
Perioperative intravenous lidocaine in thoracoscopic surgery for improved postoperative pain control: a randomized, placebo-controlled, double-blind, superiority trial
Highlight box
Key findings
• Perioperative intravenous lidocaine reduces pain scores after video-assisted thoracoscopic surgery. It has limited effect on acute but not on chronic pain.
What is known and what is new?
• Lidocaine is a known local and regional anesthetic and also used systemically.
• Perioperative intravenous lidocaine may be helpful as part of a multimodal analgesia protocol or with patients in whom other analgesics are contraindicated.
What is the implication, and what should change now?
• The small number of included patients resulted in the confirmation of the primary hypothesis, but not of the other clinically important secondary endpoints. Further prospective randomized trials are needed.
Introduction
Background
Pain after thoracic surgery is associated with alterations in the lung function, potentially leading to pulmonary complications (e.g., atelectasis, pneumonia) and increased perioperative morbidity (1). Furthermore, thrombosis, endocrine and cardiac complications are potential consequences of insufficiently treated postoperative pain (2-4). Optimal perioperative pain management is therefore crucial (2) especially when patients should be discharged at the earliest possible time after surgery (3).
Rationale and knowledge gap
Although we have introduced video-assisted thoracoscopic surgery (VATS) in our daily practice, we still see patients experiencing different levels of postoperative pain. To avoid excessive use of opiates, we are looking for alternatives. Lidocaine is a known local and regional anesthetic and used systemically, primarily as an antiarrhythmic drug. Given intravenously, lidocaine exerts an analgesic and anti-inflammatory effect, however, responsive mechanisms are not fully understood (5). The plasma half-life is 90-120 minutes, but the analgesic and anti-inflammatory effects might last longer than expected (5). In the Cochrane-based review, Weibel and colleagues were uncertain whether lidocaine has a beneficial impact on pain scores in the early postoperative phase, on gastrointestinal recovery, length of hospital stay, postoperative nausea, and intra- and post-operative opioid requirement (6).
Objective
In our trial, we investigated the effect of perioperative lidocaine administration on the total morphine consumption (mg) and pain measured with visual analog score (VAS) after VATS. We hypothesized that VAS and morphine use will be lower in patients with perioperative lidocaine administration (7). We present this article in accordance with the CONSORT reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1438/rc).
Methods
In this prospective, randomized, double-blind, single center, superiority clinical trial, we evaluated the effect of perioperative lidocaine on the postoperative total morphine consumption and postoperative pain. This trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). With the approval by the local ethics review board EKNZ (Ethikkommission Nordwest- und Zentralschweiz) BASEC 2016-00259, 2018-07-12 and after trial registration (ClinicalTrials.gov NCT03677817), we included 52 patients scheduled for VATS with an expected operation duration of ≤90 minutes. All procedures were performed at the Thoracic Surgery Department of the University Hospital of Basel, Switzerland. Patients provided informed consent for study inclusion and study data publication.
Inclusion criteria
Patients aged ≥18 years were included if they were scheduled for VATS under general anesthesia, with a planned surgery duration ≤90 minutes, and a physical status classification I to III according to the American Society of Anesthesiologists (ASA).
Exclusion criteria
Patients with contraindications to the class of drugs under trial, pregnant or breast-feeding women, and patients receiving steroid or chronic pain therapy were excluded. Similarly, patients with known or suspected noncompliance, drug or alcohol abuse, grade II to III atrioventricular block, congestive heart failure and/or liver insufficiency, inability to follow the study procedures and participants in another study within the 30 days before our study were excluded.
Intervention and pain management
The trial addressed the analgesic potential of perioperative lidocaine infusion in addition to standard analgesia. To evaluate this, patients either received intravenous lidocaine 2% (lidocaine group) or saline 0.9% (placebo group). In the lidocaine group, the administered volumes of lidocaine were calculated using the actual body weight, resulting in a lidocaine bolus of 1.5 mg/kg 30 minutes before surgical incision, followed by a continuous intravenous infusion of 3.0 mg/kg/hour until two hours after skin closure. If the patient was randomized to the placebo group, the volumes of saline were calculated and administered at an identical infusion rate. As both liquids are clear and administered at the same volume, the anesthesiologist and the study personnel were effectively blinded to the study drug.
Using this regimen for lidocaine, it would result in a plasma concentration of 1.9±0.7 µg/mL (8) to 2.1±0.4 µg/mL (8,9), which is clearly below the toxicity-inducing plasma levels of 5–10 µg/mL (9,10).
