
Incidental detection of ground glass nodules and primary lung cancer in patients with breast cancer: prevalence and long-term follow-up on chest computed tomography
Highlight box
Key findings
• Incidental ground glass nodules (GGNs) were found in 5.0% of patients with breast cancer who underwent chest computed tomography.
• Older age, part-solid nodules and total number of GGNs (≥2) were risk factors for growth of GGNs.
• Lung cancer was confirmed in 0.9% of patients, all adenocarcinoma with high prevalence of epidermal growth factor receptor mutation (69.2%).
What is known and what is new?
• Incidence of lung cancer is higher in patients with breast cancer than general population.
• In this study, prevalence of GGNs and lung cancer was 5.0% and 0.9% in patients with breast cancer.
• Older age and total number of GGNs (≥2) were risk factors for growth of GGNs.
What is the implication, and what should change now?
• Persistent GGNs in older individuals, part-solid nodules, and multiple GGNs (≥2) be adequately monitored for the early detection of GGN growth and the treatment of lung cancer.
Introduction
Breast cancer is the most commonly diagnosed malignant tumor and it was the 4th leading cause of death worldwide in 2020, according to the International Agency for Research on Cancer (1). Based on the 2020 Korean Central Cancer Registry database, breast cancer is the most frequently occurring cancer among women in Korea, accounting for 21.1% of all cancers, and ranks 5th overall at 10.1% (2). Breast cancer patients have a high survival rate, attributed to advances in treatment and surgical techniques. Additionally, the incidence of early stage of breast cancer is increasing, which is expected to further improve the survival rate (3). However, with prolonged survival, approximately 10% of breast cancer patients are diagnosed with a second malignancy within 10 years after their initial diagnosis of cancer, of which lung cancer is the most common (4-13).
For breast cancer patients, contrast-enhanced chest computed tomography (CT) is recommended to monitor for lung metastasis during treatment (14). Incidental detection of ground glass nodules (GGNs) or lung cancer may occur during this process. If persistent GGNs are identified on chest CT, these are a cause for concern, due to the possibility of pre-invasive (atypical adenomatous hyperplasia, adenocarcinoma in situ) or invasive adenocarcinoma, unlike transient GGNs (15,16). A previous study established that 14.8% of breast cancer patients had persistent GGNs and 4.3% of these were confirmed as pre-invasive or invasive adenocarcinoma (17). Additionally, lung cancer was diagnosed in approximately 0.8% of breast cancer patients who underwent chest CT and lung cancer demonstrated a high rate (78.5%) of epidermal growth factor receptor (EGFR) mutation (18). Only two studies have been conducted on Chinese patients to evaluate the incidental findings of GGNs or lung cancer after breast cancer diagnosis (17,18). Therefore, in the study, we evaluated the prevalence of persistent GGNs and the clinical and radiological characteristics of GGNs showing interval growth. We also examined the clinicopathologic features of lung cancer, including EGFR mutation in patients with breast cancer. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1605/rc).
Methods
Study population
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The retrospective study was approved by the Institutional Review Board of Kyung Hee University Hospital at Gangdong (approval No. 2023-02-016) and informed consent was waived owing to the retrospective nature of the study. We examined the medical records of 2,077 patients with pathologically confirmed breast cancer between January 2008 and December 2022. Since, January 2008, chest CT have been performed in patients with breast cancer to evaluate asymptomatic lung metastasis. A preoperative chest CT was recommended for tumor, node, metastasis (TNM) staging to all patients. Subsequently, chest CT scans were performed at 6-month intervals within the first 12 months after operation. Afterward, annual follow chest CT scans were recommended. All data were anonymized after collecting from the medical records. Among them, 1,409 patients (surgical: 1,309, nonsurgical: 100) underwent chest CT. A total of 25 cases were excluded from the study based on the following criteria: male patients (n=9), patients with GGN who were either not followed up after CT or had a follow-up period of fewer than 90 days (n=8), patients with no thin-section chest CT (≤3 mm slice thickness) (n=5), and patients with diffuse lung metastasis (n=3) due to limited evaluation for detection of GGN. Finally, 1,384 patients were included to calculate the prevalence of GGN and lung cancer detected on chest CT. To evaluate GGN growth, we only included GGNs that met two specific criteria: (I) persistence for at least 3 months after the initial CT scan, which defined them as persistent GGNs; and (II) the largest diameter of less than 3 cm on the initial CT scan.
