A comparison of balloon angioplasty of native coarctation versus surgical repair for short segment coarctation associated with ventricular septal defect—a single-center retrospective review of 92 cases
Introduction
Coarctation (CoA) with a ventricular septal defect (VSD) has been recognized as one of the most serious congenital cardiac malformations (1), commonly leading to congestive cardiac failure and metabolic acidosis in very young infants (2). One-stage surgery through a midline incision (3) has been proposed to be the routine approach for repairing the CoA and VSD, with an acceptable early-phase mortality. However, complications associated with the surgery, such as left bronchial compression (4), recurrent laryngeal nerve injury (5), and neurological sequelae (6,7) influence the recovery and prognosis of these patients.
Hybrid approaches, combining interventional catheterization and surgical procedures, can minimize the exposure to cardiopulmonary bypass (CPB) and thus improve outcomes, as suggested by Baba et al. (8) for hypoplastic left heart syndrome (HLHS). We hypothesized that a hybrid technique can also be performed on carefully selected CoA and VSD patients to decrease the incidence of the complications noted above and to improve the prognosis. Considering the high possibility of recurrence of CoA with balloon angioplasty (9), patients with short-segment (≤5 mm) CoA may be more suitable for balloon angioplasty. Therefore, in this study, we investigated the efficacy of combined balloon angioplasty and surgical repair for cases of short-segment CoA with VSD and compared outcomes with those of patients who received traditional surgical treatment.
Methods
Study design
We retrospectively reviewed all patients who underwent complete repair for CoA and VSD between January 2004 and July 2014 in our institution. Overall, 189 patients were identified. All of the patients were examined by echocardiography for evaluation of intracardiac malformations and contrast-enhanced computed tomography (CT) scanning to evaluate the aortic arch and length of CoA before surgery. In total, 92 patients were selected for inclusion in our study according to the following criteria: (I) the length of CoA was less than 5 mm; (II) the transverse aortic arch-to-ascending aorta diameter ratio was more than 0.3; and (III) the size of the patent ductus arteriosus (PDA) was less than 50% of the descending aorta diameter. The patients were divided into two groups according to the treatment applied; 39 patients were treated with the hybrid procedure (group A), and the other 53 by traditional midline surgical repair (group B). All data were retrospective reviewed and compared between the two groups. The study protocol was approved by the institutional review board of our institution before performance of the study.
Surgical procedures
Group A
Balloon angioplasty for 35 patients with CoA was performed in the catheter lab; however, four procedures were performed in the operating room through the ascending aorta with an open heart procedure due to the low weight of the infants. The size of the catheter was chosen depending on the mean size of aortic arch diameter and the aortic diameter as measured at the level of the diaphragm. Usually, a 4- to 8-mm (mean, 7±1.0 mm) balloon was chosen, and the procedure repeated to dilate the aortic CoA three times with the maximum pressure of 8–10 kPa. All procedures were performed successfully without any bleeding or rupture. Success of balloon angioplasty was defined as a peak systolic gradient ≤20 mmHg after balloon angioplasty. The patients were then immediately transferred to the operating room. Moderate-to-large VSD patch closure and PDA division through a midline incision with CPB was performed
Group B
Fifty-three patients underwent one-stage repair of short segment aortic CoA and VSD through a midline approach under CPB. With respect to the method of aortic CoA repair, 13 patients received extended end-to-end anastomosis, whereas the other 40 received end-to-end anastomosis. Deep hypothermic circulatory arrest (DHCA) was performed in nine patients. Antegrade regional cerebral perfusion (ACP) was applied in 25 patients. Hypothermic low flow at 30~50 mL/kg was applied in the other 19 patients.
Follow-up
Echocardiographic examination was performed to investigate the aortic arch and isthmus. Contrast-enhanced CT scanning or magnetic resonance imaging (MRI) was performed if further information was needed regarding the aortic arch. An aneurysm was defined as an area of dilatation that was 150% of the aortic diameter at the level of the diaphragm. Re-CoA was defined as a peak pressure gradient over 30 mmHg identified by echocardiography during follow-up. Re-intervention was defined as any surgical- or catheter-based balloon intervention performed only for residual or re-CoA that occurred after discharge.
Statistical analysis
Demographic data, perioperative factors, and postoperative events were recorded. Continuous variables are presented as mean [± standard deviation (SD)] for normally distributed data or median and range otherwise. For subgroup analyses, the Student t test was used to compare the parametric values, and the Mann-Whitney U test was used to compare the nonparametric values. The re-CoA rate and re-intervention rate were analyzed using Kaplan-Meier analysis as categorical data. A probability value of <0.05 was considered significant in all analyses. In addition, possible factors for re-CoA in the hybrid group were analyzed. Variables with P values less than 0.1 were assessed using multivariate logistic regression to evaluate risk factors for re-CoA after balloon angioplasty. Statistical analyses were performed using SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA).
