2016 Respiratory Effectiveness Group Annual Summit Report—impact & influence of real-world respiratory evidence
The Respiratory Effectiveness Group (REG) (www.effectivenessevaluation.org) is a global network of respiratory clinicians, researchers, outcomes researchers and allied health professionals with a shared interest in real-world research. Founded in early 2013, the REG is an investigator-led collaborative organisation that works to raise the quality and profile of real-life research by standardising methodologies, setting standards and applying high-quality methods to address evidence gaps and unmet research needs.
The REG’s third annual summit took place on the 15–16th of April 2016 in Lyon, France. The event was scheduled to dovetail with the final symposium of the European Union Funded ASTRO-LAB Study (http://www.astrolab-project.eu; Seventh Framework Program under Grant Agreement n 282593). The ASTRO-LAB symposium was the culmination of a 4-year assessment of the long-acting beta2-agonist (LABA) benefit/risk ratio in asthma in routine care by combining healthcare databases and direct patient follow-up. The coincident timing of the two events reflected the close working relationships of ASTRO-LAB investigators and REG collaborators and their shared ethos—that there is a need to look beyond the classical registration randomised controlled trial (RCT) evidence and to consider longer-term studies (in broader patient populations) conducted in routine care settings to understand the true effectiveness and safety profiles of respiratory interventions.
Previous REG Summit themes focussed on the evolving role and value of real-life research in Respiratory Medicine (1) and on available observational research resources around the world (2). As the REG is maturing as an organisation, its Executive Members felt that the theme for 2016 should explore the extent to which real-life research is, likewise, maturing and starting to influence and have an impact on decision making across different stakeholder groups. This was reflected in the 2016 Summit theme: Impact and Influence of Real-World Research on Respiratory Medicine.
The resultant programme was developed in collaboration with the REG Chairman, Prof. David Price (Singapore, Singapore) and an international REG Summit Committee: Dr. Nemr Eid (Louisville, Kentucky, USA), Prof. George Christoff (Sofia, Bulgaria); Dr. Bernardino Alcázar-Navarrete (Granada, Spain) and Mr Aji Barot (London, UK).
The programme spanned 2 days and included a range of different session formats (e.g., plenaries, pro/con debates, oral and poster abstract presentations) and topics. The ‘impact and influence’ of real-world respiratory evidence were considered with respect to: individualised care/personalised medicine; market access and payor perspectives and guideline development. The scope and potential impact of the studies currently underway through REG’s speciality working groups was also discussed.
Individualised care/personalised medicine
The opening plenary of the summit considered real-world respiratory evidence in terms of its potential to inform clinical management decisions and help realise ‘precision medicine’ in chronic respiratory diseases. Dr. Alan Kaplan (Ontario, Canada) considered opportunities in the context of chronic obstructive pulmonary disease (COPD) and Prof. David Price (Singapore, Singapore) in relation to asthma.
Within the context of asthma, Professor Price highlighted the clear disconnect with the trial reports of efficacious therapies and persistent asthma mortality rates. This unmet disease burden suggests a need to understand more about how licensed therapies are currently being used, including quantifying inappropriate prescribing practices that may add to patient risk [e.g., long-acting bronchodilators without concomitant inhaled corticosteroid (ICS) therapy; failing to step-up patients who remain uncontrolled on existing therapy] and how to tailor management more explicitly to the individual lifestyle, control and risk profiles of the patient. While RCTs provide evidence on the efficacy and safety of interventions when used optimally in ideal patients, the broader inclusion criteria of real-world studies (both in terms of eligible patients and ecologies of care) can unlock important evidence on routine care practices and patient characteristics (demographic, clinical, biometric, social and psychosocial) associated with future risk and/or potential treatment response.
