
Wedge resection, segmentectomy, and lobectomy: oncologic outcomes based on extent of surgical resection for ≤2 cm stage IA non-small cell lung cancer
Highlight box
Key findings
• In patients with clinical stage IA non-small cell lung cancer (NSCLC) ≤2 cm, overall survival, disease-free survival, and lung cancer-specific survival were similar between wedge resection, segmentectomy, and lobectomy.
What is known and what is new?
• Randomized trials published by Japanese Clinical Oncology Group (JCOG) 0802 and Cancer and Leukemia Group B (CALGB) 140503 have demonstrated that sublobar resection is non-inferior to lobectomy in well-selected patients with early-stage, small lung cancers with no nodal involvement.
• Our study demonstrates patients who have inferior performance status and worse pulmonary function are more likely to be selected to undergo sublobar resection, without compromising oncological outcomes.
What is the implication, and what should change now?
• Sublobar resection has similar survival outcomes and recurrence patterns to lobectomy despite being used more often in patients with inferior pulmonary and performance status and is an appropriate surgical approach in patients with clinical stage IA (T1a and T1bN0M0) NSCLC.
Introduction
There has long been controversy over whether sublobar resections are an adequate oncologic procedure for early-stage non-small cell lung cancer (NSCLC). Since the Lung Cancer Study Group published randomized trial results in 1995 demonstrating higher rates of local recurrence and worse survival for “limited pulmonary resection” than lobectomy for T1N0 ≤3 cm NSCLC, lobectomy has been firmly established as the standard of care for patients with adequate pulmonary reserve (1,2). Still, sublobar resections in the form of segmentectomies and wedge resections were still used in older patients and those with limited pulmonary reserve or poor performance status (3).
Improvements in diagnostic imaging modalities and the advent of lung cancer screening have led to better detection and staging of small, early-stage NSCLC over the past two decades, and so the use of sublobar resection to preserve lung function has maintained steady interest. Saji et al. recently published results from Japanese Clinical Oncology Group (JCOG) 0802, a randomized trial comparing oncologic outcomes of patients with stage IA NSCLC ≤2 cm after undergoing segmentectomy versus lobectomy. The results demonstrated improved 5-year overall survival (OS) for segmentectomy over lobectomy, challenging the notion of whether lobectomy should remain the standard of care for small early-stage IA NSCLC (4). Soon thereafter, Altorki et al. published the long-anticipated results of the Cancer and Leukemia Group B (CALGB) 140503 trial, a multicenter, international, randomized trial comparing oncological outcomes between sublobar resection and lobectomy in patients with stage IA NSCLC ≤2 cm, demonstrating comparable 5-year disease-free survival (DFS) and OS between sublobar resections and lobectomy, with hazard ratio (HR) 1.01 [90% confidence interval (CI): 0.83–1.24] and 0.95 (95% CI: 0.72–1.26), respectively (5). CALGB 140503 left the type of sublobar resection to the discretion of the surgeon, with 59.1% of those randomized receiving a wedge resection. The CALGB group also reported that there were no statistical differences between lobectomy and sublobar resection in 30- and 90-day mortality and morbidity (6). In the current study, we sought to further characterize oncologic outcomes for patients with stage IA NSCLC ≤2 cm using a large, real-world, single-institutional experience. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1693/rc).
Methods
We conducted a retrospective review of a prospectively maintained database to identify all patients who underwent sublobar resection or lobectomy for clinical stage IA NSCLC with tumor size ≤2 cm (Tumor-Node-Metastasis 8th Edition) between 2011 and 2020. Patients were excluded if the tumor measured >2 cm in size and if they had prior history of lung cancer. Carcinoid tumors and ground glass lesions with less than 50% solid component were also excluded (Figure S1). General baseline demographics and clinical variables, along with performance status, pulmonary function, histology, surgical approach, and recurrence patterns were evaluated. The surgical approach was grouped into video-assisted thoracoscopic surgery (VATS), robot-assisted thoracoscopic surgery (RATS), thoracotomy, and VATS converted to thoracotomy. The primary outcomes of interest were OS and DFS, and secondary outcomes included lung cancer-specific survival (LCSS), recurrence patterns, and perioperative morbidity and mortality. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board (IRB) of Weill Cornell Medicine and NewYork-Presbyterian Hospital (IRB 22-10025327) and patient written consent was waived for the retrospective analysis.
