
Comparisons of short-term outcomes between robot-assisted, video-assisted, and open esophagectomy for resectable esophageal cancer after neoadjuvant treatment: a retrospective study
Highlight box
Key findings
• The difference between robot-assisted esophagectomy (RAE) and video-assisted minimally invasive esophagectomy for neoadjuvant esophagectomy is not significant, and open esophagectomy has a longer postoperative hospital stay compared to them.
What is known and what is new?
• Most reported clinical studies have focused on comparing two of these three types of surgery for esophageal cancer until now.
• Our study rarely compared three surgical methods and included cases of neoadjuvant immunotherapy combined with chemotherapy for treatment in recent years.
What is the implication, and what should change now?
• The advantages of RAE among the two minimally invasive surgical methods are not clear and require further clinical research to verify.
Introduction
Esophageal cancer (EC) ranks seventh in the incidence of tumors and sixth in tumor-associated mortality worldwide according to the “Global Cancer Statistics 2022” (1). EC is composed of esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC), with the former more common in Asia and the latter more common in Europe and the United States (2). Despite significant progress in the diagnosis and treatment, the 5-year overall survival (OS) rate for EC ranges only from 20% to 35% (3). Currently, the standard treatment for patients with locally advanced EC is neoadjuvant treatment followed by surgical resection with radical lymphadenectomy (4).
So far, surgical approaches include open esophagectomy (OE), video-assisted minimally invasive esophagectomy (VAMIE), and robot-assisted esophagectomy (RAE). OE was initially the standard surgical approach (5); however, the incidence of cardiopulmonary complications is high (6). VAMIE was designed to ameliorate the postoperative outcomes, thereby lowering morbidity and mortality rates (7,8). Compared with OE, VAMIE has been associated with fewer postoperative complications, shorter intensive care unit (ICU) and hospital stay, reduced postoperative pain, and better quality of life with comparable oncologic results in randomized clinical trials (9,10). Therefore, VAMIE has gradually become the preferred surgical approach globally. However, it may not be adequate for delicate dissections of the esophagus and lymph nodes particularly in surgery after neoadjuvant treatment or salvage surgery following definitive chemoradiotherapy due to its two-dimensional vision and decreased degrees of motion (11). In 2003, RAE was initiated to solve these limitations of VAMIE with a steady three-dimensional enlarged vision and articulated instruments which can realize accurate dissection via seven degrees of freedom of motion (12,13). With the recent developments in technology, the application of RAE in the surgical treatment of EC has exhibited an obvious upward trend worldwide. In terms of oncological outcomes, previous studies have shown that RAE was at least comparable to OE and VAMIE (14-16). The ROBOT trial and a meta-analysis comparing robot-assisted minimally invasive thoraco-laparoscopic esophagectomy with OE revealed that RAE as the surgical approach to EC could result in lower postoperative complications and pain, better quality of life, and faster postoperative functional recovery (17,18). The RAMIE trial, a randomized trial comparing RAE with thoraco-laparoscopic esophagectomy, demonstrated that RAE could improve lymph node dissection in patients undergoing neoadjuvant therapy (19). Several studies have also shown that RAE could reduce the risk of recurrent laryngeal nerve (RLN) injury during paratracheal lymph node dissection compared with VAMIE (11,16,20).
To the best of our knowledge, most reported clinical studies (15-17,19,20) have focused on comparing two of these three types of surgery for EC until now. However, there have been few comparisons between RAE, VAMIE, and OE for resectable EC after neoadjuvant treatment. Therefore, we aimed to compare short-term outcomes between RAE, VAMIE, and OE for resectable EC after neoadjuvant treatment. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-75/rc).
Methods
Patients
A retrospective study was performed at the Department of Thoracic Surgery, the First Affiliated Hospital, Zhejiang University School of Medicine. All patients underwent neoadjuvant treatment before surgery and were evaluated as suitable for surgical treatment after neoadjuvant therapy. The inclusion criteria of this study were as follows: (I) age over 18 and under 80 years; (II) histopathologically diagnosed EC by endoscopy; (III) neoadjuvant treatment for their newly diagnosed EC; (IV) Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1; and (V) ample organ function with sufficient cardiopulmonary function. We excluded patients with the following situations: (I) incomplete basic information and therapeutic information in our hospital; (II) other concomitant malignant tumors; and (III) distant metastases.
This study was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine (No. 2022 IIT-1165), and conformed with the Declaration of Helsinki (as revised in 2013) and Good Clinical Practice Guidelines. Written informed consent was provided by all patients to allow access to their electronic medical record information.