In asthma, Prof. Price recommended use of routine care data to risk stratify patients and to ensure high-risk patients receive timely specialist referral, in line with current guideline recommendations (3-5).For the broader population, he proposed it was perhaps time to abolish step 1 of the guidelines—as needed short-acting beta2-agonist therapy—and move directly to regular anti-inflammatory therapy or even combination anti-inflammatory therapy and beta2-agonist therapy for symptom relief. The choice of anti-inflammatory therapy, he continued, should be guided by clinical need and patient preference, and agreed in collaboration with the patient. While twice-daily ICS would be the standard of care, once-daily ICS could be considered where it might help to optimise adherence, as could leukotriene receptor antagonists [which have been shown to be comparable to ICS when used in routine care (6)] or, in patients with more mild disease, as-needed ICS/LABA therapy (7) and/or fast-acting formulations, which have been shown to be preferred by patients (8).
In the context of COPD, Dr. Kaplan focused on the potential for real-world studies to guide the safe and effective use of ICS in COPD. Contrary to guideline recommendations (9), ICS therapy is widely used in the routine care of patients with COPD (10,11) outside its guideline and licensed position. Well-designed real-world studies could play an important role in understanding their optimum use. They could, he proposed, help to understand exacerbation risk predictors and so identify COPD patients in whom ICS can be safely stepped-down (or withdrawn) and to understand the potential involvement of comorbid conditions on treatment outcomes and the role of eosinophilia as a predictor of treatment response.
Both presenters agreed that at the core of successful precision medicine is good clinical care, where clinicians try to: take full patient histories (including comorbidities), understand attitudes and beliefs to therapies, identify common inhaler errors, tailor patient education, retrain inhaler technique, optimise regimens (simplifying them as required), reassess and adjusting therapy (as required) and engage their patients in shared decision-making so that both parties work towards reaching agreement and are invested in the final decision made.
Regulatory implications & opportunities
The role of real-life research in guiding regulatory change was discussed in a number of Summit’s presentations. To date, regulatory interest in real-world research has largely focussed on the opportunities it presents in the post-licensing phase to provide broader and longer-term safety evaluations of licensed therapies.
In a plenary session entitled, “Safety & Risk outside the idealised trial environment”,Dr. Bernardino Alcázar-Navarrete(Granada, Spain) proposed thatRCTs with a 52-week outcome period may not be the best option to evaluate the full safety profile of pharmacological interventions, particularly when those RCTs are designed to include ≤10% of the routine care respiratory population (12). This, he suggested, was particularly true when considering serious adverse events, which can be uncommon and difficult to detect. Citing examples of research conducted by REG collaborators and/or being presented in the Summit abstract sessions (all currently in press), Dr. Alcázar-Navarrete went on to show worked examples of important clinical safety questions that real-world studies are well placed to address. These included evaluation of: the cardiovascular risk profile of nicotine replacement therapy (13); the risk of beta-blockers in COPD and asthma (see abstracts in this issue: Lahousse et al., AB014 and Verhamme et al., AB029) and the potential metabolic consequences of long-term ICS use. He concluded with some unmet safety questions and targets for future real-life studies, including: pneumonia risk associated with ICS use in COPD; risk of major cardiovascular events in patients with COPD on dual bronchodilator therapy (long-acting bronchodilator and muscarinic antagonists) and long-term cardiovascular safety of macrolides in patients with COPD.
It was also proposed during the Summit that real-life study approaches can inform relevant endpoints for emerging therapies. A lively pro/con debate involving Drs. Omar Usmani (London, United Kingdom) and Ronald Dandurand (Montreal, Canada) titled “To FEV1 or not?” debated the relevance of forced expiratory volume in one second (FEV1) as a key regulatory endpoint for respiratory interventions. Dr. Osmani explained that FEV1 is measured via a “wholly unnatural manoeuvre” (requiring rapid, maximal exhalation of the lungs). Furthermore, he continued, it is difficult to assess with any degree of consistency in the clinic (requiring repetition and the best of three measures to be recorded) and of little interest to patients (who care more about symptoms and exacerbations), yet it remains a gold standard trial endpoint. A compelling argument was put forward for impulse oscillometry (IOS) to replace spirometry as it is a simple, non-invasive method using the forced oscillation technique. It also requires minimal patient cooperation and is suitable for use in both children and adults. The method can be used to assess obstruction in the large and small peripheral airways and has been used to measure bronchodilator response and bronchoprovocation testing (14-20). IOS also boasts better predictive value than spirometry in identifying patients with potential loss of asthma control; greater ability to differentiate between asthma and COPD; more accurate lung function assessment in paediatric patients, and is more sensitive than spirometry at identifying pathology in the peripheral airways (21).However, while extolling the theoretical virtues of IOS, both presenters also acknowledged that further validation work is required to optimise the reliability of IOS data by improving agreement between evaluation devices. Dr. Dandurand is now bringing together a together a collective of IOS experts to address this need with a view to providing the confidence required to see greater uptake of IOS in the future, by practicing clinicians and potentially regulators.