Statistical analysis
The categorical data identified was compared using chi-square tests and continuous variables were presented using median and interquartile range or means and standard deviation, which were subsequently compared between the groups using analysis of variance test (ANOVA). Patients were followed per our institutional protocol based on National Comprehensive Cancer Network guidelines. They had a postoperative visit 10–14 days after surgery, followed by visits and computed tomography (CT) scan of the chest for radiographic surveillance every 6 months for 2–3 years, and annually thereafter. The OS was estimated from date of surgery to last known date of follow-up or date of death. The DFS was estimated from date of surgery to last known date of thoracic surgery follow-up, last known date of chest CT imaging, or date of death. The LCSS was estimated from date of recurrence or death from cancer. The 5-year survival probabilities were estimated using Kaplan-Meier survival analysis and compared between the groups using log-rank analysis. All statistical analyses were two-sided with statistical significance identified as P<0.05. Univariable and multivariable predictors of mortality were evaluated using Cox proportional hazards regression analysis. Variables that were included in the model were age, smoking status, Eastern Cooperative Oncology Group (ECOG) performance status, histologic subtype, pulmonary function tests, pathologic N stage, procedure, and surgical approach. These variables were added in a sequential fashion and are presented as HRs with 95% CIs. Univariate predictors that had P values >0.20 were excluded from multivariable analysis. All analyses were performed using IBM SPSS software (version 25.0; SPSS, Inc., Chicago, IL, USA).
Results
Between January 2011 and December 2020, there were 480 patients who met the inclusion criteria. Of these 480 patients, 93 (19.4%) underwent wedge resection, 90 (18.7%) received segmentectomy, and 297 (61.9%) underwent lobectomy. Baseline demographic and clinical characteristics are presented in Table 1. The three groups were comparable in terms of age, sex, race, and smoking status. Patients who underwent wedge resection had worse ECOG performance status (23.7% ECOG 1 or 2 vs. 5.6% among segmentectomy and 5.4% among lobectomy, P<0.05). Both wedge resection and segmentectomy patients had lower preoperative mean percentage of predicted forced expiratory volume in one second (%FEV1) compared to the lobectomy group (81.8% and 82.6% vs. 89.6%, P=0.002), a higher proportion of patients with chronic obstructive pulmonary disease (COPD) and interstitial lung disease (ILD), and a higher Charlson Comorbidity Index (Table 2). Patients who underwent wedge resection had smaller mean pathologic tumor size compared to segmentectomy and lobectomy; 1.23 vs. 1.50 and 1.76 cm, respectively (P=0.001). Patients who underwent wedge resection also had the smallest median margin length of 1.20 cm compared to 1.80 cm in segmentectomy and 3.00 cm in lobectomy (P<0.001) Furthermore, patients who underwent wedge resection had the lowest median number of lymph nodes resected compared to segmentectomy and lobectomy; 4 vs. 9 and 13, respectively (P=0.001) and lower number of N2 stations sampled. Nodal upstaging was significantly greater for lobectomy compared with sublobar resection. N1 upstaging was observed in 1.1% of wedge resection, 3.3% of segmentectomy, and 6.4% of lobectomy, and N2 upstaging was observed in 1.1%, 4.4%, and 5.1%, respectively (P=0.05, Table 3).