Surgical approaches
All surgeries are performed by the same surgical team, including McKeown and Ivor-Lewis esophagectomy. Prior to conducting this study, the surgical team had completed a large number of EC resection surgeries, including OE, VAMIE, and RAE, with over 100 cases of RAE completed. In the VAMIE group, we only included the cases that successfully underwent combined thoracoscopic and laparoscopic esophagectomy. In the RAE group, any cases with conversions from minimally invasive to open were not included. Whether to undergo minimally invasive surgery was determined by the surgeon based on the patient’s wishes and a comprehensive evaluation of the patient’s tumor condition. For proximal EC, the distance from the tumors margins to the resection margins should be larger than 2 cm in principle, and for middle and distal EC, the distance should be larger than 5 cm. All the cases should conduct intraoperative frozen section analysis of the recovery margins performed.
RAE
The patient was intubated with a double-lumen endotracheal tube under general anesthesia, with unilateral pulmonary ventilation during the procedure. In the thoracic part, the patient was placed in a left lateral decubitus position. We created three operation ports to introduce the robotic arm at the third intercostal space on the anterior axillary line, the fifth intercostal space on the middle axillary line, and the ninth intercostal space on the posterior median line respectively. The observation port was made at the seventh intercostal space on the posterior axillary line to introduce the camera. The assistant port was created at the seventh intercostal space on the middle axillary line (Figure 1A). In the abdominal part, the patient was placed in a supine position. If the study team found the abdominal cavity had obvious adhesion due to the neoadjuvant treatment, open surgery was adopted and the incision was created in the middle of the upper abdomen. In the cervical part, the patient was placed in the supine position with head to the left. A 5 cm incision was made in the right neck.
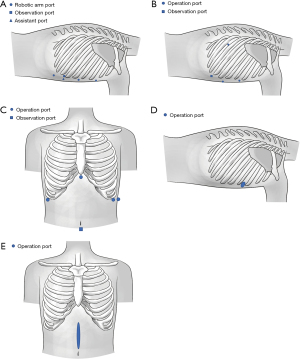
VAMIE
Under general anesthesia, the patient was intubated with a double-lumen endotracheal tube for split-lung ventilation during surgery. In the thoracic part, the patient was placed in a left lateral decubitus position. We created three operation ports at the fourth intercostal space on the middle axillary line, the sixth intercostal space on the anterior axillary line, and the ninth intercostal space on the subscapular line respectively. The observation port was made at the eighth intercostal space on the posterior axillary line (Figure 1B). In the abdominal part, the patient was placed in a supine position. A 1 cm incision was made below the umbilicus as the observation port to introduce the camera. We created four operation ports at the anterior axillary line under the right and left costal edge, the middle axillary line under the left costal edge, and the lower edge of the xiphoid respectively (Figure 1C).
In the cervical part, the patient was placed in a supine position. The incision was created along the anterior edge of the left sternocleidomastoid muscle.
OE
The patient was intubated with a double-lumen endotracheal tube for unilateral pulmonary ventilation during surgery. In the thoracic part, the patient was placed in a left lateral decubitus position. The incision was created at the posterolateral side of the fourth intercostal space (Figure 1D). In the abdominal part, the patient was placed in a supine position. The incision was made in the middle of the upper abdomen (Figure 1E). In the cervical part, the patient was put in a supine position. The incision was created along the anterior edge of the left sternocleidomastoid muscle.
Surgical methods included McKeown and Ivor-Lewis. A cervical or intrathoracic anastomosis was performed during the operation. All patients underwent two-field lymph node dissection (thoracic and abdominal lymph nodes). The procedure of McKeown esophagectomy was as follows. First, the thoracic esophagus with the surrounding lymph nodes was totally resected. The esophagus was severed at the top of the chest and above the esophageal hiatus. The paraesophageal, carina, paratracheal, and bilateral RLN lymph nodes were removed. Second, the tubular stomach was made by removing the lesser curvature of the stomach with a linear cutting suture device. Lymph nodes along the celiac trunk, the left gastric and splenic artery, and the lesser omentum were removed. Finally, anastomosis of the esophageal stump and tubular stomach was performed in the neck with the stapler. The procedure of Ivor-Lewis esophagectomy was as follows. First, the stomach was dissociated carefully and the tubular stomach was created with a linear cutting suture device. Abdominal lymph node dissection was performed. Second, the thoracic esophagus was resected en bloc with the surrounding thoracic lymph nodes. Intrathoracic anastomosis of the esophageal stump and tubular stomach was conducted above the arch of the azygos vein. All end-to-side anastomoses were performed with the same surgical technique using a 25 mm circular stapler.