Results
Overall, 92 selected patients with short-segment CoA and VSD, including 67 males and 25 females, aged from 9–190 days (median, 55.4 days) and weighing from 1.9–7 kg (median, 4.0 kg) were included. There were no significant differences in the preoperative demographic data between the two groups (Table 1).
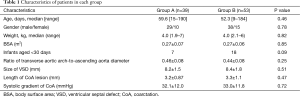
Full table
Balloon angioplasty
Successful balloon angioplasty was achieved in all patients in group A. The diameter of the CoA increased from 2.0±0.6 to 3.8±0.8 mm immediately after balloon angioplasty, while the peak gradient pressure decreased from 32.1±12.0 to 7.4±5.0 mmHg (Table 2).

Full table
Periprocedural outcomes
Compared with patients in group B, patients in group A experienced a shorter aortic clamp time (28.1±6.7 vs. 43.2±9.2 minutes, P<0.001) and shorter CPB time (52.9±10.7 vs. 86.2±23.8 minutes, P<0.001). No DHCA or SCP was needed for patients in group A. Thus, patients in group A experienced quicker recovery with a shorter time on mechanical ventilation (47.0 vs. 73.7 hours, P=0.002) and shorter intensive care unit stay (6.2 vs. 9.1 days, P=0.019) compared to patients in group B. In addition, all patients in group A remained alive after the perioperative period, whereas three early deaths occurred in group B, which occurred 9, 16, 25 days after surgery respectively. More importantly, injury of the recurrent laryngeal nerve was avoided in group A, whereas six patients in group B experienced nerve injury as evidenced by laryngoscopy (Table 3). Recurrent laryngeal nerve injury leads to the symptoms of hoarseness and choking.

Full table
Follow-up data
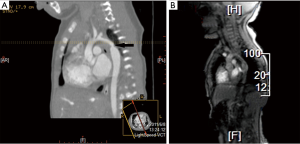
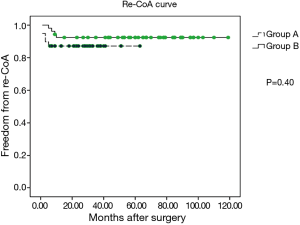
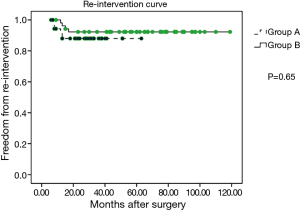
The media follow-up for patients in group A was 33 months (range, 6–63 months), whereas that for patients in group B was 62 months (range, 9–119 months). During the follow-up period, no cases of late mortality occurred in the two groups. In group A, no residual VSD and pseudoaneurysm was observed during the follow-up (Figure 1). However, re-CoA was detected in five patients in group A, and each case occurred in the first six months after performance of the hybrid procedure. Moreover, four patients of these re-CoAs in group A received re-intervention (three cases of repeated balloon angioplasty at 8, 12, and 13 months and one case underwent end-to-end anastomosis through left thoracotomy) for the CoA. In group B, re-CoA was confirmed in four patients between the first 6 and 12 months after surgery, and all cases were successfully treated by repeated balloon angioplasty. The percentages of patients in group A who were free of aortic re-CoA at 6 months, 1 year, and 5 years were 87.2%, 87.2%, and 87.2%, respectively, and these percentages in group B were 98.1%, 92.4%, and 92.4%, respectively (P=0.40, Figure 2). The percentages patients who did not require re-intervention at six months, one year, and five years were 94.3%, 91.1%, and 88.0%, respectively, in group A compared to 98.1%, 96.2%, and 92.3%, respectively, in group B (P=0.65, Figure 3).
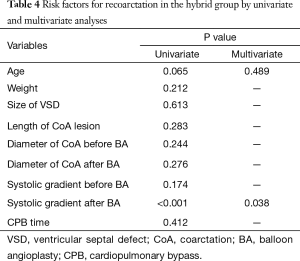
Risk factors for re-CoA in the hybrid group
Univariate and multivariate logistic analyses revealed that a systolic pressure gradient after balloon angioplasty was a significant risk factor for re-CoA in the hybrid group (odds ratio 2.5, 95% confidence interval: 1.1–6.1, P=0.038). Eighty percent (4/5) of re-CoA cases occurred in patients with a systolic gradient ≥15 mmHg after balloon angioplasty. In contrast, age was not found to be significantly associated with re-CoA (P=0.489; Table 4).