Evaluation of clinical and radiological characteristics
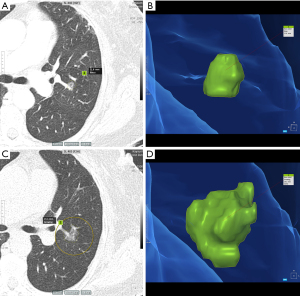
In the study, clinical information including age, history of malignancy other than breast or lung cancer, TNM stage of breast cancer, and history of radiation therapy was evaluated. We primarily examined formal chest CT reports to identify GGNs and one thoracic radiologist (H.N.L.) with 9 years of experience in chest radiology reviewed the initial chest CT images to find persistent GGNs. Following that, radiologic features of GGNs were assessed, including nodule type (pure or part-solid), location, total number, presence of air-bronchogram, and follow-up duration post-CT. Two thoracic radiologists (H.N.L. with 9 and J.I.K. with 13 years of experience in thoracic CT imaging, respectively) independently measured the long diameter of GGNs on initial and follow-up chest CT on lung windows on Picture Archiving and Communication Systems (PACS); in case of measurement discrepancies, GGN growth categorization was reached by discussion. GGN growth was defined as an interval increase in long diameter greater than 2 mm on follow-up chest CT (19,20). If a patient had multiple GGNs, we selected one representative GGN with the largest diameter or solid component. In addition, we also calculated volume doubling time (VDT) for GGNs with growth group using a semi-automatic software program (AVIEW, version 1.1.42.7, Coreline Soft, Seoul, Republic of Korea) (Figure 1). VDT was calculated using the Schwartz formula , where is the natural logarithm, and are initial and final tumor volumes, and is the time elapsed between initial and final scans.
Pathologic and molecular analysis
For patients diagnosed with lung cancer, the results of pathology and pathologic TNM stage were investigated. The pathologic stage of lung cancer was determined using the 8th edition TNM classification by the Union Internationale Contre le Cancer and American Joint Committee on Cancer (AJCC) (21). Molecular analysis, including EGFR mutation, anaplastic lymphoma kinase gene (ALK) mutation, and c-ros oncogene 1 (ROS1) mutation, was performed using a commercially available polymerase chain reaction-based kit. In addition, programmed death-ligand 1 (PD-L1) expression was examined using the PD-L1 immunohistochemical (IHC) 22C3 pharmDx assay (Agilent Technologies, Santa Clara, CA, USA) that uses tumor proportion score (TPS), which is the percentage of viable tumor cells showing partial or complete membrane staining, to determine PD-L1 protein expression. A TPS of ≥1% is considered positive, while a TPS of <1% is considered negative. In the positive group, TPS scores of 1% to 49% are classified as low PD-L1 expression, and TPS scores of ≥50% are classified as high PD-L1 expression (22,23).
The pathological staging of breast cancer was based on the AJCC staging system 8th edition (24). We evaluated standard IHC markers, including estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor (HER2). Subsequently, we classified breast cancer into luminal A, luminal B, HER2-positive, and triple-negative subtypes based on surrogate definitions of intrinsic subtypes of breast cancer (25).
Chest CT scan
Chest CT was performed using 16- or 64-row multi-detector CT (MDCT) (Brilliance 16- and 64-row CT scanners; Philips Medical Systems, Cleveland, OH, USA), and 256-row MDCT (Revolution CT; GE Healthcare, Milwaukee, WI, USA). The CT parameters for 16-/64-row MDCT were as follows: detector collimation =0.75/0.625 mm; pitch =1.188/1.014; gantry rotation time =0.75/0.5 s; matrix size =512×512 pixels; tube voltage =120 kVp; tube current time product =30–200 mAs; slice thickness =3 mm. For 256-row MDCT, the parameters were: collimation =0.625 mm; pitch =0.992; gantry rotation time =0.5 s; matrix size =512×512; tube voltage =120 kVp; tube current time product =50–175 mAs; slice thickness =2.5 mm. In clinical practice, when GGNs are detected in chest CT images, thin section images of 1 or 1.25 mm are examined on another workstation and then it was sent to the PACS.