Regulatory bodies are also increasingly interested in the patient voice and ensuring therapies have positive impact on patients’ health-related quality of life. Prof. Thys van der Molen spoke about questionnaires as a valuable and straightforward means of evaluating disease impact and interventions from the patient’s perspective. He explained that ‘hard’ and objective trial endpoints in Respiratory Medicine (e.g., mortality, FEV1) miss the disease impact of greatest importance to patients—dyspnoea. They also fail to capture many of the realities of living with a chronic respiratory disease that patients report in questionnaires, such as tiredness, emotions (e.g., depression), functional status, social status, cough, side effects of medication, hospitalisations. These patient perceptions of disease and patients’ desired treatment outcomes, Prof. van der Molen insisted, cannot be overlooked when making treatment decisions. For example: if a patient is a committed smoker and told they must give up smoking for their prescribed treatment to have greatest effect, it is quite probable that they will continue to smoke, regardless. Similarly, if a patient has an aversion to steroid use and concerns about potential side-effects of long-term steroid treatment, then it would be appropriate to consider licensed alternatives to ICS as the standard of care. Optimum treatment should be selected in collaboration with the patient, taking into account their attitudes, beliefs, lifestyle and desired outcomes.
Another emerging way of capturing the patient’s voice and informing future patient-centric outcomes is through engagement with technology based-health solutions (TBS). The Technologies Plenary Session: “Setting the Standards for Remote Assessment (lung function, inhaler technique) & Monitoring (adherence) in Respiratory Medicine” considered some of the opportunities and risks that TBS pose for respiratory medicine and also the potential role of REG’s technology working group within this evolving environment. Professor John Blakey (Liverpool, UK)—REG technologies working group Lead and Session Chair—prefaced the discussions with the thought that “just because we can, doesn’t mean we should”. At their best, technologies can potentially alleviate some of the current pressures facing healthcare systems by helping to facilitate increased and improved patient self-management. Yet developers (particularly for Apps and wearables) are reticent to invest in research and development as the return is typically small. Reflecting on this, the session presenters drew attention to the important opportunities for REG as a network of respiratory experts and multi-disciplinary stakeholders. It was agreed that the REG is well positioned to work with developers to create priority lists of outline end user needs and to propose standards for data collection and ethical data management.
Chris Chen (Associate Director of Digital Strategy at Mundipharma Asia Pacific, Latin America, Middle East, Africa) shared with the summit delegates a preview of their next generation respiratory healthcare App and how it could help support patients, particularly with mastering inhaler technique. The App uses facial recognition software and augmented reality tools (already being used widely in consumer apps) to support greater patient engagement and support self-management challenges. A multi-disciplinary panel of REG collaborators will be working alongside Mundipharma to provide clinical and data management expertise to help guide the development and implementation of the App.
Market access
Regulatory approval has traditionally required demonstration of clearing three hurdles: safety, efficacy, and production quality. With many pharmaceutical innovations gaining regulatory approval in past generations, healthcare costs and treatment alternatives are on the rise, and a fourth hurdle—demonstration of cost effectiveness—is increasingly evident.