Table 1
| Variables | Wedge resection (N=93) | Segmentectomy (N=90) | Lobectomy (N=297) | P value |
|---|---|---|---|---|
| Age (years) | 72±9 | 70.2±9 | 69.9±9 | 0.15 |
| Sex | 0.57 | |||
| Female | 51 (54.8) | 56 (62.2) | 169 (56.9) | |
| Male | 42 (45.2) | 34 (37.8) | 128 (43.1) | |
| Race | 0.085 | |||
| White | 56 (60.2) | 56 (62.2) | 187 (63.0) | |
| African American | 10 (10.8) | 9 (10.0) | 24 (8.1) | |
| Asian | 7 (7.5) | 9 (10.0) | 50 (16.8) | |
| Other | 20 (21.5) | 16 (17.8) | 36 (12.1) | |
| Smoking status | 0.85 | |||
| Never | 17 (18.3) | 20 (22.2) | 71 (23.9) | |
| Former | 52 (55.9) | 49 (54.4) | 158 (53.2) | |
| Current | 24 (25.8) | 21 (23.3) | 68 (22.9) | |
| ECOG performance status | <0.05 | |||
| 0 | 71 (76.3) | 85 (94.4) | 281 (94.6) | |
| 1 or 2 | 22 (23.7) | 5 (5.6) | 16 (5.4) | |
| Pulmonary function test | ||||
| %FVC | 88.9±19.8 | 85±16 | 89.8±19.4 | 0.17 |
| %FEV1 | 81.8±26 | 82.6±19.8 | 89.6±18.9 | 0.002 |
| %DLCO | 77.9±28.5 | 80±19.1 | 85.2±23.1 | 0.05 |
| Surgical approach | ||||
| Thoracotomy | 1 (1.1) | 2 (2.2) | 7 (2.4) | 0.003 |
| VATS | 92 (98.9) | 81 (90.0) | 258 (86.9) | |
| VATS converted to thoracotomy | 0 | 0 | 1 (0.3) | |
| RATS | 0 | 7 (7.8) | 31 (10.4) | |
| Histologic subtype | 0.72 | |||
| Adenocarcinoma | 74 (79.6) | 74 (82.2) | 257 (86.5) | |
| Squamous cell carcinoma | 16 (17.2) | 14 (15.6) | 32 (10.8) | |
| Other | 3 (3.2) | 2 (2.2) | 8 (2.7) | |
| Type of nodule | 0.400 | |||
| Solid | 83 (89.2) | 77 (85.6) | 269 (90.6) | |
| Part-solid | 10 (10.8) | 13 (14.4) | 28 (9.4) | |
| Recurrence | 0.36 | |||
| No recurrence | 76 (81.7) | 78 (86.6) | 259 (87.2) | |
| Local | 8 (8.6) | 6 (6.7) | 11 (3.7) | |
| Distant | 9 (9.7) | 6 (6.7) | 27 (9.1) |
Data are presented as frequency (%) or mean ± SD. ECOG, Eastern Cooperative Oncology Group; %FVC, percentage of predicted forced vital capacity; %FEV1, percentage of predicted forced expiratory volume in one second; %DLCO, percentage of predicted diffusing capacity of the lungs for carbon monoxide; VATS, video-assisted thoracoscopic surgery; RATS, robot-assisted thoracoscopic surgery; SD standard deviation.