Statistical analyses
Statistical analysis was performed using SPSS version 29 (IBM Corporation, Armonk, NY, USA). Measurement data are expressed as mean ± standard deviation or median with an interquartile range.
To compare the clinical and radiologic features of GGNs with or without growth, we used Pearson’s chi-squared and Fisher’s exact tests for categorical variables and the independent samples t-test or Mann-Whitney U test for continuous data. Multivariable logistic regression analyses were used to identify independent factors for GGN growth. All covariates with P<0.05 in univariate analysis were included in multivariate analysis.
Results
Prevalence and long-term follow-up of GGNs in patients with breast cancer
Among 1,384 patients who underwent chest CT for breast cancer, 69 (5.0%) had at least one persistent GGN and the remaining 1,315 patients including 20 patients with transient GGNs, had no persistent GGNs. Among 69 persistent GGNs, 27 (39.1%) exhibited interval growth and 42 (60.9%) were stable in size on follow-up chest CT. For 27 GGNs with growth group, the median VDT was 1,006.0 days (interquartile range, 622.0–1,528.0 days) during the median 959.0 follow-up days (interquartile range, 612.0–1,645.0 days). The clinicopathologic characteristics of patients with breast cancer with GGNs are summarized in Table 1.
Table 1
| Characteristics | Total patients (n=69) | Patients with GGN growth (n=27) | Patients with GGN stable (n=42) | P value |
|---|---|---|---|---|
| Age (years) | 58.1±10.6 | 63.3±11.5 | 54.7±8.4 | 0.002 |
| History of malignancy† | 0.299‡ | |||
| Yes | 9 (13.0) | 5 (18.5) | 4 (9.5) | |
| None | 60 (87.0) | 22 (81.5) | 38 (90.5) | |
| Smoking history | 0.391‡ | |||
| None | 68 (98.6) | 26 (96.3) | 42 (100.0) | |
| Ex smoker | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Current smoker | 1 (1.4) | 1 (3.7) | 0 (0.0) | |
| Family history of lung cancer | 0.663‡ | |||
| None | 66 (95.7) | 26 (96.3) | 40 (95.2) | |
| Yes | 3 (4.3) | 1 (3.7) | 2 (4.8) | |
| T stage | n=66 | n=26 | n=40 | 0.107‡ |
| Tis | 4 (6.1) | 0 (0.0) | 4 (10.0) | |
| T1, T2 | 56 (84.8) | 22 (84.6) | 34 (85.0) | |
| T3, T4 | 6 (9.1) | 4 (15.4) | 2 (5.0) | |
| N stage | n=58 | n=22 | n=36 | 0.529‡ |
| N0 | 45 (77.6) | 16 (72.7) | 29 (80.6) | |
| N1–3 | 13 (22.4) | 6 (27.3) | 7 (19.4) | |
| Intrinsic subtype§ | n=66 | n=26 | n=40 | 0.504‡ |
| Luminal A | 27 (40.9) | 11 (42.3) | 16 (40.0) | |
| Luminal B | 24 (36.4) | 7 (26.9) | 17 (42.5) | |
| HER2 overexpression | 6 (9.1) | 3 (11.5) | 3 (7.5) | |
| Triple negative | 9 (13.6) | 5 (19.2) | 4 (10.0) | |
| Radiation therapy | n=66 | n=26 | n=40 | 0.607 |
| Yes | 48 (72.7) | 18 (69.2) | 30 (75.0) | |
| No | 18 (27.3) | 8 (30.8) | 10 (25.0) |
Data are presented as mean ± standard deviation or n (%). †, history of malignancy: papillary thyroid cancer (n=5), carcinoma of cervix (n=2), squamous cell carcinoma of bladder cancer (n=2); ‡, Fisher’s exact test; §, surrogate definitions of intrinsic subtypes of breast cancer classification. GGN, ground glass nodule; HER2, human epidermal growth factor receptor 2.
