6) | 74 (68.5) | 0.864 |
ICI, immune checkpoint inhibitor; ORR, overall response rate; DCR, disease control rate.
After IPTW adjustment, the effective sample size was 129 in the surgery group and 134 in the non-surgery group. The mean weight for adjustment was 1.31 (standard deviation, 0.19; range, 1.11–1.91) for the surgery group and 8.86 (standard deviation, 4.88; range, 2.05–38.6) for the non-surgery group. Kaplan-Meier curves for the IPTW-adjusted ICI-PFS and OS are shown in Figure 2. The 2-year ICI-PFS did not significantly differ (P=0.19) between the surgery (31.2%) and non-surgery (27.3%) groups (Figure 2A). In terms of OS, similar to the ICI-PFS, there was a trend toward better prognosis in the surgery group (69.6%) than in the non-surgery group (62.2%), although no statistically significant difference was observed (P=0.10) (Figure 2B).
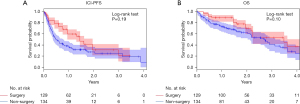
Figure 3 illustrates the ICI-PFS and OS in the surgery group according to the extent of LND. Patients who underwent selective LND had a significantly (P=0.03) higher 2-year ICI-PFS rate (45.7%) than that among patients who underwent systematic LND (20.0%) (Figure 3A). Although the 2-year OS rate was higher in the selective group (80.0%) than that in the systematic group (66.5%), the difference was not statistically significant (P=0.295) (Figure 3B). Moreover, there were no significant differences in the DCR (80% in the selective group and 68.7% in the systematic group; P=0.529) or the ORR (60.0% in the selective group and 37.5% in the systematic group; P=0.263) according to the extent of LND.

Relationship between ICI efficacy and LND
The results of the univariate and multivariate analyses of ICI-PFS in the surgery group are presented in Table 3. In univariate analysis, the extent of LND [hazard ratio (HR), 3.323; 95% confidence interval (CI): 1.057–10.452; P=0.040], late treatment line (HR, 3.070; 95% CI: 1.008–9.357; P=0.048), use of ICI monotherapy (HR, 3.384; 95% CI: 1.142–10.024; P=0.028), and without disease control (HR, 6.544; 95% CI: 1.775–24.130; P=0.005) were found to be significant poor prognostic factors in the surgery group. In multivariate analysis, the extent of LND (HR, 3.709; 95% CI: 1.101–12.500; P=0.034) and without disease control (HR, 9.338; 95% CI: 1.922–45.373; P=0.006) remained independent significant poor prognostic factors in the surgery group.
Table 3
| Variables | Reference | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |||
| Sex | Female | 0.721 | 0.234–2.449 | 0.721 | – | – | – | |
| Age | ≥75 years | 2.638 | 0.918–7.583 | 0.072 | – | – | – | |
| Pack years | ≥200 | 1.348 | 0.465–3.911 | 0.582 | – | – | – | |
| BMI | >22 kg/m2 | 0.391 | 0.133–1.151 | 0.088 | – | – | – | |
| NLR | ≥5 | 0.903 | 0.197–1.147 | 0.895 | – | – | – | |
| TPS | 1–49% | 0.569 | 0.160–2.030 | 0.385 | – | – | – | |
| ≥50% | 1.201 | 0.362–3.983 | 0.765 | – | – | – | ||
| Clinical stage | II/III/IV | 1.107 | 0.401–3.059 | 0.845 | – | – | – | |
| Recurrence site | Distant | 3.286 | 0.739–14.618 | 0.118 | – | – | – | |
| DFI | <2 years | 0.794 | 0.293–2.147 | 0.649 | – | – | – | |
| Line of treatment | ≥2nd | 3.070 | 1.008–9.357 | 0.048 | 1.200 | 0.285–5.050 | 0.804 | |
| ICI treatment | ICI monotherapy | 3.384 | 1.142–10.024 | 0.028 | 2.651 | 0.683–10.295 | 0.159 | |
| LND | Systematic | 3.323 | 1.057–10.452 | 0.040 | 3.709 | 1.101–12.500 | 0.034 | |
| Objective response | PD | 6.544 | 1.775–24.130 | 0.005 | 9.338 | 1.922–45.373 | 0.006 | |
ICI, immune checkpoint inhibitor; HR, hazard ratio; CI, confidence interval; BMI, body mass index; NLR, neutrophil-to-lymphocyte ratio; TPS, tumor proportion score; DFI, disease-free interval; LND, lymph node dissection; PD, progression disease.