Study procedures and endpoints
Before neoadjuvant therapy and surgery, systematic imaging evaluations were conducted, including computed tomography (CT) of the chest and abdomen, endoscopic ultrasound, positron emission tomography (PET)-CT, bone emission CT, and brain magnetic resonance imaging (MRI) for all patients. Before surgery, we performed the echocardiogram, electrocardiogram, and pulmonary function tests to evaluate the cardiopulmonary function. We acquired clinicopathological and follow-up data from patients through their regular examinations or therapeutic process in our hospital. Data included the baseline characteristics of patients [gender, age, body mass index (BMI), ECOG PS, smoking status, drinking status, hypertension, diabetes, histological type, pathological grade, regimen of neoadjuvant therapy, tumor location, surgical method, and clinical tumor-node-metastasis (cTNM)], pathological outcomes [post-neoadjuvant pathologic tumor-node-metastasis (ypTNM) and tumor regression grade (TRG)], intraoperative and postoperative outcomes (operation time, blood loss, ICU stay status, drainage duration, volume of drainage, R0 resection rate, lymph node dissection status, postoperative hospital stay, and total cost), and postoperative complications (pneumonia, pneumothorax, RLN injury, anastomotic leakage, chylothorax, thoracic empyema, wound infection, atrial fibrillation, postoperative bleeding and re-surgery). We adopted the eighth edition of the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging to determine the location of the dissected lymph nodes, tumor, degree of differentiation, cTNM, and ypTNM (21). Independent analyses were done by two investigators according to the pathological report and photographs so that we could acquire the pathological type, degree of differentiation, depth of invasion, resection margins, lymph nodes, and TRG. TRG was defined by calculating the estimated proportion of residual viable tumor cells in the original cancer area based on the College of American Pathologists (CAP)/the National Comprehensive Cancer Network (NCCN) guidelines: TRG 0 (no residual viable tumor cells), TRG 1 (remaining active tumor cells ≤10%), TRG 2 (>10% to ≤50% remaining viable tumor cells), and TRG 3 (>50% residual active tumor cells). Evaluations of postoperative complications were performed on the basis of definitions proposed by the Esophagectomy Complications Consensus Group (ECCG) (22). Follow-up ended 3 months after surgery. The main endpoints of interest were operation time, blood loss, number of dissected lymph nodes and patients in whom right/left RLN lymph nodes were removed (achievement rate), and total cost. Additional metrics of interest were the length of ICU stay, postoperative hospital stay, and postoperative complications.
Statistical analysis
Baseline data and perioperative outcomes were analyzed. The results were presented as mean ± standard deviation (SD) or median with ranges for continuous variables and numbers (percentages) for categorical variables. One-way analysis of variance (ANOVA) or Kruskal-Wallis test was used for three-way comparison of groups, depending on the normality of distribution. Pearson’s chi-squared test or Fisher’s exact test was applied for categorical parameters. Bonferroni correction was used for multiple testing. Statistical significance was defined at P<0.05. Statistical analyses were performed using SPSS software version 26.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism version 9.0 (GraphPad Software, San Diego, CA, USA).
Results
Characteristics at baseline
From January 2021 to August 2022, a total of 98 patients were included in our study: 31 patients underwent RAE, 31 underwent VAMIE, and 36 patients underwent OE. All patients received neoadjuvant treatment (including immunochemotherapy and chemoradiotherapy) before surgery. The flowchart of the study is reported in Figure 2. The baseline characteristics of these patients are listed in Table 1. No significant differences were detected among the three groups in any of the basic characteristics.
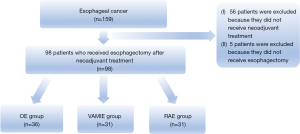
Table 1