Professor Jon Campbell (Denver, Colorado, USA) and Dr. Brett McQueen (Denver, Colorado, USA) opened a panel discussion on the role of real-life studies in informing relevant and meaningful cost-effectiveness evaluations to help address this fourth hurdle.
There are different sources of evidence available to inform ‘fourth-hurdle decision making’—effectiveness evidence from real-life studies and efficacy evidence from RCTs. The challenge facing Health Technology Appraisal committees is to understand how to use these different data sources optimally to inform therapeutic coverage and reimbursement decisions, Dr. McQueen explained.
Prof. Campbell then illustrated the implications of using different types of evidence within cost effectiveness analysis (CEA) models with an interesting example from everyday respiratory practice. Using omalizumab as a worked example, he showed two CEA models: one informed by an efficacy trial data and the other primarily based on evidence from observational effectiveness study data (22). Although there was reasonable agreement between the two models, the incremental cost-effectiveness ratio (ICER) generated by the real-world data model was more favourable for omalizumab than the RCT-based model. Prof. Campbell advised that Health Technology Assessment (HTA) bodies and payers must use their judgment to determine which components of efficacy-based and effectiveness-based CEA evidence are most closely aligned with their goals.
The panel discussion that followed included market access experts from the academic and commercial sectors. A number of issues were raised, one being the question of affordability rather than cost-effectiveness as for some chronic, degenerative conditions the cost-benefit of a treatment can be accrued over a long period of time, but there is often a substantial upfront payment, i.e., raising an affordability rather than a cost-effectiveness barrier. To address this, pharmaceutical companies can work with HTA bodies to agree acceptable patient access schemes, which often waive (or reimburse) payment for patients who do not respond to therapy. Such schemes, however, tend to come with an additional administrative burden that is not without its own associated costs.
The extent to which such indirect costs are factored into cost-effectiveness models (anecdotally not always well) were also discussed, as was the need for good data quality in order to establish causality (e.g., whether a condition may result as a side effect of treatment or was already pre-existing) to help inform accurate indirect cost evaluations. The importance of clearly defining ‘cost to whom’ was also outlined as CEA models need to be developed with key stakeholders in mind. For example: an intervention may reduce acute care costs at the expense of an increase in primary care costs while resulting in a cost-neutral position for the system as a whole. The benefit of the intervention will be perceived differently within primary care compared with a secondary care stakeholders and differently again by a payor working across the system.
Key research needs flagged during the discussions for the future attention of the REG cost-effectiveness working group, included: quantification of the cost-implications associated with oral steroid comorbidities [building on Sweeney et al.’s steroid-related comorbidity prevalence work in severe asthma (23)] as a means to inform future HTA of severe asthma therapies and refinement of current models (e.g., ICERs) to better reflect conditions (and the benefit of therapies) where symptom management is the primary outcome rather than disease modification.
Guideline development opportunities & implications
Responding to numerous calls for a more integrated approach to evidence evaluation to be taken when developing guidelines, REG joined forces with the European Academy of Asthma and Clinical Immunology (EAACI) to establish a taskforce to develop tools and worked examples to support this process.
Professors Nicolas Roche (Paris, France; Taskforce Lead) Jon Campbell (Denver, Colorado, USA; Taskforce Co-Lead) reviewed the traditional GRADE approach to evidence evaluation, which is used by over 70 guideline developers. GRADE’s quality assessment starts with RCTs as high-quality evidence, and observational studies as low-quality evidence (24). Factors that can lower the quality of RCT evidence include: poorly detailed design and execution, inconsistency, indirectness, reporting bias, and imprecision. Factors that can increase the quality of evidence from observational studies include use of appropriate and robust methods of handling potential sources of bias (selection bias, recall bias, information bias, detection bias) a priori study registration.
The REG-EAACI Taskforce did not set out to challenge the GRADE approach—it is well recognised and robust—rather to offer practical suggestions and solutions to support integration of observational study evidence in future guidelines. The taskforce has recently completed a systematic review and quality appraisal of the asthma observational comparative effectiveness literature from the last decade. The review focused on PICOT questions that had not been addressed by previous RCTs and where real-world studies have an opportunity to address remaining evidence gaps. On these grounds, the taskforce members selected the following areas of interest, the comparative effectiveness of different: (I) particle size ICS therapies; (II) inhaler device types; (III) adherence levels; (IV) management options in smokers.