Table 2
| Variables | Wedge resection (N=93) | Segmentectomy (N=90) | Lobectomy (N=297) | P value |
|---|---|---|---|---|
| Comorbidities | ||||
| Hypertension | 50 (53.8) | 50 (55.6) | 178 (59.9) | 0.51 |
| Hypercholesterolemia | 43 (46.2) | 43 (47.8) | 123 (41.4) | 0.48 |
| Coronary artery disease | 15 (16.1) | 9 (10.0) | 47 (15.8) | 0.36 |
| Interstitial lung fibrosis | 5 (5.4) | 6 (6.7) | 4 (1.3) | 0.02 |
| Myocardial infarction | 8 (8.6) | 0 | 15 (5.1) | 0.004 |
| Peripheral vascular disease | 7 (7.5) | 3 (3.3) | 14 (4.7) | 0.42 |
| Congestive heart failure | 6 (6.5) | 1 (1.1) | 4 (1.3) | 0.03 |
| Chronic obstructive pulmonary disease | 31 (33.3) | 28 (31.1) | 54 (18.2) | 0.002 |
| Atrial fibrillation | 7 (7.5) | 10 (11.1) | 23 (7.7) | 0.57 |
| Diabetes mellitus | 19 (20.4) | 20 (22.2) | 45 (15.2) | 0.22 |
| Renal disease | 9 (9.7) | 4 (4.4) | 14 (4.7) | 0.17 |
| Charlson Comorbidity Index | 0.03 | |||
| 0 | 29 (31.2) | 28 (31.1) | 143 (48.2) | |
| 1 | 22 (23.7) | 30 (33.3) | 68 (22.9) | |
| 2 | 20 (21.5) | 21 (23.3) | 55 (18.5) | |
| 3 or greater | 22 (23.6) | 11 (12.2) | 31 (10.4) |
Data are presented as frequency (%).
Table 3
| Variables | Wedge resection (N=93) | Segmentectomy (N=90) | Lobectomy (N=297) | P value |
|---|---|---|---|---|
| Pathologic stage | 0.36 | |||
| Stage 0 | 1 (1.1) | 0 | 2 (0.7) | |
| Stage I | 87 (93.5) | 82 (91.1) | 257 (86.5) | |
| Stage II | 3 (3.2) | 4 (4.4) | 23 (7.7) | |
| Stage III | 2 (2.2) | 4 (4.4) | 15 (5.1) | |
| Pathologic tumor stage | 0.539 | |||
| Tis | 1 (1.1) | 0 | 2 (0.7) | |
| T1 | 76 (81.7) | 79 (87.8) | 239 (80.5) | |
| T2 | 13 (14.0) | 10 (11.1) | 52 (17.5) | |
| T3/T4 | 3 (3.2) | 1 (1.1) | 4 (1.3) | |
| Pathologic tumor size, cm | 1.23±0.50 | 1.50±0.50 | 1.76±0.80 | 0.001 |
| Margin length, cm | 1.20 [0.60–2.00] | 1.80 [0.98–3.10] | 3.00 [1.50–5.00] | <0.001 |
| Pathologic nodal stage | 0.05 | |||
| Nx | 6 (6.5) | 1 (1.1) | 0 | |
| N0 | 85 (91.4) | 82 (91.1) | 263 (88.6) | |
| N1 | 1 (1.1) | 3 (3.3) | 19 (6.4) | |
| N2 | 1 (1.1) | 4 (4.4) | 15 (5.1) | |
| Number of LN sampled | 4 [3–8] | 9 [5–13] | 13 [9–18] | 0.001 |
| Number of N2 stations sampled | 0.012 | |||
| 0 | 8 (8.6) | 4 (4.4) | 6 (2.0) | |
| 1 | 23 (24.7) | 14 (15.6) | 39 (13.1) | |
| 2 | 43 (46.2) | 49 (54.4) | 157 (52.9) | |
| 3 | 15 (16.1) | 18 (20.0) | 79 (26.6) | |
| 4 | 3 (3.2) | 3 (3.3) | 15 (5.1) | |
| 5 | 1 (1.1) | 2 (2.2) | 1 (0.3) |
Data are presented as frequency (%), mean ± SD, or median [IQR]. LN, lymph node; SD, standard deviation; IQR, interquartile range.
The median follow-up for the entire cohort was 49 months. There was no statistical difference in 5-year OS, DFS, or LCSS between the groups: 90%, 61%, and 78% for wedge resection compared with 85%, 75% and 86% for segmentectomy, and 87%, 77% and 87% for lobectomy, respectively (Figures 1-3). Recurrence of any type was observed in 17 patients (18.3%, 8 local, 9 distant) who underwent wedge resection, 12 patients (13.4%, 6 local, 6 distant) who received segmentectomy, and 38 patients (12.8%, 11 local, 27 distant) who underwent lobectomy, which was not statistically different (P=0.36).