| Variables | OE (n=36) | VAMIE (n=31) | RAE (n=31) | P value |
|---|---|---|---|---|
| Gender | 0.72 | |||
| Male | 32 (88.9) | 29 (93.5) | 29 (93.5) | |
| Female | 4 (11.1) | 2 (6.5) | 2 (6.5) | |
| BMI (kg/m2) | 21.7±2.9 | 22.1±3.1 | 21.9±3.1 | 0.90 |
| Age (years) | 65.0±7.4 | 61.8±8.3 | 62.9±9.3 | 0.29 |
| ECOG | 0.72 | |||
| PS 0 | 19 (52.8) | 14 (45.2) | 17 (54.8) | |
| PS 1 | 17 (47.2) | 17 (54.8) | 14 (45.2) | |
| Once smoking | 20 (55.6) | 18 (58.1) | 15 (48.4) | 0.73 |
| Once drinking | 17 (47.2) | 16 (51.6) | 12 (38.7) | 0.58 |
| Underlying conditions | ||||
| Hypertension | 11 (30.6) | 5 (16.1) | 9 (29.0) | 0.35 |
| Diabetes | 6 (16.7) | 2 (6.5) | 5 (16.1) | 0.40 |
| Type of carcinoma | 0.27 | |||
| Squamous cell carcinoma | 35 (97.2) | 31 (100.0) | 28 (90.3) | |
| Adenocarcinoma | 1 (2.8) | 0 (0.0) | 1 (3.2) | |
| Others | 0 (0.0) | 0 (0.0) | 2 (6.5) | |
| Pathological grade | 0.17 | |||
| G1 | 11 (30.6) | 4 (12.9) | 7 (22.6) | |
| G2 | 16 (44.4) | 19 (61.3) | 11 (35.5) | |
| G3 | 9 (25.0) | 8 (25.8) | 13 (41.9) | |
| Neoadjuvant treatment | 0.52 | |||
| Immunochemotherapy | 35 (97.2) | 31 (100.0) | 29 (93.5) | |
| Chemoradiotherapy | 1 (2.8) | 0 (0.0) | 2 (6.5) | |
| Tumor location | 0.30 | |||
| Proximal | 5 (13.9) | 8 (25.8) | 5 (16.1) | |
| Middle | 12 (33.3) | 9 (29.0) | 5 (16.1) | |
| Distal | 19 (52.8) | 14 (45.2) | 21 (67.7) | |
| Method | 0.32 | |||
| McKeown | 31 (86.1) | 30 (96.8) | 28 (90.3) | |
| Ivor-Lewis | 5 (13.9) | 1 (3.2) | 3 (9.7) | |
| cTNM stage | 0.15 | |||
| II | 4 (11.1) | 6 (19.4) | 4 (12.9) | |
| III | 18 (50.0) | 21 (67.7) | 21 (67.7) | |
| IVA | 14 (38.9) | 4 (12.9) | 6 (19.4) |
Data are presented as n (%) or mean ± SD. OE, open esophagectomy; VAMIE, video-assisted minimally invasive esophagectomy; RAE, robot-assisted esophagectomy; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; PS, performance status; cTNM, clinical tumor-node-metastasis; SD, standard deviation.
Pathological outcomes
After neoadjuvant therapy, we could see dramatic downstaging of T/N stage (concluded by comparing ypTNM with cTNM) among the three groups. The number of patients with stage IVA decreased and the number of patients with stage I increased. No significant differences were detected among the three groups in terms of ypTNM stage, downstaging of T/N stage, and pathological response (Table 2).
Table 2
| Variables | OE (n=36) | VAMIE (n=31) | RAE (n=31) | P value |
|---|---|---|---|---|
| ypTNM stage | 0.70 | |||
| I | 9 (25.0) | 9 (29.0) | 13 (41.9) | |
| II | 7 (19.4) | 7 (22.6) | 3 (9.7) | |
| III | 18 (50.0) | 13 (41.9) | 14 (45.2) | |
| IVA | 2 (5.6) | 2 (6.5) | 1 (3.2) | |
| Downstaging of T/N stage† | 26 (72.2) | 23 (74.2) | 25 (80.6) | 0.77 |
| Pathological response | 0.29 | |||
| TRG 0 | 3 (8.3) | 2 (6.5) | 5 (16.1) | |
| TRG 1 | 2 (5.6) | 1 (3.2) | 3 (9.7) | |
| TRG 2 | 20 (55.6) | 24 (77.4) | 15 (48.4) | |
| TRG 3 | 11 (30.6) | 4 (12.9) | 8 (25.8) |
Data are presented as n (%). †, determined by comparing ypTNM with cTNM within each operative group. OE, open esophagectomy; VAMIE, video-assisted minimally invasive esophagectomy; RAE, robot-assisted esophagectomy; ypTNM, post-neoadjuvant pathologic tumor-node-metastasis; TRG, tumor regression grade.
Intraoperative and postoperative outcomes
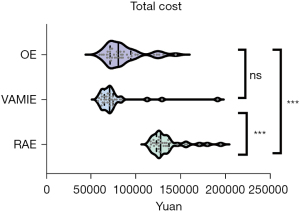
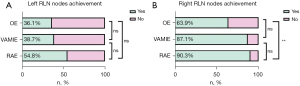
No significant differences were observed in the operation time, blood loss, length of ICU stay, and R0 resection between the three groups (Table 3). There was a significant difference in the total cost (RAE: ¥127,512, VAMIE: ¥70,742, OE: ¥80,513, P<0.001) (Figure 3). No significant differences were found in the total, thoracic, abdominal, right RLN, and left RLN dissected lymph node numbers between the three groups (P>0.05). There was no significant difference in the achievement rate of the left RLN lymph node (RAE: 54.8%, VAMIE: 38.7%, OE: 36.1%, P>0.05) (Figure 4A), but a significant difference was seen in the achievement rate of the right RLN lymph node (RAE: 90.3%, VAMIE: 87.1%, OE: 63.9%, P=0.01) (Figure 4B). The postoperative hospital stay of OE was significantly longer compared to RAE and VAMIE (P<0.05).