The mean age of patients with persistent GGNs was 58.1±10.6 years and 68 of 69 patients were non-smoker. When comparing the two groups, the mean age of patients with GGN growth was older than those with stable GGN (63.3±11.5 vs. 54.7±8.4 years, P=0.002). Breast cancer stage and its pathologic characteristics were not significantly different between the two groups (P>0.05). Furthermore, there was no significant difference in the proportion of prior radiation therapy between the two groups (P=0.607). The characteristics of total patients are present in Table S1. The mean age of patients with persistent GGNs or lung cancer was older than the other group (64.5±10.4 vs. 60.3±11.7 years, P=0.001).
Radiological characteristics of persistent GGNs
The radiological characteristics of GGNs in patients with breast cancer are presented in Table 2. The initial GGN diameter was 6.3±3.6 mm, and the majority (85.5%, n=59) were pure GGNs. When the radiological findings between the two groups were compared, the GGN growth group had a larger nodule size (7.6±5.3 vs. 5.5±1.6 mm, P=0.046), a higher ratio of the solid component (33.3% vs. 2.4%, P=0.001), and were more likely to have more than two GGNs than the stable GGN group (37.0% vs. 11.9%, P=0.014). Additionally, the GGN growth group was more likely to show an air-bronchogram (44.4% vs. 16.7%, P=0.012).
Table 2
| Characteristics | Total patients (n=69) | Patients with GGN growth (n=27) | Patients with GGN stable (n=42) | P value |
|---|---|---|---|---|
| Initial diameter (mm) | 6.3±3.6 | 7.6±5.3 | 5.5±1.6 | 0.046 |
| Initial nodule size (mm) | 0.017† | |||
| Size <5 | 28 (40.6) | 10 (37.0) | 18 (42.9) | |
| 5≤ size <10 | 36 (52.2) | 12 (44.4) | 24 (57.1) | |
| 10≤ size <30 | 5 (7.2) | 5 (18.5) | 0 (0.0) | |
| Initial nodule type | 0.001† | |||
| Pure ground-glass | 59 (85.5) | 18 (66.7) | 41 (97.6) | |
| Part-solid | 10 (14.5) | 9 (33.3) | 1 (2.4) | |
| Location of GGN | 0.674 | |||
| Upper, middle lobe (RUL, LUL, RML) | 43 (62.3) | 16 (59.3) | 27 (64.3) | |
| Lower lobe (RLL, LLL) | 26 (37.7) | 11 (40.7) | 15 (35.7) | |
| Numbers of GGN | 0.014 | |||
| 1 | 54 (78.3) | 17 (63.0) | 37 (88.1) | |
| ≥2 | 15 (21.7) | 10 (37.0) | 5 (11.9) | |
| Air-bronchogram | 0.012 | |||
| Yes | 19 (27.5) | 12 (44.4) | 7 (16.7) | |
| No | 50 (72.5) | 15 (55.6) | 35 (83.3) | |
| Interval time (years) | 0.336† | |||
| Time <1 | 7 (10.1) | 4 (14.8) | 3 (7.1) | |
| 1≤ time <3 | 22 (31.9) | 10 (37.0) | 12 (28.6) | |
| Time ≥3 | 40 (58.0) | 13 (48.1) | 27 (64.3) | |
| Location of GGO related with RT | n=48 | n=18 | n=30 | 0.881 |
| Ipsilateral | 22 (45.8) | 8 (44.4) | 14 (46.7) | |
| Contralateral | 26 (54.2) | 10 (55.6) | 16 (53.3) | |
| GGN detection before RT | n=48 | n=18 | n=30 | 0.282† |
| Yes | 38 (79.2) | 16 (88.9) | 22 (73.3) | |
| None/unknown | 10 (20.8) | 2 (11.1) | 8 (26.7) |
Data are presented as mean ± standard deviation or n (%). †, Fisher’s exact test. GGN, ground glass nodule; RUL, right upper lobe; LUL, left upper lobe; RML, right middle lobe; RLL, right lower lobe; LLL, left lower lobe; RT, radiotherapy.
Regarding radiation therapy, we observed no significant correlation between the direction of the radiation therapy field and the detected GGNs (P=0.881). Moreover, in the majority of patients (79.2%, 38/48), the GGN was detected on CT before undergoing radiation therapy.