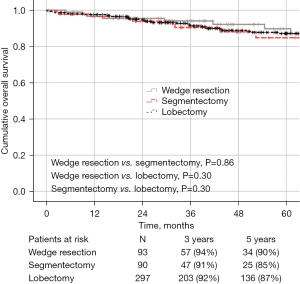
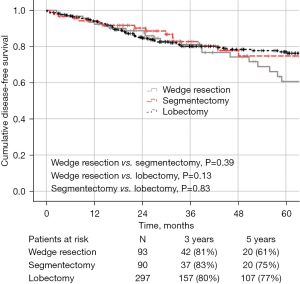
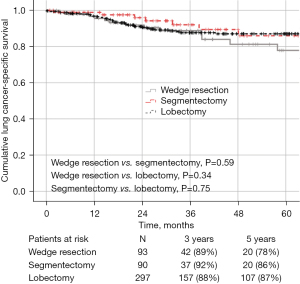
Factors associated with worse DFS on univariate analysis were older age (HR 1.056; 95% CI: 1.020–1.093, P=0.002), squamous histology (HR 3.024; 95% CI: 1.622–5.636, P<0.001), thoracotomy (HR 3.959; 95% CI: 0.661–23.706, P=0.13), pathologic upstaging to stage III/IV (HR 3.199; 95% CI: 1.260–8.122, P=0.01), and solid nodules (HR 2.558; 95% CI: 1.044–6.268, P=0.040). On multivariable analysis, older age (HR 1.042; 95% CI: 1.017–1.068, P<0.001), squamous histology (HR 1.800; 95% CI: 1.079–3.002, P=0.024), pathologic upstaging to stage II (HR 3.430; 95% CI: 1.877–6.269, P<0.001) and pathologic upstaging to stage III/IV (HR 4.128; 95% CI: 1.944–8.769, P<0.001) remained independent predictors of worse DFS (Table S1). The extent of resection was not a predictor of worse DFS.
There was no difference between the three groups in the incidence of postoperative complications (Table S2). In the wedge resection group, 9.7% of patients had any type of complication compared to 15.6% in segmentectomy and 14.8% in lobectomy, which was not statistically significant (P=0.41). The number of patients who had an unexpected return to the operating room during their admission was comparable between the groups (3.2% in wedge resection, 3.3% in segmentectomy, and 3% among lobectomy). Perioperative mortality was not significantly different between the groups: 1.1% 30-day mortality and 1.1% 90-day mortality after wedge resection, 0% and 2.2% after segmentectomy, and 0.7% and 1.4% after lobectomy.
Discussion
In this retrospective study of a sizable, single institution cohort, OS, DFS, and LCSS following wedge resection, segmentectomy, and lobectomy performed for ≤2 cm clinical stage IA NSCLC were not significantly different, even though wedge resections and segmentectomies were more likely to be performed on patients with worse baseline performance status and lung function. This makes intuitive sense as surgeons can be expected to have a selection bias towards a smaller extent of resection for patients who are more marginal surgical candidates. Importantly, this study demonstrates that while this selection bias favoring wedge resections and segmentectomies for patients with poor performance status and lung function may exist in real-world settings outside of controlled clinical trials, it does not appear to come at the cost of inferior oncologic or survival outcomes.
There have been other retrospective studies from large cancer databases that reported noninferiority of sublobar resection compared to lobectomy (7-9). Li et al. reviewed the Surveillance, Epidemiology, and End Results (SEER) database from 2004–2015 to compare outcomes of lobectomy versus segmentectomy in patients with pathologic stage IA NSCLC. This study showed that the OS and LCSS were similar in both groups; 5-year OS after lobectomy compared to segmentectomy was 75.8% and 76.4% (P=0.16) and LCSS was 82.7% and 82.9%, respectively (P=0.02) (7).