Table 3
| Variables | OE (n=36) | VAMIE (n=31) | RAE (n=31) | P value |
|---|---|---|---|---|
| Operating time (min) | 300.0±99.9 | 321.2±74.1 | 347.0±66.9 | 0.07 |
| Blood loss (mL) | 100 [50–500] | 100 [20–400] | 100 [20–500] | 0.11 |
| ICU stay | 11 (30.6) | 2 (6.5) | 6 (19.4) | 0.04† |
| Length of ICU stay (days) | 0 [0–7] | 0 [0–4] | 0 [0–8] | 0.05 |
| Drainage duration (days) | 11 [7–34] | 9 [6–38] | 10 [7–27] | 0.02† |
| Volume of drainage (mL) | 2,172 [680–11,370] | 1,350 [220–9,045] | 1,840 [210–10,605] | 0.02† |
| R0 resection | 35 (97.2) | 31 (100.0) | 29 (93.5) | 0.52 |
| Number of dissected lymph node | ||||
| Total number | 30 [11–51] | 28 [13–48] | 28 [14–48] | 0.74 |
| Thoracic | 15 [3–38] | 15 [5–29] | 14 [5–32] | 0.94 |
| Abdominal | 12 [0–31] | 12 [2–23] | 12 [3–25] | 0.92 |
| Right RLN nodes | 2 [0–9] | 3 [0–7] | 2 [0–8] | 0.41 |
| Left RLN nodes | 0 [0–10] | 0 [0–8] | 2 [0–8] | 0.15 |
| Achievement rate¶ | ||||
| Right RLN nodes | 23 (63.9) | 27 (87.1) | 28 (90.3) | 0.01‡ |
| Left RLN nodes | 13 (36.1) | 12 (38.7) | 17 (54.8) | 0.26 |
| Postoperative hospital stay (days) | 13 [9–26] | 11 [9–55] | 12 [8–28] | 0.01† |
| Total cost (¥) | 80,513 [59,585–144,311] |
70,742 [57,761–191,062] |
127,512 [115,833–194,555] |
<0.001‡,§ |
Data are presented as mean ± SD, median [IQR], or n (%). †, represents a significant statistical difference between OE group with VAMIE group; ‡, represents a significant statistical difference between OE group with RAE group; §, represents a significant statistical difference between VAMIE with RAE group; ¶, refers to number of patients in whom right/left RLN lymph nodes are removed. OE, open esophagectomy; VAMIE, video-assisted minimally invasive esophagectomy; RAE, robot-assisted esophagectomy; ICU, intensive care unit; RLN, recurrent laryngeal nerve; SD, standard deviation; IQR, interquartile range.
Postoperative complications
No significant differences were seen between the three surgical approaches in terms of postoperative complications (RAE: 41.9%, VAMIE: 35.5%, OE: 30.6%, P=0.63) (Table 4). There was no anastomotic leakage, chylothorax, thoracic empyema, wound infection, postoperative bleeding, and revision surgery occurring in the RAE group. The postoperative pneumonia rate was higher in the VAMIE group (RAE: 16.1%, VAMIE: 19.4%, OE: 8.3%). The incidence of RLN injury was higher in OE (RAE: 3.2%, VAMIE: 3.2%, OE: 5.6%). The incidence of atrial fibrillation was comparable between the three groups. There were no postoperative deaths within 90 days among the three groups.
Table 4
| Variables | OE (n=36) | VAMIE (n=31) | RAE (n=31) | P value |
|---|---|---|---|---|
| Any complication in 90 days | 11 (30.6) | 11 (35.5) | 13 (41.9) | 0.63 |
| Pneumonia | 3 (8.3) | 6 (19.4) | 5 (16.1) | 0.44 |
| Pneumothorax | 4 (11.1) | 3 (9.7) | 4 (12.9) | >0.99 |
| RLN injury | 2 (5.6) | 1 (3.2) | 1 (3.2) | >0.99 |
| Anastomotic stenosis | 1 (2.8) | 2 (6.5) | 1 (3.2) | 0.83 |
| Anastomotic leakage | 1 (2.8) | 1 (3.2) | 0 (0.0) | >0.99 |
| Chylothorax | 1 (2.8) | 0 (0.0) | 0 (0.0) | >0.99 |
| Thoracic empyema | 1 (2.8) | 1 (3.2) | 0 (0.0) | >0.99 |
| Wound infection | 2 (5.6) | 0 (0.0) | 0 (0.0) | 0.33 |
| Atrial fibrillation | 2 (5.6) | 2 (6.5) | 2 (6.5) | >0.99 |
| Postoperative bleeding | 2 (5.6) | 0 (0.0) | 0 (0.0) | 0.33 |
| Re-surgery | 2 (5.6) | 1 (3.2) | 0 (0.0) | 0.77 |
Data are presented as n (%). OE, open esophagectomy; VAMIE, video-assisted minimally invasive esophagectomy; RAE, robot-assisted esophagectomy; RLN, recurrent laryngeal nerve.
Discussion
In recent years, minimally invasive surgery, including VAMIE and RAE, is gradually replacing OE in the management of EC with reduced postoperative complications and fast recovery. However, there have been few comparisons between these three surgical approaches for resectable EC after neoadjuvant treatment and this study compared short-term outcomes in this setting.