In order to deliver this work, the Taskforce needed to develop a means of assessing the quality of the published literature (25,26). Tool development drew on existing literature and expertise from the taskforce and involved iterative refinement of the tool through taskforce testing and then a wider pilot within the REG network (involving approximately 50 respiratory clinicians and clinical and/or health outcomes researchers) before rolling it out for full application to the literature identified for appraisal. The results of the work are now being prepared for publication and will provide guideline developers with two useful tools: the first a thorough review of the quality of the observational study literature they may wish to consider for inclusion in future revisions of the asthma guidelines; the second, a practical, easily implementable and peer tested tool to support future quality appraisals.
REG working groups: impact of current projects
To drive forward a number of specialist research areas—areas where real-life research methods have particular utility—the REG has established a number of working groups (see Table 1). These working groups first identify research needs, then work collaboratively to develop protocols and to deliver research that not only addresses those evidence gaps, but also provides worked examples of REG quality standards ‘in practice’. The work of groups was integrated through the summit programme, but also featured in a special Working Group Update Plenary Session. Session highlights included updates from the ACOS, COPD, Small Airways and Interstitial Lung Disease (ILD) working groups.
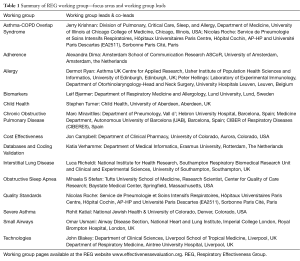
Full table
ACOS working group
Presence of a mixed asthma-COPD phenotype is a growing area of interest within current respiratory research. Such patients—those with ‘mixed disease’—have routinely been excluded from both asthma and COPD RCTs and, as a result, there is limited evidence as to the potential management implications (particularly the potential response to ICS) that a mixed phenotype may represent. Understanding such management implications warrants evaluation in a well-characterized ACOS population, however, there is currently no standard definition of what constitutes ACOS. The first joint publication from GINA and GOLD recognized the presence of patients with characteristics of both conditions and provided a working definition to support clinicians in appropriate diagnosis of the condition (see Table 2) (27), but it is a definition that includes so many permutations that it is not feasible to operationalize in a research context.
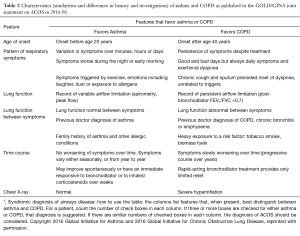
Full table
As a first step to developing standardized definitions and tools for ACOS research, particularly database research, the ACOS working group are evaluating the prevalence and clinical characteristics of a series of ACOS diagnostic cohorts and (in a later phase) will explore the clinical impact of such variations in definitions. The definitions explore whether defining a patient with ACOS as (I) a patient with asthma who has some evidence of COPD (evidence by age, smoking history and presence of fixed airflow obstruction) results in a different ‘type’ of patient than defining an ACOS patient as (II) someone with COPD and some degree of airway reversibility. The study will also explore whether either of these approaches results in a different type of patient than a patient who (diagnostically) appears to have an equal footing in the asthma and COPD camps. Study results from the United Kingdom—using the Optimum Patient Care Research Database—were presented at the summit and are now being prepared for publication. Plans are now underway to leverage the different national databases available to the REG network to repeat the work so as to be able to compare not only within-database difference in prevalence and clinical characteristics and implications, but also between database differences. In time, it is anticipated that the work will provide valuable insights into the optimum definition(s) and databases for use in future ACOS-related research and, importantly, a means of standardizing study design and comparability of results across different studies.