Clinical and radiologic factors associated with GGN growth
In multivariate logistic regression analysis, older age [odds ratio (OR), 1.081; 95% confidence interval (CI): 1.009–1.157; P=0.026], part-solid GGN (OR, 29.642; 95% CI: 2.681–327.775; P=0.006) and total number of GGNs (≥2) (OR, 7.361; 95% CI: 1.729–31.338; P=0.007) were significant independent factors for GGN growth.
Prevalence and clinicopathologic characteristics of lung cancer
In our study of 1,384 patients, lung cancer was confirmed in 13 patients (0.9%). Moreover, eight of the 13 patients were diagnosed with lung cancer during follow-up after detection of GGN. Among these, six patients were in the group with GGN growth and two were in the group with stable GGN size. The remaining five patients were diagnosed with lung cancer after the initial detection of a solid nodule or mass during the evaluation of breast cancer. All 13 patients were diagnosed with adenocarcinoma, with one case being minimally invasive adenocarcinoma (Table 3).
Table 3
| Characteristics | Total patients (n=13) |
|---|---|
| Confirmation method | |
| PCNB | 3 (23.1) |
| Wedge resection | 4 (30.8) |
| Segmentectomy | 1 (7.7) |
| Lobectomy | 4 (30.8) |
| Pneumonectomy | 1 (7.7) |
| Histology (n=10)† | |
| MIA (n=1) | 1 (10.0) |
| Predominant lepidic pattern | 1 (100.0) |
| IAC (n=9) | 9 (90.0) |
| Acinar/papillary pattern | 7 (77.8) |
| Acinar pattern | 1 (11.1) |
| Papillary dominant pattern | 1 (11.1) |
| TNM staging (n=10)† | |
| pT1a(mi)N0M0 | 1 (10.0) |
| pT1bN0M0 | 4 (40.0) |
| pT1bNxM0 | 2 (20.0) |
| pT2bN0M0 | 1 (10.0) |
| pT3N0M0 | 1 (10.0) |
| cT3aN1M0 | 1 (10.0) |
| EGFR mutation detection | |
| Negative | 4 (30.8) |
| Positive | 9 (69.2) |
| Exon 19 mutation (E19del) | 4 (44.4) |
| Exon 21 mutation (L858R) | 5 (55.6) |
| ALK mutation detection (n=11) | |
| Negative | 11 (100.0) |
| Positive | 0 (0.0) |
| ROS1 mutation detection (n=7) | |
| Negative | 6 (85.7) |
| Positive | 1 (14.3) |
| PD-L1 expression (n=10) | |
| Negative (TPS <1%) | 4 (40.0) |
| Positive | 6 (60.0) |
| Low PD-L1 expression (1%≤ TPS ≤49%) | 5 (83.3) |
| High PD-L1 expression (TPS ≥50%) | 1 (16.7) |
Data are presented as n (%). †, for patients who were confirmed with PCNB, indeterminate results were obtained. PCNB, percutaneous needle biopsy; MIA, minimally invasive adenocarcinoma; IAC, invasive adenocarcinoma; TNM, tumor, node, metastasis; pT, pathologic T; cT, clinical T; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; ROS1, c-ros oncogene 1; PD-L1, programmed death-ligand 1; TPS, tumor proportion score.
All patients were tested for EGFR gene mutation, and 9 of 13 patients (69.2%) demonstrated EGFR mutation including exon 19 mutation (E19del) (n=4) and exon 21 mutation (L858R) (n=5). All 11 patients who were tested for ALK mutation were negative. Furthermore, 6 of 10 patients (60.0%) were positive for PD-L1 expression.
Discussion
In the study, we evaluated the prevalence of GGN and the risk factors for its growth in patients with breast cancer. Incidental GGN was identified in 5% of patients with breast cancer. Older age, part-solid nodules, and more than two GGNs were significant risk factors for its growth.