Currently, the main management for patients with locally advanced EC is still neoadjuvant treatment followed by surgical resection with radical lymphadenectomy. Neoadjuvant therapy (including immunochemotherapy and chemoradiotherapy) has been shown to improve survival in patients with locally advanced EC (23-26). After neoadjuvant treatment, the EC may shrink and even be downstaged, enabling radical tumor resection that would otherwise not be possible with upfront surgery. In the meta-analysis by He et al., the authors noted that neoadjuvant chemoimmunotherapy was associated with both lower pCR and treatment-related grade 3–4 adverse events than neoadjuvant immunotherapy with chemoradiation (26). They noted that neoadjuvant chemoimmunotherapy pCR rates appeared higher than historic ones with neoadjuvant chemotherapy alone, but lower than historic pCR rates with chemoradiation. In the present study, we included 98 consecutive patients with EC who met the inclusion criteria including having a clinical stage in the range T1–4N0–3M0. All of these enrolled patients received neoadjuvant treatment before surgery and were evaluated as capable of surgical treatment after neoadjuvant therapy. In this study, the vast majority received neoadjuvant chemoimmunotherapy, with only 3% receiving chemoradiation. We determined the number of patients with stage IVA decreased and the number of patients with stage I increased after neoadjuvant therapy. Further, the R0 resection rate of these three methods was all over 90%, which ensured the safety and integrity of tumor resection. Several studies have reported that neoadjuvant therapy seemed to have no impact on surgery (27,28). In our study, we adopted open surgery in the abdominal part of RAE because the abdominal cavity had obvious adhesion due to the neoadjuvant treatment. RAE was only performed in the thoracic part, which was different from other studies (11,29). This may produce an effect on the intraoperative and postoperative outcomes of RAE.
Lymphadenectomy has a significant role in the management of EC, which can have an effect on short- and long-term survival (30). Currently, the consensus on the extent of lymphadenectomy is not uniform worldwide. In the present study, all patients underwent two-field lymph node dissection (thoracic and abdominal lymph nodes). The ROBOT trial revealed that the total dissected lymph node number in the OE and RAE groups was 25 and 27, respectively (P=0.41) (17). Chen et al. reported no significant difference in the total lymph node number between VAMIE and RAE (VAMIE: 24.7±11.2, RAE: 25.4±7.5) (11). Gong et al. showed that there were no significant differences in the OE, VAMIE, and RAMIE groups (OE: 24.1, VAMIE: 23.1, RAE: 22.8, P=0.68) with regard to the total lymph node number (29). These findings were in accordance with our study. In our study, the total dissected lymph node number in the OE, VAMIE, and RAE group was 30, 28, and 28, respectively (P=0.74). Bilateral RLN lymphadenectomy is considered an essential step because of its high frequency of metastasis (31). However, due to the narrow surgical space, it is technically challenging. The robot-assisted technique solves this problem, which enables operation in a tight space and more delicate dissections of the lymph nodes via its three-dimensional vision and flexible instruments (32). Chao et al. showed that the total number of lymph nodes dissected from the left RLN was greater in RAE compared with VAMIE (P=0.001) (33). Deng et al. revealed a higher lymph node yield along the right RLN (P=0.03) (34). In the present study, dissected lymph node number in the right RLN and left RLN was comparable between the three groups and no significant differences were found (P>0.05). This may be due to our limited experience in robotic surgery for EC. We compared the achievement rate of dissection along the RLN lymph node area between these three groups. Gong et al. found that the achievement rate of the right RLN area was comparable in these three groups, but the achievement rate along the left RLN area was significantly high in RAE (OE: 37.7%, VAMIE: 52.1%, RAMIE: 59.3%, P=0.02) (29). This was different from our findings. There was no significant difference in the achievement rate of the left RLN lymph node (RAE: 54.8%, VAMIE: 38.7%, OE: 36.1%, P>0.05), but a significant difference was revealed in the achievement rate of the right RLN lymph node (P=0.01) and this variable was higher in RAE than the other two groups (RAE: 90.3%, VAMIE: 87.1%, OE: 63.9%). Given the complete bilateral RLN lymph nodes dissection by RAE, there may be more risks of RLN injury. We often pay close attention to whether there is RLN injury after surgery. Several studies have reported that the postoperative RLN injury rate ranged from 11.5% to 27.0%, 14.3% to 28.8%, and 9.5% to 21.6% in the OE, VAMIE, and RAE groups, respectively (7,10). The incidence of RLN injury was lower in our study (RAE: 3.2%, VAMIE: 3.2%, OE: 5.6%).
