
Ectopic mediastinal thyroid tissue: an indelible imitator
Introduction
Forming during the third and fourth weeks of gestation, the thyroid is the first endocrine organ to develop within the human body. The thyroid primordium arises as an invagination of endodermal tissue near the base of the tongue. From there, the newly formed thyroid diverticulum descends caudally via the thyroglossal tract, which atrophies following migration of the formed gland to its normal anatomic position anterior to the trachea. Two types of endocrine cells comprise the thyroid parenchyma: follicular cells, which secrete triiodothyronine (T3) and thyroxine (T4), and calcitonin-producing parafollicular cells, also called C cells. Occasionally, abnormal migration of the thyroid gland results in seeding of thyroid tissue in sites other than the organ’s normal pretracheal position, forming ectopic thyroid tissue (ETT). Ninety percent of the time, ETT develops in the midline, somewhere along the path of its primordial descent. In rare cases, ETT can be found elsewhere, such as the heart, diaphragm, esophagus, or mediastinum. Mediastinal ETT is possibly formed from remnants of thyroid tissue that are pulled along with the heart and great vessels into the chest as the embryo transforms (1,2). Mediastinal ETT comprises less than one percent of reported ETT cases, of which most were located within the anterior mediastinum, with rare reports of posterior localization (3). Mediastinal ETT has no connection to the cervical thyroid and possesses its own intrathoracic vascular supply. Less than one percent of all mediastinal tumors are ETT (4-8).
Autopsy studies estimate the prevalence of ETT to be between seven and ten percent, the highest in patients with existing thyroid pathology (8). ETT has a female predominance and can be discovered at any age (2). The risk of developing malignancy from ETT is estimated to be less than one percent (9-11). Santangelo and colleagues (2) found ETT in 28 out of 3,092 specimens (0.9%) of patients who underwent thyroidectomy between 2000 and 2013. Among these, one case of ETT (3.5%) was mediastinal and showed papillary thyroid carcinoma.
While the mechanisms underlying abnormal thyroid migration are not fully understood, multiple genes may be involved. Recent genetic research shows that the gene transcription factors TTF-1 (Nkx2-1), FOXE1 (TTF-2), NKX2-5 and PAX-8 are essential for thyroid development and differentiation, suggesting that mutation in one or more of these genes could increase susceptibility to thyroid dysgenesis (6,12). In studies, mice with homozygous FOXE1 mutations developed lingual thyroid, although this mutation has not been described in humans. Polymorphisms in the length of the polyalanine tract of FOXE1 gene may contribute to thyroid dysgenesis (4,12-14).
Patients with ETT typically do not exhibit symptoms (47%), although local signs related to the upper airway, including dysphagia, dry cough, dyspnea, hemoptysis, and obstruction, are possible (6). ETT can also present with uncontrolled hyperthyroidism or harbor malignancy, either of which may warrant operative intervention. Hypothyroidism was reported up to one-third of ETT cases, although intrathoracic ETT cases are usually euthyroid (1). Thyroid function tests should be performed as part of the workup.
Diagnosis
Imaging
Scintigraphy using technetium-99m pertechnetate, iodine-123, or iodine-131 is the gold standard for detecting ETT size, location, and activity; however, pathologic uptake can occur in other conditions including meningiomas, dacryocystitis, sinusitis, ocular prostheses, and dental disease (1) and thus is not specific for ETT. Conversely, lack of iodine uptake does not exclude the presence of ectopic tissue that may be necrotic or malignant. On computed tomography (CT), ETT demonstrates increased attenuation without contrast due to its high iodine content and is markedly enhanced with contrast. On magnetic resonance imaging, ETT can appear mildly hyperintense compared to skeletal muscle on both T1- and T2-weighted images. On fluorine-18 fluorodeoxyglucose positron emission tomography (F18-FDG PET), normal thyroid tissue demonstrates low uptake while hypermetabolic conditions such as carcinoma and nodular hyperplasia show local or diffuse uptake. The heterogeneity of findings on PET/CT makes diagnosing ETT a challenge. Nevertheless, ETT should be considered as a differential diagnosis in patients with clinically apparent thyroid disease (1,15-19).
Pathology
Fine needle aspiration (FNA) is frequently used to characterize an unknown lesion, but ETT diagnosed with FNA is exceedingly rare (11,20-22). FNA should be used preoperatively to exclude thyroid carcinoma before diagnosing ETT. However, FNA of ETT could be a potential diagnostic pitfall due to limited sampling (21). Hu et al. reported a case of papillary thyroid carcinoma of a mediastinal ETT diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) (11). In a case, FNA of a submandibular mass revealed a papillary thyroid lesion; however, ETT with focal chronic thyroiditis was demonstrated in excision specimen evaluation (21). There are also reports describing neck malignancies initially diagnosed via FNA as benign ETT that later proved to be papillary thyroid microcarcinomas (10). Malignant transformation of benign ETT has rarely been reported (23). Ultimately, most cases of ETT are diagnosed via histopathologic examination following surgical resection.