COPD working group
The concept of disease control is well accepted in asthma (28),but traditionally less so in COPD. However, a new definition of COPD Control has recently been proposed with a view to developing a clinical tool to help guide therapeutic management of patients whose short-term disease state can vary (without changing overall severity category) (29). The new definition of control combines a cross-sectional evaluation of current disease impact with a longitudinal assessment of disease stability (over 3 months). Led by Dr. Marc Miravitlles (Barcelona, Spain) the REG COPD working group is undertaking a prospective validation of this concept of control, involving 300 patients from six countries to evaluate the effect of baseline control status on outcomes over the following 2 years. Dr. Miriam Barrecheguren (Barcelona, Spain; colleague of Dr. Miravitlles) provided an update on the status of the study—six countries had now confirmed their involvement and had either secured or were awaiting confirmation of ethics approval at the time of the Summit. The electronic clinical review form had also been developed (and relevant investigators trained) and patient recruitment was due to commence in late April.
In addition, Dr. Barrecheguren noted a number of parallel ‘sub-studies’ that are underway to support the main validation study and explore the optimum thresholds used in the current definition of COPD Control (30). These include one database pilot using the UK’s Optimum Patient Care Research Database [(OPCRD); www.optimumpatientcare.org] and two small, short-term prospective studies in Spain to compare changes in control with changes in (I) COPD severity and (II) COPD symptoms.
Small airways
The most established of the REG working groups, the Small Airways working group, has a broad portfolio of comparative effectiveness studies (in asthma, COPD, paediatrics, smoking asthmatics) that explores the potential benefits of extra-fine particle ICS therapy compared with larger standard particle ICS (30-33). The work is motivated by the principle that there may be additional benefit afforded by using a therapy that can access inflammation present in the small airways, particularly in patient subgroups who may be expected to have more small airways involvement (e.g., smokers, paediatrics). The studies conducted by the group have shown a consistent trend for at least as good, if not better, outcomes for extra-fine (vs. non-extra fine) particle ICS at significantly lower equivalent ICS dose.
A systematic review of the RCT evidence presented at the British Thoracic Society in 2014, however, concluded particle size had no effect on outcomes (34). To address the apparent contradiction, the Small Airways working group are now carrying out a systematic review of the real-world literature (using the REG-EAACI Taskforce work as a quality inclusion threshold) to synthesize the available real-life study evidence and discuss the differences in research questions being asked by the RCT and real-world approaches to help clinicians understand differences in results and to interpret them for the benefit of the patients they see in routine clinical practice.
Interstitial Lung Disease
The newest REG working group is the ILD working group, led by Professor Luca Richeldi (Southampton, UK). ILD is an area of particular interest following the recent licensing (in 2014) of two first in class efficacious therapies for idiopathic pulmonary fibrosis (IPF), the most common and lethal of the idiopathic interstitial pneumonias (35-38).With the advent of these new therapies, which slow rather than reverse disease progression, there is increased focus on early and accurate accurate diagnosis to ensure patients are identified early in the disease course and receive timely therapeutic intervention. Motivated by this, the working group is undertaking two studies to help better understand the real-world path to IPF diagnosis.
The first study is a global undertaking to characterize diagnostic decision making (and the extent to which the gold standard multi-disciplinary team approach to diagnostic decision making is implemented) across centres worldwide. The second is a database study to explore the pathway to an IPF diagnosis with a view to identifying missed diagnostic opportunities and the potential for earlier intervention. Prof. Richeldi shared the preliminary results of this database study with Lyon delegates, which suggest clear trends in respiratory-related healthcare resource utilization in the years preceding an IPF diagnosis. The study results are currently being prepared for publication.
Abstract sessions
The scope of the research being conducted by REG and by the wider respiratory community was evident in the Summit’s thematic abstract sessions (six oral abstract sessions and one poster session).
The abstracts are featured within this issue and include 30 examples of how real-world study methodologies can and are being used (see Table 3).