Two previous studies from China stated that the prevalence of GGN was 14.8% and 18.4% in patients with breast cancer, respectively. However, the prevalence of GGN was relatively low in our study compared with previous studies (17,18). This may be because our study excluded chest CT scans with follow-up of fewer than 3 months, only if GGN was detected, whereas one study excluded all chest CT scans with follow-up of fewer than 3 months regardless of GGN detection, with a smaller estimation of denominator (17). Additionally, another study included GGNs including transient opacity (18). While primary lung cancer rates are modestly higher in breast cancer patients than in the general female population (12), to date, no definitive evidence has been reported regarding whether the detection rates of GGNs in breast cancer patients are higher than those in the general women population. Previous studies have reported that the incidental detection rate of GGNs in females ranges from 5.2% to 10.2% (26,27). However, this value may include transient GGNs or ground-glass opacities (GGOs) for calculation, making a challenge for direct comparisons. Further investigations are necessary to elucidate the potential relationship between GGN detection rates and the prevalence of primary lung cancer in breast cancer patients.
Multiple or synchronous GGOs are defined as more than two GGOs simultaneously observed in one patient (28). According to the Fleischner Society, if there is a GGO at least measuring 6 mm or larger that persists on follow-up CT, multiple primary adenocarcinoma should be considered, and follow-up for 2 to 4 years is recommended to confirm stability (29). In our study, we observed that 15 of 69 (21.7%) patients presented with multiple GGNs, and among these, 11 patients had nodules larger than 6 mm in size. The presence of multiple GGNs suggests a higher malignant potential and analyzing only the representative nodule may have influenced the results. This study is the first report suggesting a correlation between the presence of multiple GGNs and GGN growth.
Lung cancer was detected in 0.9% of patients with breast cancer. Moreover, all diagnosed cases of lung cancer were adenocarcinoma. Lung adenocarcinoma in females who are non-smokers has been recognized as a distinct entity due to its unique epidemiologic, clinical and biological features. In these patients, EGFR mutations are identified as driver mutations (30). Consistent with this finding, our study reveals that nearly all patients, except for one, were non-smokers and demonstrated a notable 69.2% frequency of EGFR mutations.
There are limited studies on EGFR mutations of lung cancer in patients with breast cancer. Although our study has limitations due to the small sample size of confirmed lung cancer cases and the lack of a comparison with lung cancer patients without breast cancer, regarding the EGFR mutation rate, we observed a high incidence of EGFR mutations of 69.2% compared with previous study which reported the highest EGFR mutation frequency of 47% in Asia-Pacific patients with non-small cell lung cancer/adenocarcinoma subgroup (31). A study conducted in China, similar to our research, demonstrated a high EGFR mutation rate of 78.5% in breast cancer patients with secondary lung cancer (18). Other studies have provided evidence of frequent EGFR overexpression in breast cancer patients (32), the involvement of EGFR/HER1 activation in ER signaling, and consequently, a correlation between ER expression and EGFR mutation. The aforementioned findings imply that EGFR signaling might have a significant role in the concurrent development of lung and breast cancers (18,33,34).
We observed that 79.2% (38/48) of GGNs were present before radiation therapy and there was no correlation between a history of radiation therapy and the occurrence of GGN growth. Many studies have investigated the association between radiation therapy and the risk of developing lung cancer. Previous studies demonstrated a substantially increased risk of developing lung cancer in the ipsilateral lung, with smoking being an important risk factor in those receiving radiotherapy (35,36). However, there is currently no definite evidence of an elevated risk of lung cancer associated with advanced radiotherapy techniques (12).
Our study had several limitations. First, it was a retrospective study conducted at a single medical center with a small sample size, which may limit the generalizability of our findings. Second, manual measurement of GGNs can be subject to measurement errors. However, it is a method used in clinical practice and we performed discussion between two experienced radiologists to minimize measurement errors. Third, while our cases of GGNs lacked pathological confirmation, the pure GGNs with stable size have a higher possibility of preinvasive or invasive lung cancer (37). Lastly, chest CT was not dedicated to automated volume measurement, as it was obtained from various vendors and protocols.