ETT lesions are typically well-demarcated and well-circumscribed, especially when benign. Goiters often have a nodular, semi-translucent and tan-brown cut surface, which can demonstrate areas of fibrosis, hemorrhage, and cystic degeneration. In contrast, malignant ETT tumors show more infiltrative growth as well as areas of hemorrhage and necrosis.
The cytologic, histopathologic, and molecular features of ETT lesions in the mediastinum closely resemble those of the thyroid gland proper. Goiter and papillary thyroid carcinoma are the most common benign and malignant ETT lesions, respectively. However, medullary carcinoma and anaplastic thyroid carcinomas have been described in literature (Figure 1) (4,24,25). Immunohistochemically, thyroid tumors of follicular origin are positive for pancytokeratin, CK7, thyroglobulin, TTF1, and PAX8, whereas medullary thyroid carcinomas are positive for neuroendocrine markers (CD56, synaptophysin, chromogranin A and INSM1) and calcitonin and variably positive for TTF1 and PAX8.
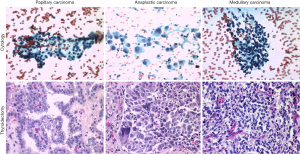
In addition to ETT, other differential diagnoses for mediastinal masses include enlarged lymph nodes, germ cell tumors, neurogenic tumors, lymphomas, thymic lesions, and mesenchymal tumors. In patients being worked up for staging of a malignant tumor, a mediastinal ETT may be mistaken for metastasis involving a lymph node (5). Further studies, such as scintigraphy using technetium-99m or tissue sampling for pathologic examination with appropriate immunohistochemical studies, would be helpful. Malignant ETT tumors may be difficult to differentiate from primary thymic carcinomas, especially neuroendocrine neoplasms, due to somewhat similar histopathological and immunohistochemical features.
Treatment
Due to its rarity and variable clinical course, the optimal strategy to manage mediastinal ETT is not standardized (25). When mediastinal ETT is euthyroid and asymptomatic (as it often is), regular follow-up is recommended to detect any interval changes. Definitive treatment of symptomatic lesions is surgical excision with histopathological assessment. However, in 70% to 90% of cases, the ETT is the only functioning thyroid tissue in the body (4,6), an important consideration when evaluating the appropriateness of surgical resection. Most authors agree that the decision to treat ETT surgically should take into consideration factors such as patient age and thyroid function status, as well as features of the ETT mass such as size, location, risk of malignant transformation, and the presence of complications such as ulceration, compressive symptoms, and bleeding. When appropriate, surgical treatment options for symptomatic mediastinal ETT includes thoracotomy, sternotomy, and uniportal video-assisted thoracoscopic surgery (U-VATS). Minimally invasive approaches such as radioiodine ablation can be implemented in recurrent cases (2-4,8,9).
Conclusions
While rare, mediastinal ETT needs to be kept in mind for the differential diagnosis of a mediastinal mass. Recognition of an intrathoracic ETT is imperative for appropriate clinical management. Diagnostic imaging and thyroid function should be evaluated preoperatively if ETT is suspected. FNA, augmented by immunohistochemical and molecular workup as needed, is commonly used for initial evaluation of a suspected ETT, although low cellularity and/or suboptimal specimens could pose a diagnostic challenge. When indicated, the definitive diagnosis of ETT is a surgical excision with thorough pathologic examination and ancillary studies. However, the presence of other functioning thyroid tissue must be ascertained preoperatively via imaging and thyroid function tests to ensure patients remain euthyroid.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Thoracic Disease. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-200/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siddique M, Bashir H. 99mTc Sodium Pertechnetate Uptake in Ectopic Mediastinal Thyroid Tissue on Hybrid Thyroid Scintigraphy. Clin Nucl Med 2018;43:820-2. [Crossref] [PubMed]
- Santangelo G, Pellino G, De Falco N, et al. Prevalence, diagnosis and management of ectopic thyroid glands. Int J Surg 2016;28:S1-6. [Crossref] [PubMed]
- Motlaghzadeh Y, Nesbit S, Guo HH, et al. Surgical resection of mediastinal ectopic thyroid tissue: a case series. J Thorac Dis 2023;15:1473-81. [Crossref] [PubMed]
- Noussios G, Anagnostis P, Goulis DG, et al. Ectopic thyroid tissue: anatomical, clinical, and surgical implications of a rare entity. Eur J Endocrinol 2011;165:375-82. [Crossref] [PubMed]
- Tsai A, Rafferty W, Ren S. Mediastinal ectopic thyroid tissue, an imitator of an enlarged lymph node with metastatic pulmonary neoplasia. Diagn Cytopathol 2021;49:E471-4. [Crossref] [PubMed]
- Ibrahim NA, Fadeyibi IO. Ectopic thyroid: etiology, pathology and management. Hormones (Athens) 2011;10:261-9. [Crossref] [PubMed]
- Uludag M, Isgor A, Yetkin G, et al. Ectopic mediastinal thyroid tissue: cervical or mediastinum originated? BMJ Case Rep 2009;2009:bcr09.2008.1004.