Intraoperative analgesia was given as deemed necessary by the anesthetist, using fentanyl boluses and a continuous infusion of remifentanil: fentanyl was administered at a dose of 3–4 µg/kg of fentanyl intravenously until the start of surgery and no more than 1 µg/kg of fentanyl per hour intravenously, thereafter. The last dose of fentanyl was administered at least 60 minutes before the end of the surgical procedure. For additional analgesia requirements, the anesthesiologist adjusted the remifentanil infusion according to the patients’ needs. Administration of morphine during surgery and infiltration with local anesthetics were not allowed. At the end of surgery, after hemostasis and if not contraindicated, metamizole (dipyrone) 1 g intravenous was given.
Postoperative pain treatment: non-steroidal anti-inflammatory drugs (NSAIDs) and paracetamol (acetaminophen) according to a standardized pain treatment protocol adjusted to the patient’s requirements. In addition, patient-controlled analgesia (PCA) pain pump (CADD®-Solis VIP, Smiths Medical, Switzerland) after surgery according to hospital standards: demand dose of 2 mg morphine, a lockout of 12 minutes and a dose limitation of 10 mg per hour, which could be adjusted to patients need if required for 48 hours. The PCA pain pump recorded the total morphine consumption in mg up to 48 hours after skin closure. Pain intensity using the VAS score was evaluated when patients were immobile, thereafter patients were asked to cough and again asses their pain.
Clinical outcomes
Primary endpoints were pain score (VAS) while coughing and morphine consumption (mg) in the first 24 hours measured at 1, 2, 4, 8, 16 and 24 hours after skin closure.
Secondary endpoints were pain intensity (VAS) at rest over 48 hours, VAS at coughing and total morphine consumption (in mg) 48 hours and later after skin closure, time from skin closure to first defecation (in hours), and occurrence of vomiting, length of hospital stay (in days), development of chronic pain (>3 months), and 30-day mortality. Pain intensity (VAS) was assessed (with and without cough), 14, 90, and 180 days after surgery in our outpatient clinic.
Methods of minimizing bias
Patients were assigned to trial arms (1:1) using blocked randomization, as implemented in the electronic data capture software secuTrial®. The trial drug was prepared by an uninvolved anesthesiologist before surgery. The designated thoracic anesthesiologists, blinded to the randomization, performed general anesthesia to further prevent bias. The trial-specific data were collected by two researchers, blinded to the patient allocation, aiming to minimize inter-observer bias.
Statistical analysis
The analyses were performed on the premise of the intention-to-treat setting. VAS and morphine consumption (in mg) were combined in a Silverman integrated approach (SIA) as suggested by Dai et al. to perform statistical evaluations and are presented in Appendix 1 (SIA score calculation, Figure S1) (7). The sample size was calculated to achieve an 80% power to find an SIA difference associated with a pain reduction of 1.5 points on the VAS and a reduction of morphine consumption of 2 mg.
For the primary analysis, SIA over the first 24 hours was summarized by calculating the area under the curve that connects the measurements at 1, 2, 4, 8, 16 and 24 hours (interpolating possible missing observations). The total area was then divided by the total time of observation. A similar procedure was applied to calculate the secondary time-averaged variables. For the primary outcome, as well as for the secondary outcomes, a regression model was fitted, regressing the obtained time-average SIA score on the trial group, correcting for two covariates: the number of trocars and operation duration. Model validity was checked by visually inspecting residuals vs. the fitted values and a normal Q-Q plot of the residuals. No severe violations of model assumptions were observed. The estimated treatment effect, the 95% confidence interval thereof and the P value are presented. For the primary analysis we allowed a 5% alpha-level (two-sided). Statistical analyses were performed using R Statistical Software (v4.2.2; R Core Team 2022) (11).
Results
Between November 2020 and March 2022, 52 patients were included, 23 (44.2%) were female and 29 (55.8%) were male, with 28 patients assigned to the lidocaine group (CONSORT 2010 Flow Diagram, Figure S2). The patients’ baseline characteristics and preoperative pain scores are shown in Table 1.
Table 1
| Characteristics | Lidocaine (N=28) | Placebo (N=24) | P value; test method |
|---|---|---|---|
| Gender | 0.95; χ2 test | ||
| Male | 15 (53.6) | 14 (58.3) | |
| Female | 13 (46.4) | 10 (41.7) | |
| Age (years) at operation | 52.71±18.50 | 57.54±17.93 | 0.34; t-test |
| Height (cm) | 169.50±9.83 | 170.04±10.39 | 0.85; t-test |
| Weight (kg) | 73.00±15.36 | 73.42±13.51 | 0.92; t-test |
| BMI (kg/m2) | 25.33±4.61 | 25.51±5.04 | 0.89; t-test |
| Preoperative pain (VAS) | 0.14±0.45 | 0.00±0.00 | 0.12; t-test |
Baseline characteristics in all patients and by study group. Data are presented as n (%) or mean ± SD. N, total number; BMI, body mass index; VAS, visual analog score; SD, standard deviation.