Discussion
Key findings
In an era where ICIs are being widely used even in the perioperative period, it is critical to understand the impact of surgical intervention on tumor immunity. Moreover, a preclinical study suggested that TDLN dissection could attenuate the efficacy of ICIs (12). Therefore, a comprehensive understanding of the patient’s condition is necessary before treatment begins to increase the chances of curing the cancer and improve patient prognosis. Therefore, this study was designed to provide indirect insights into the effects of LND on ICI efficacy. Through this retrospective analysis of patients with NSCLC treated with ICIs, we found no significant impact of surgery on survival outcomes, whereas a significant improvement in 2-year ICI-PFS was found in cases involving selective LND compared with that in cases involving systematic LND. These findings suggest that the extent of LND can impact the efficacy of ICI therapy for lung cancer, with implications for determining the most appropriate timing of treatment.
Strengths and limitations
To the best of our knowledge, this is the first study to investigate the effect of LND on the efficacy of ICIs in patients with NSCLC. Although the results of the present study can provide profound insights into perioperative ICI indications and surgical decisions, there are several limitations to acknowledge. First, this was a retrospective study conducted at a single center with only 26 patients included in the surgery group. The study design and small sample size may have introduced a selection bias. To mitigate this, the patients were matched based on their backgrounds using IPTW. However, regarding the analysis of the impact of the extent of LND on patient outcome, IPTW could not be performed within the surgery group because of the small sample size. Therefore, further validation at larger centers is necessary to confirm these results. Second, this study did not evaluate the performance status (PS) score of patients, which is an essential predictor of response in patients receiving ICI therapy (16). Unfortunately, data on the PS at the time of postoperative recurrence are unavailable. However, most patients who received chemotherapy and/or ICI at Tokyo Medical University Hachioji Medical Center typically had a PS of 0–1, indicating relatively normal activity and ability to care for oneself, making it unlikely that this factor significantly affected the results.
Comparison with similar research
Two previous studies have also aimed to identify the prognostic factors in patients with postoperative NSCLC recurrence (17,18). Shimada et al. (17) identified several prognostic factors for postoperative recurrence, including chemotherapy and molecular targeted therapy, adenocarcinoma subtype, and the absence of bone and liver metastases. In 2009, Sekine et al. (18) conducted a study to compare the prognosis of patients with postoperative recurrence with that of patients with stage IV lung cancer and reported that patients with postoperative recurrence had a more favorable prognosis, whereas the response to chemotherapy was comparable between the two groups. These results are similar to those of our study. One possible reason for the trend toward a better prognosis in the surgery group may be that the cancer was diagnosed at an earlier stage in these patients than in the non-surgery group, which is linked to a difference in tumor volume between these two groups at the time of ICI initiation. Tumor volume is correlated with the detection rate of cell-free DNA, which is established as a poor prognostic factor (19). We assumed that patients with postoperative recurrence had a better prognosis than patients initially diagnosed with unresectable lung cancer because the former group received regular follow-up after surgery and, therefore, their recurrent lesions were found earlier when the tumor volumes were relatively smaller. In this study, 35% of patients in the surgery group had no measurable lesions, such as small pulmonary metastases.
Some studies have also investigated the impact of immunotherapy in patients with postoperative recurrence. Yuasa et al. (20) found that among 87 patients, the median PFS and OS were 3.2 and 17.5 months, respectively. Kuroda et al. (21) investigated the effectiveness of ICI monotherapy in patients with postoperative recurrence of lung cancer and reported efficacy in both first and later lines of treatment. However, no study has directly compared the prognoses of patients with postoperative recurrence and advanced lung cancer in terms of ICI efficacy. Given the observed differences in patient characteristics between the two groups, adjusting for these differences was necessary. We adjusted for the variation in these patient characteristics using IPTW, which we believe provides statistical reliability for interpreting our results. Considering the potential of LND to exert a confined impact on the aggregate patient population, we further examined the ramifications of ICI on the LND scope solely in individuals exhibiting postoperative relapse.