- El Haj NI, Hafidi S, Khoaja A, et al. Ectopic mediastinal thyroid removed by U-VATS approach. A case report. Int J Surg Case Rep 2021;78:284-7. [Crossref] [PubMed]
- Shah BC, Ravichand CS, Juluri S, et al. Ectopic thyroid cancer. Ann Thorac Cardiovasc Surg 2007;13:122-4.
- Borges A, Martins M, André S. Double thyroid ectopia (with incidental papillary thyroid microcarcinoma) (2010: 8b). Eur Radiol 2010;20:2768-71. [Crossref] [PubMed]
- Hu J, Li M, Xu L. Ectopic thyroid cancer diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration. Thorac Cancer 2017;8:703-5. [Crossref] [PubMed]
- Montanelli L, Tonacchera M. Genetics and phenomics of hypothyroidism and thyroid dys- and agenesis due to PAX8 and TTF1 mutations. Mol Cell Endocrinol 2010;322:64-71. [Crossref] [PubMed]
- Pimentel CP, Cortinhas-Alves EA, de Oliveira EHC, et al. Does the Polymorphism in the Length of the Polyalanine Tract of FOXE1 Gene Influence the Risk of Thyroid Dysgenesis Occurrence? J Thyroid Res 2017;2017:2793205. [Crossref] [PubMed]
- Hermanns P, Grasberger H, Refetoff S, et al. Mutations in the NKX2.5 gene and the PAX8 promoter in a girl with thyroid dysgenesis. J Clin Endocrinol Metab 2011;96:E977-81. [Crossref] [PubMed]
- Hammond RJ, Meakin K, Davies JE. Case report: lateral thyroid ectopia--CT and MRI findings. Br J Radiol 1996;69:1178-80. [Crossref] [PubMed]
- Hummel J, Wachsmann J, Carrick K, et al. Ectopic Thyroid Tissue in the Mediastinum Characterized by Histology and Functional Imaging with I-123 SPECT/CT. Case Rep Radiol 2017;2017:9084207. [Crossref] [PubMed]
- Zander DA, Smoker WR. Imaging of ectopic thyroid tissue and thyroglossal duct cysts. Radiographics 2014;34:37-50. [Crossref] [PubMed]
- Juanpere S, Cañete N, Ortuño P, et al. A diagnostic approach to the mediastinal masses. Insights Imaging 2013;4:29-52. [Crossref] [PubMed]
- Oh JR, Ahn BC. False-positive uptake on radioiodine whole-body scintigraphy: physiologic and pathologic variants unrelated to thyroid cancer. Am J Nucl Med Mol Imaging 2012;2:362-85.
- Saleh HA, Hammoud J, Shah MB. Aspiration biopsy cytology of ectopic thyroid tissue in the lateral chest wall: a case report. Acta Cytol 2009;53:313-5. [Crossref] [PubMed]
- Paksoy N. Ectopic lesions as potential pitfalls in fine needle aspiration cytology: a report of 3 cases derived from the thyroid, endometrium and breast. Acta Cytol 2007;51:222-6. [Crossref] [PubMed]
- Ozturk A, Cicek T, Aktas Z, et al. Mediastinal ectopic thyroid diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration: Report of three cases. J Clin Ultrasound 2018;46:299-301. [Crossref] [PubMed]
- Vuorisalo A, Tommola E, Eloranta P, et al. Ectopic thyroid in EBUS: experience from a quality assurance programme. APMIS 2023;131:217-25. [Crossref] [PubMed]
- Nguyen D, Htun NN, Wang B, et al. An Anaplastic Thyroid Carcinoma of the Giant-Cell Type from a Mediastinal Ectopic Thyroid Gland. Diagnostics (Basel) 2023;13:2941. [Crossref] [PubMed]
- Caroço TV, Saraiva RP, Baião JM, et al. Mediastinal papillary thyroid carcinoma treated by video-assisted thoracic surgery - Case report. Int J Surg Case Rep 2023;106:108140. [Crossref] [PubMed]