Pain scores (VAS) when coughing over 24 hours were statistically significantly lower in the lidocaine group compared to the placebo group (4.60±1.64 vs. 5.52±1.65; P=0.02) (Figure 1). Morphine consumption over 24 hours was not statistically significantly lower in the lidocaine group (lidocaine 18.22±12.87 vs. placebo 21.26±9.39 mg; P=0.26). When comparing the lidocaine group to the placebo group at 48 hours after surgery, a positive effect of lidocaine was seen on VAS when coughing (lidocaine 3.93±1.66 vs. placebo 4.87±1.62; P=0.02) (Figure 1, Table 2) but not on morphine consumption (lidocaine 21.88±16.06 vs. placebo 26.50±12.48 mg; P=0.20) (Table 2). The number of VATS ports or the duration of the surgery did not affect postoperative pain, intraoperative parameters were similar in both groups (Table 2). Patients could be distributed into three main procedural groups: pleural, lung and mediastinal surgery, with a median duration of 69.50 [interquartile range (IQR), 56.00; 104.50] minutes in lidocaine group vs. 66.00 (IQR, 52.75; 113.00) minutes in placebo group (P=0.93) (Figure 2, Table 2). Patients received remifentanil (µg/kg) throughout surgery, with no significant difference between the trial groups.
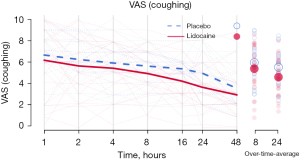
Table 2
| Summary | Lidocaine (N=28) | Placebo (N=24) | P value; test method |
|---|---|---|---|
| Operation | |||
| Type of operation | 0.51; χ2 test | ||
| Pleural surgery | 2 (7.1) | 4 (16.7) | |
| Lung surgery | 22 (78.6) | 16 (66.7) | |
| Mediastinal surgery | 4 (14.3) | 4 (16.7) | |
| Duration (min) | 69.50 [56.00; 104.50] | 66.00; [52.75; 113.00] | 0.93; Wilcoxon |
| Number of trocars | 0.46; χ2 test | ||
| 2 | 12 (42.9) | 7 (29.2) | |
| 3 | 16 (57.1) | 17 (70.8) | |
| Analgesia | |||
| Fentanyl total (µg) | 300.0 [268.75; 350.0] | 325.0 [287.5; 400.0] | 0.55; Wilcoxon |
| Fentanyl (µg/kg) | 4.32 [3.79; 5.03] | 4.67 [3.77; 5.19] | 0.49; Wilcoxon |
| Remifentanil (µg/kg) | 12.36 [9.17; 17.84] | 14.51 [9.34; 21.7] | 0.38; Wilcoxon |
| Pain | |||
| Over 8 hours | |||
| VAS | 3.89±1.98 | 3.99±1.79 | 0.80; linear regression |
| VAS (coughing) | 5.39±2.03 | 5.96±1.87 | 0.25; linear regression |
| Morphine (mg) | 12.78±8.86 | 14.32±6.99 | 0.45; linear regression |
| Over 16 hours | |||
| VAS | 3.34±1.78 | 3.35±1.49 | 0.87; linear regression |
| VAS (coughing) | 4.95±1.73 | 5.71±1.75 | 0.07; linear regression |
| Morphine (mg) | 16.1±11.21 | 18.39±8.32 | 0.33; linear regression |
| Over 24 hours | |||
| VAS | 3.02±1.65 | 2.96±1.31 | 0.97; linear regression |
| VAS (coughing) | 4.60±1.64 | 5.52±1.65 | 0.02; linear regression |
| Morphine (mg) | 18.22±12.87 | 21.26±9.39 | 0.26; linear regression |
| Over 48 hours | |||
| VAS | 2.35±1.54 | 2.30±1.12 | >0.99; linear regression |
| VAS (coughing) | 3.93±1.66 | 4.87±1.62 | 0.02; linear regression |
| Morphine (mg) | 21.88±16.06 | 26.50±12.48 | 0.20; linear regression |
| Hospitalization | |||
| Time to defecation (hours) | 44.92±16.92 | 54.27±20.74 | 0.10; linear regression |
| Vomit count | 0 | 0 | |
| Length of stay in hospital (days) | 4.50±2.17 | 4.91±2.52 | 0.67; Wilcoxon |
Operation, pain (VAS) at rest/while coughing, morphine consumption and hospitalization related events by all patients and by study group. Data are presented as n (%), median [IQR] or mean ± SD. N, total number; VAS, visual analog score; IQR, interquartile range; SD, standard deviation.