On the other hand, Speicher et al. conducted a retrospective review of the National Cancer Database (NCDB) from 2003–2011 and demonstrated that in patients with clinical stage IA NSCLC, the 5-year OS in the lobectomy group was significantly better compared to sublobar resection (66.2% vs. 51.2% respectively, P<0.001) (10). Of note, 84.7% of patients in the sublobar resection group underwent a wedge resection and perhaps the decreased OS in the sublobar resection group can in part be explained by the fact that more than 25% of patients had no hilar or mediastinal lymph nodes assessed compared to the lobectomy group. In contrast, within our cohort, only 7.6% of patients undergoing sublobar resection had no lymph nodes assessed (6.5% in wedge resection and 1.1% in segmentectomy). Taken together with what we have learned from JCOG 0802 and CALGB 140503, adequate lymph node dissection at the time of surgery remains essential to maintaining good oncological outcomes.
There have also been variations in survival outcomes in the literature when comparing similar patient population from the SEER database over time. Yendamuri et al. examined the effect of time trends on survival outcomes in the SEER database and showed worse survival outcomes for sublobar resection compared to lobectomy for an earlier cohort [1988–1997]. However, there was no difference in terms of OS between the two groups when examining a more modern cohort from 2005–2008. This observed improvement in OS in the same population is likely secondary to better mediastinal staging prior to resection as the SEER database enhanced the quality of reporting on mediastinal staging by 60% from 2000 to 2005 (11). Harrison et al. conducted a literature review that suggested the survival differences that have been observed in studies between sublobar resection and lobectomy are likely secondary to more frequent nodal upstaging from thorough mediastinal dissections in lobectomies, which might be lacking in wedge resections (12). Additionally, many studies have reported a higher rate of locoregional recurrence in patients who undergo sublobar resection (4,10,13-16). In Divisi et al.’s literature review, the highest reported risk of recurrence was 53.4% in the sublobar resection group compared to 32% in lobectomy, which they attributed primarily to the decreased access to thorough lymph node assessment seen among patients who underwent a sublobar resection (14). Although in our study we observed a higher median number of lymph nodes resected and increased rates of nodal upstaging in lobectomy compared with wedge resection and segmentectomy, this did not translate to any difference in survival or recurrence between the groups.
Our findings corroborate the results of JCOG 0802 and CALGB 140503, the only two randomized trials comparing extent of surgical resection for small early-stage lung cancer in the modern era. JCOG 0802 compared oncological outcomes of patients who underwent segmentectomy versus lobectomy in stage IA NSCLC ≤2 cm, which showed superior 5-year OS in the segmentectomy group and similar relapse free survival between the groups (94.3% vs. 91.1% and 88% vs. 87.9% respectively) (4). CALGB 140503, a multicenter, international randomized noninferiority trial reported equivalent 5-year DFS and OS between sublobar resection and lobectomy (63.6% vs. 64.1% and 80.3% vs. 78.9% respectively) in patients with small peripheral NSCLC (5). A post hoc analysis of the CALGB 140503 study comparing lobectomy, wedge resection, and segmentectomy independently demonstrated 5-year DFS, OS, and LCSS were similar regardless of the extent of pulmonary resection (17). Our current findings remain in line with these two randomized trials, and further suggests that even though wedge resection and segmentectomy may be favored in patients with lower baseline performance and lung function, this does not compromise oncological outcomes.
Our study is limited as it is a retrospective study of a single-institutional experience, and therefore allows for the possibility of unanticipated biases. Those biases likely reflect selection biases and clinical judgments that surgeons make in a real-world setting in response to perceived patient performance status and pulmonary function, outside of the strict confines of a clinical trial. The conclusions made in this study are also limited to patients with clinical stage IA NSCLC tumors ≤2 cm who are deemed node negative by preoperative positron emission tomography (PET)/CT or invasive mediastinal staging.