There are several possible reasons to explain observed differences in ICI efficacy depending on the extent of dissection. The lymph nodes contain a cluster of plasma cells and B cells contained in high endothelial venules, which are specialized blood vessels for lymphocyte recruitment known as the germinal center (22,23). These clusters of plasma and B cells in the tumor tissue are considered tertiary lymphoid structures (TLSs), which have been identified as prognostic factors for surgically resected NSCLC (24). The presence or absence of TLSs has also been reported to influence the effectiveness of ICI in other cancers, indicating that lymph nodes with similar structures may be associated with ICI efficacy (25). The total number of lymph nodes in the body may have been attenuated, resulting in fewer TLSs that could exert an immune response to these tumors. However, a previous study reported no difference in prognosis between patients with complete LND and selective lymph node sampling (26).
Explanations of findings
We postulated that LND may adversely affect the effectiveness of ICI. Therefore, we designed this study to investigate the potential negative impact of LND on the effectiveness of ICI by comparing the prognosis of patients who experienced recurrence after surgery with and without complete LND. The surgery group showed no significant differences in ICI-PFS or OS compared with those of the non-surgery group. Although this result was contrary to our general expectations, we found that the extent of LND had a significant impact on prognosis in subgroup analysis for patients in the surgery group with postoperative recurrence.
Implications and actions needed
With the growing use of ICI combined with neoadjuvant or adjuvant chemotherapy, the findings of the current study provide deeper insights into the indications for the use of ICIs in the perioperative period. Our results suggest that for the selected patient population, a treatment strategy that can reduce the extent of LND may be helpful to increase the effectiveness of ICI at the time of recurrence. A randomized phase III trial is ongoing for patients diagnosed with early-stage NSCLC treated with a range of LNDs (27). Because ICIs are key drugs in the treatment of patients with postoperative recurrence, it is anticipated that the patients enrolled in this trial will also receive ICI therapy at recurrence. Therefore, the results of this trial will provide further important insights into the efficacy of ICI in patients with postoperative recurrence.
Conclusions
In conclusion, ICI appear to be equally effective in patients with postoperative recurrence and advanced lung cancer, suggesting that the treatment protocols should be similar for these two groups. However, our results suggest that the extent of LND can affect the ICI efficacy.
Acknowledgments
AI use statement: during the preparation of this work, the authors used ChatGPT and Paperpal to improve the quality of the manuscript and make it more grammatically sophisticated. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication. We gratefully acknowledge Editage for providing editorial assistance in the preparation of this manuscript.
Funding: This research was supported by a
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1806/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1806/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1806/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1806/coif). N.I. received grants and contracts from Chugai Pharma and Ono Pharma; payments or honoraria for lectures, presentations, speakers, manuscript writing, or educational events from Chugai Pharma, Ono Pharma, Bristol Meyers, and MSD; and has leadership or fiduciary roles in the Japan Surgical Society and the Japan Lung Cancer Society. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of this work and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of Tokyo Medical University Hospital (approval No. T2023-0074), which waived the requirement for written informed consent in accordance with the retrospective study protocol.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg 1995;109:120-9. [Crossref] [PubMed]
- al-Kattan K, Sepsas E, Fountain SW, et al. Disease recurrence after resection for stage I lung cancer. Eur J Cardiothorac Surg 1997;12:380-4. [Crossref] [PubMed]
- Martin J, Ginsberg RJ, Venkatraman ES, et al. Long-term results of combined-modality therapy in resectable non-small-cell lung cancer. J Clin Oncol 2002;20:1989-95. [Crossref] [PubMed]
- Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2019;381:2020-31. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Johnson ML, Cho BC, Luft A, et al. Durvalumab With or Without Tremelimumab in Combination With Chemotherapy as First-Line Therapy for Metastatic Non-Small-Cell Lung Cancer: The Phase III POSEIDON Study. J Clin Oncol 2023;41:1213-27. [Crossref] [PubMed]
- Hanagiri T, Shigematsu Y, Shinohara S, et al. Clinical significance of the frequency of regulatory T cells in regional lymph node lymphocytes as a prognostic factor for non-small-cell lung cancer. Lung Cancer 2013;81:475-9.