In our study, the majority of the incidentally detected GGNs exhibited a pure density and small size. Tumors of pure GGNs have excellent survival after surgical resection (38). Therefore, presence of pure GGNs with small size is not expected to impact long-term survival in most cases. While, there are no definite guidelines for surgical indication for pure GGN, surgery may be considered if the nodule grows in size or if a solid component area forms (37). According to a previous study, among GGNs smaller than 6 mm and stable for 5 years, 13% exhibited growth during follow-up. In our study, 39.1% of patients showed interval growth (19). Hence, given the increasing incidence of breast cancer in young patients, it is advisable to conduct thorough follow-up until the growth of any nodules is confirmed, especially when GGNs risk factors are identified.
Conclusions
We observed incidental findings of GGNs and lung cancer in 5% and 0.9% of patients with breast cancer, respectively. Furthermore, confirmed cases of lung cancer were adenocarcinoma with a high prevalence of EGFR mutation. It is recommended that persistent GGNs in older individuals, part-solid nodules, and multiple GGNs be continuously monitored for the early detection of GGN growth and the treatment of lung cancer.
Acknowledgments
We would like to thank Editage for editing and reviewing this manuscript for English language.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1605/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1605/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1605/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1605/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The retrospective study was approved by the Institutional Review Board of Kyung Hee University Hospital at Gangdong (approval No. 2023-02-016) and informed consent was waived owing to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: An overview. Int J Cancer 2021; Epub ahead of print. [Crossref]
- 2020 National Cancer Registry Statistics. 2022. Accessed April 1, 2023. Available online: https://www.cancer.go.kr
- Kang SY, Lee SB, Kim YS, et al. Breast Cancer Statistics in Korea, 2018. J Breast Cancer 2021;24:123-37. [Crossref] [PubMed]
- Ricceri F, Fasanelli F, Giraudo MT, et al. Risk of second primary malignancies in women with breast cancer: Results from the European prospective investigation into cancer and nutrition (EPIC). Int J Cancer 2015;137:940-8. [Crossref] [PubMed]
- Molina-Montes E, Requena M, Sánchez-Cantalejo E, et al. Risk of second cancers cancer after a first primary breast cancer: a systematic review and meta-analysis. Gynecol Oncol 2015;136:158-71. [Crossref] [PubMed]
- Curtis RE. New malignancies among cancer survivors: SEER cancer registries, 1973-2000. Bethesda: US Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2006.
- Schaapveld M, Visser O, Louwman MJ, et al. Risk of new primary nonbreast cancers after breast cancer treatment: a Dutch population-based study. J Clin Oncol 2008;26:1239-46. [Crossref] [PubMed]
- Mellemkjaer L, Friis S, Olsen JH, et al. Risk of second cancer among women with breast cancer. Int J Cancer 2006;118:2285-92. [Crossref] [PubMed]
- Sung H, Hyun N, Leach CR, et al. Association of First Primary Cancer With Risk of Subsequent Primary Cancer Among Survivors of Adult-Onset Cancers in the United States. JAMA 2020;324:2521-35. [Crossref] [PubMed]
- Parhizgar P, Bahadori Monfared A, Mohseny M, et al. Risk of second primary cancer among breast cancer patients: A systematic review and meta-analysis. Front Oncol 2022;12:1094136. [Crossref] [PubMed]
- Li C, Liu M, Li J, et al. Relationship between metastasis and second primary cancers in women with breast cancer. Front Oncol 2022;12:942320. [Crossref] [PubMed]
- Wang R, Yin Z, Liu L, et al. Second Primary Lung Cancer After Breast Cancer: A Population-Based Study of 6,269 Women. Front Oncol 2018;8:427. [Crossref] [PubMed]
- Nobel TB, Carr RA, Caso R, et al. Primary lung cancer in women after previous breast cancer. BJS Open 2021;5:zrab115. [Crossref] [PubMed]
- NCCN. NCCN Clinical Practice Guidelines in Oncology. Version 4. 2023. Accessed April 1, 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204-23.