Conclusions
Survival and recurrence patterns for patients with clinical stage IA NSCLC ≤2 cm after wedge resection, segmentectomy, and lobectomy are comparable, even though wedge resection and segmentectomy are more likely to be performed for those with worse baseline performance status and pulmonary function. Given these findings and along with the recently published randomized trials, sublobar resection should be considered an appropriate, and likely optimal, surgical option for clinical stage IA NSCLC ≤2 cm.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1693/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1693/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1693/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1693/coif). N.A. received grants or contracts from AstraZeneca and Roche/Genentech; and has ownership interest in Angiocrine Bioscience, TMRW and Viewpoint Medical. J.L.P. has leadership and ownership interest in Angiocrine Bioscience, TMRW and Viewpoint Medical. B.L. receives speaker fees from AstraZeneca. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board (IRB) of Weill Cornell Medicine and NewYork-Presbyterian Hospital (IRB 22-10025327) and patient written consent was waived for the retrospective analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang Y, Yuan C, Zhang Y, et al. Survival following segmentectomy or lobectomy in elderly patients with early-stage lung cancer. Oncotarget 2016;7:19081-6. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Villamizar N, Swanson SJ. Lobectomy vs. segmentectomy for NSCLC (T<2 cm). Ann Cardiothorac Surg 2014;3:160-6. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Altorki N, Wang X, Kozono D, et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N Engl J Med 2023;388:489-98. [Crossref] [PubMed]
- Altorki NK, Wang X, Wigle D, et al. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). Lancet Respir Med 2018;6:915-24. [Crossref] [PubMed]
- Li F, Zhao Y, Yuan L, et al. Oncologic outcomes of segmentectomy vs lobectomy in pathologic stage IA (≤2 cm) invasive lung adenocarcinoma: A population-based study. J Surg Oncol 2020;121:1132-9. [Crossref] [PubMed]
- Dziedzic R, Zurek W, Marjanski T, et al. Stage I non-small-cell lung cancer: long-term results of lobectomy versus sublobar resection from the Polish National Lung Cancer Registry. Eur J Cardiothorac Surg 2017;52:363-9. [Crossref] [PubMed]
- Wisnivesky JP, Henschke CI, Swanson S, et al. Limited resection for the treatment of patients with stage IA lung cancer. Ann Surg 2010;251:550-4. [Crossref] [PubMed]
- Speicher PJ, Gu L, Gulack BC, et al. Sublobar Resection for Clinical Stage IA Non-small-cell Lung Cancer in the United States. Clin Lung Cancer 2016;17:47-55. [Crossref] [PubMed]
- Yendamuri S, Sharma R, Demmy M, et al. Temporal trends in outcomes following sublobar and lobar resections for small (≤ 2 cm) non-small cell lung cancers--a Surveillance Epidemiology End Results database analysis. J Surg Res 2013;183:27-32. [Crossref] [PubMed]
- Harrison S, Stiles B, Altorki N. What is the role of wedge resection for T1a lung cancer? J Thorac Dis 2018;10:S1157-62. [Crossref] [PubMed]
- Kodama K, Doi O, Higashiyama M, et al. Intentional limited resection for selected patients with T1 N0 M0 non-small-cell lung cancer: a single-institution study. J Thorac Cardiovasc Surg 1997;114:347-53. [Crossref] [PubMed]
- Divisi D, De Vico A, Zaccagna G, et al. Lobectomy versus sublobar resection in patients with non-small cell lung cancer: a systematic review. J Thorac Dis 2020;12:3357-62. [Crossref] [PubMed]
- Warren WH, Faber LP. Segmentectomy versus lobectomy in patients with stage I pulmonary carcinoma. Five-year survival and patterns of intrathoracic recurrence. J Thorac Cardiovasc Surg 1994;107:1087-93; discussion 1093-4.
- Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg 1995;109:120-9. [Crossref] [PubMed]
- Altorki N, Wang X, Damman B, et al. Lobectomy, segmentectomy, or wedge resection for peripheral clinical T1aN0 non-small cell lung cancer: A post hoc analysis of CALGB 140503 (Alliance). J Thorac Cardiovasc Surg 2024;167:338-347.e1. [Crossref] [PubMed]