- Dammeijer F, van Gulijk M, Mulder EE, et al. The PD-1/PD-L1-Checkpoint Restrains T cell Immunity in Tumor-Draining Lymph Nodes. Cancer Cell 2020;38:685-700.e8. [Crossref] [PubMed]
- Yang H, Sun B, Ma W, et al. Multi-scale characterization of tumor-draining lymph nodes in resectable lung cancer treated with neoadjuvant immune checkpoint inhibitors. EBioMedicine 2022;84:104265.
- Fransen MF, Schoonderwoerd M, Knopf P, et al. Tumor-draining lymph nodes are pivotal in PD-1/PD-L1 checkpoint therapy. JCI Insight 2018;3:e124507. [Crossref] [PubMed]
- Saddawi-Konefka R, O'Farrell A, Faraji F, et al. Lymphatic-preserving treatment sequencing with immune checkpoint inhibition unleashes cDC1-dependent antitumor immunity in HNSCC. Nat Commun 2022;13:4298. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Prelaj A, Ferrara R, Rebuzzi SE, et al. EPSILoN: A Prognostic Score for Immunotherapy in Advanced Non-Small-Cell Lung Cancer: A Validation Cohort. Cancers (Basel) 2019;11:1954. [Crossref] [PubMed]
- Thoemmes F, Ong AD. A primer on inverse probability of treatment weighting and marginal structural models. Emerg Adult 2016;4:40-59.
- Sehgal K, Gill RR, Widick P, et al. Association of Performance Status With Survival in Patients With Advanced Non-Small Cell Lung Cancer Treated With Pembrolizumab Monotherapy. JAMA Netw Open 2021;4:e2037120. [Crossref] [PubMed]
- Shimada Y, Saji H, Yoshida K, et al. Prognostic factors and the significance of treatment after recurrence in completely resected stage I non-small cell lung cancer. Chest 2013;143:1626-34. [Crossref] [PubMed]
- Sekine I, Nokihara H, Yamamoto N, et al. Comparative chemotherapeutic efficacy in non-small cell lung cancer patients with postoperative recurrence and stage IV disease. J Thorac Oncol 2009;4:518-21. [Crossref] [PubMed]
- Jia B, Zhang X, Mo Y, et al. The Study of Tumor Volume as a Prognostic Factor in T Staging System for Non-Small Cell Lung Cancer: An Exploratory Study. Technol Cancer Res Treat 2020;19:1533033820980106. [Crossref] [PubMed]
- Yuasa I, Hamaji M, Ozasa H, et al. Outcomes of immune checkpoint inhibitors for postoperative recurrence of non-small cell lung cancer. Gen Thorac Cardiovasc Surg 2023;71:534-41.
- Kuroda H, Takahashi Y, Shirai S, et al. Survival benefit of immune checkpoint inhibitor monotherapy in patients with non-small cell lung cancer recurrence after completely pulmonary resection. Ann Transl Med 2021;9:1225. [Crossref] [PubMed]
- Sautès-Fridman C, Petitprez F, Calderaro J, et al. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer 2019;19:307-25. [Crossref] [PubMed]
- Fridman WH, Meylan M, Petitprez F, et al. B cells and tertiary lymphoid structures as determinants of tumour immune contexture and clinical outcome. Nat Rev Clin Oncol 2022;19:441-57. [Crossref] [PubMed]
- Fukuhara M, Muto S, Inomata S, et al. The clinical significance of tertiary lymphoid structure and its relationship with peripheral blood characteristics in patients with surgically resected non-small cell lung cancer: a single-center, retrospective study. Cancer Immunol Immunother 2022;71:1129-37.
- Asrir A, Tardiveau C, Coudert J, et al. Tumor-associated high endothelial venules mediate lymphocyte entry into tumors and predict response to PD-1 plus CTLA-4 combination immunotherapy. Cancer Cell 2022;40:318-334.e9. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Hishida T, Saji H, Watanabe SI, et al. A randomized Phase III trial of lobe-specific vs. systematic nodal dissection for clinical Stage I-II non-small cell lung cancer (JCOG1413). Jpn J Clin Oncol 2018;48:190-4. [Crossref] [PubMed]