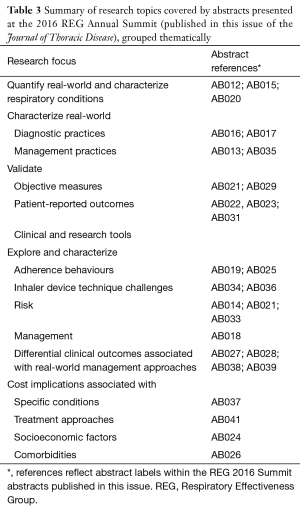
Full table
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- NPJ Primary Care Respiratory Medicine. Abstracts from the Respiratory Effectiveness Group's inaugural summit, June 2014. Available online: http://www.nature.com/public/article-assets/npg/npjpcrm/abstracts/npjpcrm201473.pdf
- Authors on behalf of the Respiratory Effectiveness Group. Meeting abstracts from the Respiratory Effectiveness Group 2015 Winter Summit—databases and registries around the world: maximizing the yield. Pragmatic and Observational Research 2015;6:13-38.
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention (2016 update). Available online: http://ginasthma.org/wp-content/uploads/2016/04/GINA-2016-main-report_tracked.pdf
- Healthcare Improvement Scotland. BTS/SIGN British guideline on the management of asthma. A national clinical guideline. Available online: https://www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-asthma-guideline-2014/
- American Academy of Allergy Asthma & Immunology (AAAAI). Consultation and referral guidelines citing the evidence: how the allergist/immunologist can help. Available online: http://www.aaaai.org/practice-resources/consultation-and-referral-guidelines.aspx
- Price D, Musgrave SD, Shepstone L, et al. Leukotriene antagonists as first-line or add-on asthma-controller therapy. N Engl J Med 2011;364:1695-707. [Crossref] [PubMed]
- Papi A, Canonica GW, Maestrelli P, et al. Rescue use of beclomethasone and albuterol in a single inhaler for mild asthma. N Engl J Med 2007;356:2040-52. [Crossref] [PubMed]
- O'Connor RD, Patrick DL, Parasuraman B, et al. Comparison of patient-reported outcomes during treatment with adjustable- and fixed-dose budesonide/formoterol pressurized metered-dose inhaler versus fixed-dose fluticasone propionate/salmeterol dry powder inhaler in patients with asthma. J Asthma 2010;47:217-23. [Crossref] [PubMed]
- Global Initiative for Obstructive Lung Disease (GOLD). Global Strategy for Diagnosis, Management, and Prevention of COPD—2016. Updated December 2015. Available online: http://www.goldcopd.org
- Postma DS, Calverley P. Inhaled corticosteroids in COPD: a case in favour. Eur Respir J 2009;34:10-2. [Crossref] [PubMed]
- Suissa S, Barnes PJ. Inhaled corticosteroids in COPD: the case against. Eur Respir J 2009;34:13-6. [Crossref] [PubMed]
- Herland K, Akselsen JP, Skjønsberg OH, et al. How representative are clinical study patients with asthma or COPD for a larger "real life" population of patients with obstructive lung disease? Respir Med 2005;99:11-9. [Crossref] [PubMed]
- Research in Real Life Limited and Respiratory Effectiveness Limited, Cambridge, UK. Data on file. Available online: http://rirl.org
- Oostveen E, MacLeod D, Lorino H, et al. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J 2003;22:1026-41. [Crossref] [PubMed]
- Schulz H, Flexeder C, Behr J, et al. Reference values of impulse oscillometric lung function indices in adults of advanced age. PLoS One 2013;8:e63366. [Crossref] [PubMed]
- Rosenfeld M, Allen J, Arets BH, et al. An Official An official American Thoracic Society workshop report: optimal lung function tests for monitoring cystic fibrosis, bronchopulmonary dysplasia, and recurrent wheezing in children less than 6 years of age. Ann Am Thorac Soc 2013;10:S1-S11. [Crossref] [PubMed]
- Frantz S, Nihlén U, Dencker M, et al. Impulse oscillometry may be of value in detecting early manifestations of COPD. Respir Med 2012;106:1116-23. [Crossref] [PubMed]
- Tomalak W, Piorunek T, Gozdzik-Spychalska J, et al. Impulse oscillometry measurements in adult patients with CF: Focus on reactance. Eur Respir J 2015;46:Suppl 59.