The existing problems of robotic surgery may be the long operation time and the high cost. Several studies have shown that the operation time and cost of RAE were higher than those of VAMIE and OE (11,17). In our study, the operation time of RAE was longer than that of the other two groups (RAE: 347.0±66.9 min, VAMIE: 321.2±74.1 min, OE: 300.0±99.9 min, P>0.05). There was a significant difference in the total cost (RAE: ¥127,512, VAMIE: ¥70,742, OE: ¥80,513, P<0.001). The cause of the long operation time may be due to our limited experience in robotic surgery for EC. We believe that the operation time can be reduced as we become increasingly skilled in robotic surgery, and robotic technology develops rapidly. The reason for the high cost is that expenses of RAE cannot be reimbursed through insurance. We believe that the cost can also be reduced as the relevant insurance covers this area and robotic instruments achieve a rapid expansion. Due to the long operation time, there may be a risk of increasing postoperative respiratory complications. However, there were no significant differences in respiratory complications between the three methods in the present study. Further, there was no significant increment in the risk of anastomotic leakage, chylothorax, thoracic empyema, atrial fibrillation, and other postoperative complications. Therefore, RAE is a feasible and secure method for resectable EC after neoadjuvant therapy with comparable postoperative complications.
Some limitations of our study are listed below. First, our experience in robotic surgery for EC is limited and this may affect the associated outcomes of RAE. Second, our study was a retrospective study. The sample size was small which may have limited the statistical power of our study and resulted in selection biases. In order to eliminate selection bias to some extent and render the results representative, we consecutively included patients who satisfied the inclusion criteria. Of importance is that the results are most relevant for patients receiving neoadjuvant chemoimmunotherapy given that only 3% received neoadjuvant chemoradiation, and it is possible that both complication rates as well as downstaging could have been further increased if more neoadjuvant radiation was used. Finally, the postoperative follow-up period was short in the study. There were no long-term and oncologic outcomes recorded.
Conclusions
In conclusion, no clear overall benefit exists for RAE in the treatment of resectable EC after neoadjuvant therapy, compared with VAMIE. OE resulted in a longer hospital stay. Although the rate of achieving right RLN node retrieval was higher after RAE, the clinical relevance for this is yet unclear. Further efforts are required to reduce the total cost of RAE. Moreover, our findings need to be validated through larger scaled randomized controlled trials. Extended follow-up is needed to evaluate long-term and oncologic outcomes.
Acknowledgments
Funding: This research was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-75/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-75/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-75/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-75/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was permitted by the Clinical Research Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine (No. 2022 IIT-1165), and was in line with the Declaration of Helsinki (as revised in 2013) and Good Clinical Practice Guidelines. Written informed consent was provided by enrolled patients for us to access to their electronic medical record information.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015;64:381-7. [Crossref] [PubMed]
- Kelly RJ. Emerging Multimodality Approaches to Treat Localized Esophageal Cancer. J Natl Compr Canc Netw 2019;17:1009-14. [Crossref] [PubMed]
- Gisbertz SS, Hagens ERC, Ruurda JP, et al. The evolution of surgical approach for esophageal cancer. Ann N Y Acad Sci 2018;1434:149-55. [Crossref] [PubMed]
- Boone J, Livestro DP, Elias SG, et al. International survey on esophageal cancer: part I surgical techniques. Dis Esophagus 2009;22:195-202. [Crossref] [PubMed]
- Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662-9. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Straatman J, van der Wielen N, Cuesta MA, et al. Minimally Invasive Versus Open Esophageal Resection: Three-year Follow-up of the Previously Reported Randomized Controlled Trial: the TIME Trial. Ann Surg 2017;266:232-6. [Crossref] [PubMed]
- Maas KW, Cuesta MA, van Berge Henegouwen MI, et al. Quality of Life and Late Complications After Minimally Invasive Compared to Open Esophagectomy: Results of a Randomized Trial. World J Surg 2015;39:1986-93. [Crossref] [PubMed]
- Yibulayin W, Abulizi S, Lv H, et al. Minimally invasive oesophagectomy versus open esophagectomy for resectable esophageal cancer: a meta-analysis. World J Surg Oncol 2016;14:304. [Crossref] [PubMed]