Full table
Discussion
The results of our study indicated a favorable mid-term efficacy of the hybrid procedure for short-segment CoA and VSD. It seems the hybrid procedure may be the best option for selected patients with CoA and VSD (10) who have a short-segment or shelf-shaped CoA and the absence of severe hypoplasia of the isthmus or transverse arch without a large PDA. Compared to the traditional surgical treatment, the hybrid procedure has many advantages (11). For example, because it requires less cross clamp time and bypass time, possible cerebral dysfunction due to DHCA or low circulatory flow can be avoided (12). In addition, the patients may experience a quicker recovery from mechanical ventilation in the intensive care unit. Moreover, the hybrid approach is an efficient way to decrease the early mortality associated with repair of the CoA and VSD, and in particular, this approach is more likely to be useful in developing nations where later diagnosis and self-selection are likely to occur (11).
Cerebral dysfunction
DHCA or SCP usually are applied in traditional aortic arch construction and have been shown to be related to postoperative cerebral dysfunction (13). The incidence of neurologic dysfunction was reported to be 1.4–5.1% (14,15). Even though several cerebral protective strategies involving selective antegrade cerebral perfusion or moderate hypothermic circulatory arrest have been suggested and applied to minimize the risk, the efficacy of these strategies requires further confirmation. In our study, neither DHCA nor SCP was needed among patients treated with the hybrid procedure. This may transfer to a significantly lower risk of cerebral dysfunction.
Recurrent laryngeal nerve injury
The recurrent laryngeal nerve or its branch can be easily injured when further aortic dissection is performed for aortic reconstruction, which can lead to symptoms like hoarseness, choking, and coughing. These complications impair the feeding and growth of young infants and increase the incidence of aspiration pneumonia. Some patients may have to live with nasogastric tube feeding for several years. No recurrent laryngeal nerve impairment was detected in patients treated by the hybrid approach, which indicated that this treatment strategy may reduce the risk of recurrent laryngeal nerve injury.
Re-CoA and re-intervention
The use of balloon angioplasty remains controversial for small infants with CoA (16,17), because of relatively high rates of re-stenosis and re-intervention as reported previously. Rao and Jureidini (18) reported an 83% rate of re-CoA in neonates after balloon angioplasty, of which 46.8% of cases occurred in infants less than 3 months old. Liang et al (19) also observed 44% re-CoA in neonates and patients whose post-balloon angioplasty systolic pressure gradient was >10 mmHg or whose CoA diameter was <3 mm. A higher gradient before dilation and a small transverse arch were implicated as risk factors for re-CoA by Ovaert et al. (20). Therefore, in our study, we selected patients carefully according to the aforementioned criteria to include only those with short-segment CoA. Our results indicate that the hybrid procedure can be performed in younger infants and neonates, because no significant association was found between re-CoA and younger age in our selected group of patients. We suppose another reason for the low incidence of re-CoA in our cohort was the division of the PDA or ligament, the retraction of which is believed to be a possible cause of re-CoA. All cases of re-CoA occurred within the first six months after balloon angioplasty in our study, a finding that is similar to the results of Rao and Jureidini (18) and Ozawa et al. (21). Repeated balloon angioplasty also was shown to be an effective treatment for patients in both groups in our study, because most cases with re-CoA could be fixed successfully by repeated balloon dilatation, except for one case in which surgical repair was required.
Aneurysm formation
Aneurysm formation is another possible complication affecting the long-term prognosis, and the incidence of aneurysm formation has varied from 0% to 6% in the recent literature (22,23). Fortunately, no aneurysm formation was detected in our study. A possible reason may be the use of a smaller balloon according to our recommendation, which was associated with a lower risk of medial tearing.
Study limitations
The present study is limited by its retrospective and non-randomized design. Large-scale, randomized controlled trials are needed to confirm our results.
In conclusion, the hybrid technique resulted in favorable outcomes in carefully selected patients with short-segment CoA and VSD, as reflected by better perioperative outcomes and an acceptable incidence of aortic re-stenosis and re-intervention. Furthermore, most cases of aortic re-stenosis could be successfully relieved via repeated balloon dilatation.
Acknowledgements
Funding: This work was supported by National Key Clinical Specialty Construction Programs of China [2014–2016]; and Medical Guide Project of Shanghai Municipal Science and Technology Commission, No. 134119a4100.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional ethics committee/ethics board of 2011-079 by Children’s Hospital of Fudan University.