- Sugiyama A, Hattori N, Haruta Y, et al. Characteristics of inspiratory and expiratory reactance in interstitial lung disease. Respir Med 2013;107:875-82. [Crossref] [PubMed]
- Fujii M, Shirai T, Mori K, et al. Inspiratory resonant frequency of forced oscillation technique as a predictor of the composite physiologic index in interstitial lung disease. Respir Physiol Neurobiol 2015;207:22-7. [Crossref] [PubMed]
- Brashier B, Salvi S. Measuring lung function using sound waves: role of the forced oscillation technique and impulse oscillometry system. Breathe (Sheff) 2015;11:57-65. [Crossref] [PubMed]
- Campbell JD, McQueen RB, Briggs A. The "e" in cost-effectiveness analyses. A case study of omalizumab efficacy and effectiveness for cost-effectiveness analysis evidence. Ann Am Thorac Soc 2014;11 Suppl 2:S105-11. [Crossref] [PubMed]
- Sweeney J, Patterson CC, Menzies-Gow A, et al. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax 2016;71:339-46. [Crossref] [PubMed]
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [Crossref] [PubMed]
- Roche N, Reddel H, Martin R, et al. Quality standards for real-world research. Focus on observational database studies of comparative effectiveness. Ann Am Thorac Soc 2014;11 Suppl 2:S99-104. [Crossref] [PubMed]
- Berger ML, Martin BC, Husereau D, et al. A questionnaire to assess the relevance and credibility of observational studies to inform health care decision making: an ISPOR-AMCP-NPC Good Practice Task Force report. Value Health 2014;17:143-56. [Crossref] [PubMed]
- GINA-GOLD Diagnosis of disease of chronic airflow limitation: Asthma, COPD and asthma-COPD overlap syndrome (ACOS), 2015. Available online: http://ginasthma.org
- Juniper EF, O'Byrne PM, Guyatt GH, et al. Development and validation of a questionnaire to measure asthma control. Eur Respir J 1999;14:902-7. [Crossref] [PubMed]
- José Soler-Cataluña J, Alcázar-Navarrete B, Miravitlles M. The concept of control in COPD: a new proposal for optimising therapy. Eur Respir J 2014;44:1072-5. [Crossref] [PubMed]
- Martin RJ, Price D, Roche N, et al. Cost-effectiveness of initiating extrafine- or standard size-particle inhaled corticosteroid for asthma in two health-care systems: a retrospective matched cohort study. NPJ Prim Care Respir Med 2014;24:14081. [PubMed]
- Postma DS, Roche N, Colice G, et al. Comparing the effectiveness of small-particle versus large-particle inhaled corticosteroid in COPD. Int J Chron Obstruct Pulmon Dis 2014;9:1163-86. [PubMed]
- Roche N, Postma DS, Colice G, et al. Differential effects of inhaled corticosteroids in smokers/ex-smokers and nonsmokers with asthma. Am J Respir Crit Care Med 2015;191:960-4. [Crossref] [PubMed]
- van Aalderen WM, Grigg J, Guilbert TW, et al. Small-particle Inhaled Corticosteroid as First-line or Step-up Controller Therapy in Childhood Asthma. J Allergy Clin Immunol Pract 2015;3:721-31.e16. [Crossref] [PubMed]
- Suarez E, Fang S, Abraham J, et al. Effect of inhaled corticosteroid particle size on asthma efficacy and safety outcomes: a systematic literature review. Thorax 2014;69:A182. [Crossref]
- Richeldi L. Idiopathic pulmonary fibrosis: moving forward. BMC Med 2015;13:231. [Crossref] [PubMed]
- Raghu G, Rochwerg B, Zhang Y, et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med 2015;192:e3-19. [Crossref] [PubMed]
- Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2071-82. [Crossref] [PubMed]
- King TE Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2083-92. [Crossref] [PubMed]