- Chen J, Liu Q, Zhang X, et al. Comparisons of short-term outcomes between robot-assisted and thoraco-laparoscopic esophagectomy with extended two-field lymph node dissection for resectable thoracic esophageal squamous cell carcinoma. J Thorac Dis 2019;11:3874-80. [Crossref] [PubMed]
- van Hillegersberg R, Boone J, Draaisma WA, et al. First experience with robot-assisted thoracoscopic esophagolymphadenectomy for esophageal cancer. Surg Endosc 2006;20:1435-9. [Crossref] [PubMed]
- Boone J, Schipper ME, Moojen WA, et al. Robot-assisted thoracoscopic oesophagectomy for cancer. Br J Surg 2009;96:878-86. [Crossref] [PubMed]
- van der Sluis PC, Ruurda JP, Verhage RJ, et al. Oncologic Long-Term Results of Robot-Assisted Minimally Invasive Thoraco-Laparoscopic Esophagectomy with Two-Field Lymphadenectomy for Esophageal Cancer. Ann Surg Oncol 2015;22:S1350-6. [Crossref] [PubMed]
- de Groot EM, van der Horst S, Kingma BF, et al. Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open esophagectomy: long-term follow-up of a randomized clinical trial. Dis Esophagus 2020;33:doaa079. [Crossref] [PubMed]
- Jin D, Yao L, Yu J, et al. Robotic-assisted minimally invasive esophagectomy versus the conventional minimally invasive one: A meta-analysis and systematic review. Int J Med Robot 2019;15:e1988. [Crossref] [PubMed]
- van der Sluis PC, van der Horst S, May AM, et al. Robot-assisted Minimally Invasive Thoracolaparoscopic Esophagectomy Versus Open Transthoracic Esophagectomy for Resectable Esophageal Cancer: A Randomized Controlled Trial. Ann Surg 2019;269:621-30. [Crossref] [PubMed]
- Esagian SM, Ziogas IA, Skarentzos K, et al. Robot-Assisted Minimally Invasive Esophagectomy versus Open Esophagectomy for Esophageal Cancer: A Systematic Review and Meta-Analysis. Cancers (Basel) 2022;14:3177. [Crossref] [PubMed]
- Yang Y, Li B, Yi J, et al. Robot-assisted Versus Conventional Minimally Invasive Esophagectomy for Resectable Esophageal Squamous Cell Carcinoma: Early Results of a Multicenter Randomized Controlled Trial: the RAMIE Trial. Ann Surg 2022;275:646-53.
- Tagkalos E, Goense L, Hoppe-Lotichius M, et al. Robot-assisted minimally invasive esophagectomy (RAMIE) compared to conventional minimally invasive esophagectomy (MIE) for esophageal cancer: a propensity-matched analysis. Dis Esophagus 2020;33:doz060. [Crossref] [PubMed]
- Rice TW, Ishwaran H, Ferguson MK, et al. Cancer of the Esophagus and Esophagogastric Junction: An Eighth Edition Staging Primer. J Thorac Oncol 2017;12:36-42.
- Low DE, Alderson D, Cecconello I, et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286-94. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Yang H, Liu H, Chen Y, et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol 2018;36:2796-803. [Crossref] [PubMed]
- Xu J, Yan C, Li Z, et al. Efficacy and Safety of Neoadjuvant Chemoimmunotherapy in Resectable Esophageal Squamous Cell Carcinoma: A Meta-analysis. Ann Surg Oncol 2023;30:1597-613. [Crossref] [PubMed]
- He W, Wang C, Li C, et al. The efficacy and safety of neoadjuvant immunotherapy in resectable locally advanced esophageal squamous cell carcinoma: A systematic review and meta-analysis. Front Immunol 2023;14:1118902. [Crossref] [PubMed]
- Ma S, Yan T, Liu D, et al. Neoadjuvant chemotherapy followed by minimally invasive esophagectomy is safe and feasible for treatment of esophageal squamous cell carcinoma. Thorac Cancer 2018;9:310-5. [Crossref] [PubMed]
- Goel A, Shah SH, Selvakumar VPP, et al. Robot-Assisted Mckeown Esophagectomy is Feasible After Neoadjuvant Chemoradiation. Our Initial Experience. Indian J Surg 2018;80:24-9. [Crossref] [PubMed]
- Gong L, Jiang H, Yue J, et al. Comparison of the short-term outcomes of robot-assisted minimally invasive, video-assisted minimally invasive, and open esophagectomy. J Thorac Dis 2020;12:916-24. [Crossref] [PubMed]
- Hagens ERC, van Berge Henegouwen MI, Cuesta MA, et al. The extent of lymphadenectomy in esophageal resection for cancer should be standardized. J Thorac Dis 2017;9:S713-23. [Crossref] [PubMed]
- Tachimori Y, Nagai Y, Kanamori N, et al. Pattern of lymph node metastases of esophageal squamous cell carcinoma based on the anatomical lymphatic drainage system. Dis Esophagus 2011;24:33-8. [Crossref] [PubMed]
- Kim DJ, Park SY, Lee S, et al. Feasibility of a robot-assisted thoracoscopic lymphadenectomy along the recurrent laryngeal nerves in radical esophagectomy for esophageal squamous carcinoma. Surg Endosc 2014;28:1866-73. [Crossref] [PubMed]
- Chao YK, Hsieh MJ, Liu YH, et al. Lymph Node Evaluation in Robot-Assisted Versus Video-Assisted Thoracoscopic Esophagectomy for Esophageal Squamous Cell Carcinoma: A Propensity-Matched Analysis. World J Surg 2018;42:590-8.
- Deng HY, Huang WX, Li G, et al. Comparison of short-term outcomes between robot-assisted minimally invasive esophagectomy and video-assisted minimally invasive esophagectomy in treating middle thoracic esophageal cancer. Dis Esophagus 2018; [Crossref]