References
- Alsoufi B, Cai S, Coles JG, et al. Outcomes of different surgical strategies in the treatment of neonates with aortic coarctation and associated ventricular septal defects. Ann Thorac Surg 2007;84:1331-6; discussion 1336-7. [Crossref] [PubMed]
- Goksel OS, Tireli E. Surgical strategy in the treatment of neonates with aortic coarctation and associated ventricular septal defects. Ann Thorac Surg 2008;86:352; author reply 352-3. [Crossref] [PubMed]
- Walters HL 3rd, Ionan CE, Thomas RL, et al. Technique of single-stage repair of coarctation of the aorta with ventricular septal defect. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2008.22-30. [Crossref] [PubMed]
- Chang YH, Sung SC, Kim H, et al. Anterior translocation of the right pulmonary artery to avoid airway compression in aortic arch repair. Ann Thorac Surg 2013;96:2198-202. [Crossref] [PubMed]
- Shaath GA, Jijeh A, Alkurdi A, et al. Ultrasonography assessment of vocal cords mobility in children after cardiac surgery. J Saudi Heart Assoc 2012;24:187-90. [Crossref] [PubMed]
- Sistino JJ, Atz AM, Simpson KN, et al. The prevalence of attention-deficit/hyperactivity disorder following neonatal aortic arch repair. Cardiol Young 2015;25:663-9. [Crossref] [PubMed]
- von Rhein M, Dimitropoulos A, Valsangiacomo Buechel ER, et al. Risk factors for neurodevelopmental impairments in school-age children after cardiac surgery with full-flow cardiopulmonary bypass. J Thorac Cardiovasc Surg 2012;144:577-83. [Crossref] [PubMed]
- Baba K, Kotani Y, Chetan D, et al. Hybrid versus Norwood strategies for single-ventricle palliation. Circulation 2012;126:S123-31. [Crossref] [PubMed]
- Ergül Y, Nişli K, Dindar A, et al. The comparison a 16-year follow-up results of balloon angioplasty for aortic coarctation in children of different age groups: a single-center experience. Anadolu Kardiyol Derg 2011;11:336-42. [PubMed]
- Harris KC, Du W, Cowley CG, et al. A prospective observational multicenter study of balloon angioplasty for the treatment of native and recurrent coarctation of the aorta. Catheter Cardiovasc Interv 2014;83:1116-23. [Crossref] [PubMed]
- Zhang HF, Jia B, Liu F, et al. The comparison of Hybrid technique to the traditional complete surgical way for CoA/VSD under the age of three months. Chin J Pediatr Surg 2012;33:565-8.
- Turkoz R, Saritas B, Ozker E, et al. Selective cerebral perfusion with aortic cannulation and short-term hypothermic circulatory arrest in aortic arch reconstruction. Perfusion 2014;29:70-4. [Crossref] [PubMed]
- Salazar JD, Coleman RD, Griffith S, et al. Selective cerebral perfusion: real-time evidence of brain oxygen and energy metabolism preservation. Ann Thorac Surg 2009;88:162. [Crossref] [PubMed]
- Ziganshin BA, Rajbanshi BG, Tranquilli M, et al. Straight deep hypothermic circulatory arrest for cerebral protection during aortic arch surgery: Safe and effective. J Thorac Cardiovasc Surg 2014;148:888-98; discussion 898-900. [Crossref] [PubMed]
- Leshnower BG, Myung RJ, Kilgo PD, et al. Moderate hypothermia and unilateral selective antegrade cerebral perfusion: a contemporary cerebral protection strategy for aortic arch surgery. Ann Thorac Surg 2010;90:547-54. [Crossref] [PubMed]
- Adjagba PM, Hanna B, Miró J, et al. Percutaneous angioplasty used to manage native and recurrent coarctation of the aorta in infants younger than 1 year: immediate and midterm results. Pediatr Cardiol 2014;35:1155-61. [Crossref] [PubMed]
- Park Y, Lucas VW, Sklansky MS, et al. Balloon angioplasty of native aortic coarctation in infants 3 months of age and younger. Am Heart J 1997;134:917-23. [Crossref] [PubMed]
- Rao PS, Jureidini SB, Balfour IC, et al. Severe aortic coarctation in infants less than 3 months: successful palliation by balloon angioplasty. J Invasive Cardiol 2003;15:202-8. [PubMed]
- Liang CD, Su WJ, Chung HT, et al. Balloon angioplasty for native coarctation of the aorta in neonates and infants with congestive heart failure. Pediatr Neonatol 2009;50:152-7. [Crossref] [PubMed]
- Ovaert C, McCrindle BW, Nykanen D, et al. Balloon angioplasty of native coarctation: clinical outcomes and predictors of success. J Am Coll Cardiol 2000;35:988-96. [Crossref] [PubMed]
- Ozawa A, Predescu D, Chaturvedi R, et al. Cutting balloon angioplasty for aortic coarctation. J Invasive Cardiol 2009;21:295-9. [PubMed]
- Ammar RI. Balloon angioplasty for native aortic coarctation in children and infants younger than 12 months: immediate and medium-term follow-up. J Invasive Cardiol 2012;24:662-6. [PubMed]
- Walhout RJ, Lekkerkerker JC, Ernst SM, et al. Angioplasty for coarctation in different aged patients. Am Heart J 2002;144:180-6. [Crossref] [PubMed]