- Xu H, Pu XH, Yu TF, et al. Incidence and natural course of CT-detected pulmonary ground-glass nodules in Chinese women with breast cancer: a retrospective, single-center, long-term follow-up study in 4682 consecutive patients. Acta Radiol 2020;61:175-83. [Crossref] [PubMed]
- Zeng T, Xu H, Liu Y, et al. High rate of epidermal growth factor receptor-mutated primary lung cancer in patients with primary breast cancer. Front Oncol 2022;12:985734. [Crossref] [PubMed]
- Lee HW, Jin KN, Lee JK, et al. Long-Term Follow-Up of Ground-Glass Nodules After 5 Years of Stability. J Thorac Oncol 2019;14:1370-7. [Crossref] [PubMed]
- Matsuguma H, Mori K, Nakahara R, et al. Characteristics of subsolid pulmonary nodules showing growth during follow-up with CT scanning. Chest 2013;143:436-43. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Marchetti A, Barberis M, Franco R, et al. Multicenter Comparison of 22C3 PharmDx (Agilent) and SP263 (Ventana) Assays to Test PD-L1 Expression for NSCLC Patients to Be Treated with Immune Checkpoint Inhibitors. J Thorac Oncol 2017;12:1654-63. [Crossref] [PubMed]
- Teichgraeber DC, Guirguis MS, Whitman GJ. Breast Cancer Staging: Updates in the AJCC Cancer Staging Manual, 8th Edition, and Current Challenges for Radiologists, From the AJR Special Series on Cancer Staging. AJR Am J Roentgenol 2021;217:278-90.
- Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013;24:2206-23. [Crossref] [PubMed]
- Wu FZ, Huang YL, Wu CC, et al. Assessment of Selection Criteria for Low-Dose Lung Screening CT Among Asian Ethnic Groups in Taiwan: From Mass Screening to Specific Risk-Based Screening for Non-Smoker Lung Cancer. Clin Lung Cancer 2016;17:e45-56. [Crossref] [PubMed]
- Woodard GA, Udelsman BV, Prince SR, et al. Brief Report: Increasing Prevalence of Ground-Glass Nodules and Semisolid Lung Lesions on Outpatient Chest Computed Tomography Scans. JTO Clin Res Rep 2023;4:100583. [Crossref] [PubMed]
- Sihoe ADL, Petersen RH, Cardillo G. Multiple pulmonary ground glass opacities: is it time for new guidelines? J Thorac Dis 2018;10:5970-3. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Yano M, Sasaki H, Kobayashi Y, et al. Epidermal growth factor receptor gene mutation and computed tomographic findings in peripheral pulmonary adenocarcinoma. J Thorac Oncol 2006;1:413-6.
- Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res 2015;5:2892-911.
- Gonzalez-Conchas GA, Rodriguez-Romo L, Hernandez-Barajas D, et al. Epidermal growth factor receptor overexpression and outcomes in early breast cancer: A systematic review and a meta-analysis. Cancer Treat Rev 2018;62:1-8. [Crossref] [PubMed]
- Levva S, Kotoula V, Kostopoulos I, et al. Prognostic Evaluation of Epidermal Growth Factor Receptor (EGFR) Genotype and Phenotype Parameters in Triple-negative Breast Cancers. Cancer Genomics Proteomics 2017;14:181-95. [Crossref] [PubMed]
- He Q, Zhang M, Zhang J, et al. Correlation between epidermal growth factor receptor mutations and nuclear expression of female hormone receptors in non-small cell lung cancer: a meta-analysis. J Thorac Dis 2015;7:1588-94. [Crossref] [PubMed]
- Grantzau T, Thomsen MS, Væth M, et al. Risk of second primary lung cancer in women after radiotherapy for breast cancer. Radiother Oncol 2014;111:366-73. [Crossref] [PubMed]
- Kaufman EL, Jacobson JS, Hershman DL, et al. Effect of breast cancer radiotherapy and cigarette smoking on risk of second primary lung cancer. J Clin Oncol 2008;26:392-8. [Crossref] [PubMed]
- Cho S, Yang H, Kim K, et al. Pathology and prognosis of persistent stable pure ground-glass opacity nodules after surgical resection. Ann Thorac Surg 2013;96:1190-5. [Crossref] [PubMed]
- Ding Y, He C, Zhao X, et al. Adding predictive and diagnostic values of pulmonary ground-glass nodules on lung cancer via novel non-invasive tests. Front Med (Lausanne) 2022;9:936595. [Crossref] [PubMed]