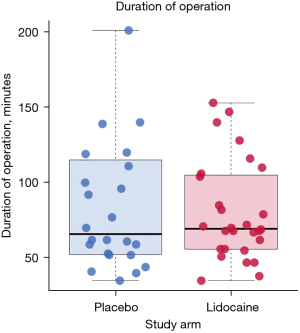
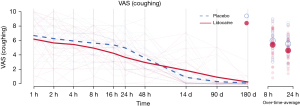
Table 3 shows the different additional analgesics given to the patients in both groups, as well as the statistical comparison of the two groups according to the number of patients in each group. Patients in both groups used similar quantities of additional analgesics. After the PCA pump was stopped, two patients in the placebo group and one patient in the lidocaine group requested additional morphine (Table 3). Figure 3 shows the development of the pain scores, including the mid- and long-term follow-up measurements (14, 90, and 180 days after operation). Some patients still experienced some pain at long-term follow-ups, nine patients after 90 days and four patients after 180 days. VAS in general were low, reaching less than VAS 3 after 180 days.
Table 3
| Analgesic | Lidocaine group | Placebo group | P | |||
|---|---|---|---|---|---|---|
| N [%] | Amount [IQR] | N [%] | Amount [IQR] | |||
| Ibuprofen (mg) | 3 [11] | 3,200 [2,600, 3,200] | 2 [8] | 3,600 [3,200, 4,000] | >0.99 | |
| Paracetamol (acetaminophen) (mg) | 14 [50] | 8,000 [3,750, 10,250] | 17 [71] | 5,000 [2,500, 12,500] | 0.21 | |
| Metamizole (dipyrone) (mg) | 25 [89] | 9,500 [6,000, 11,000] | 22 [92] | 6,500 [4,250, 11,750] | >0.99 | |
| Morphine (>48 h) IV (mg) | 1 [4] | 11 [11, 11] | 2 [8] | 97 [68.5, 125.5] | 0.89 | |
| Other (mg) | 10 [36] | 30 [12.25, 30] | 8 [33] | 23 [9, 272.5] | >0.99 | |
N, total number of patients; amount, median amount of medication taken; IQR, interquartile range; IV, intravenous.
Additional analyses using the Wilcoxon test, comparing the two arms without including the covariates show no differences in the secondary outcomes between the observed groups (Table 2). The length of stay in hospital was found to be very similar in both trial groups (Wilcoxon test W=330, P=0.67).
Complications
One patient in the lidocaine group developed bradycardia with 33 beats/minutes, a hypotension (72/36 mmHg) and a metallic taste in the mouth 90 minutes after skin closure, while receiving the investigational medicinal product (IMP) infusion as described in the protocol (until 120 minutes after skin closure). The symptoms lasted for 6 minutes, the patient had no loss of consciousness, and the IMP infusion was stopped 95 minutes after skin closure. The treatment of the patient was unblinded and a lidocaine intoxication therapy with SMOFLipid® was initiated according to institutional protocols/standard operating procedures (SOPs). The patient was monitored for an additional three and a half hours and was duly discharged from the hospital 4 days later, without additional cardio circulatory signs or symptoms. Blood samples taken immediately after first symptoms occurred, showed a normal, non-toxic level of serum lidocaine (1.9 µg/mL). This patient was excluded from the trial, nonetheless. No 30-day mortality occurred.
Discussion
Key findings
The main objective of this trial was to evaluate the superiority of intravenous lidocaine over placebo, as an adjunct to standard treatment in perioperative pain management after VATS. We saw statistically significantly lower postoperative VAS after VATS within the first 24 hours when coughing, but the anticipated difference of 1.5 points on VAS scale was not achieved. Median morphine consumption was 3 mg lower in the first 24 hours and 4.5 mg in the first 48 hours in the lidocaine group with no statistical significance (P=0.26; P=0.20). In addition, patients in the lidocaine group had their first defecation almost ten hours earlier after surgery, although, this was not statistically significant (P=0.11).
Strengths and limitations
Lidocaine is widely used for infiltration anesthesia, has an extensive safety record (10), nevertheless, some concerns remain (9). Lidocaine has a short plasma half-life. Molecular mechanisms of action of systemic lidocaine include the blocking of sodium channels and modulating several other receptors (5). Additional anti- and neuro-inflammatory effects of systemically applied lidocaine can explain the type of delayed pain modulation we observed in our trial population (5). The tendency to lower the median remifentanil dosage [lidocaine 12.36 (IQR, 9.17; 17.84) vs. placebo 14.51 (IQR, 9.34; 21.70) µg/kg, P=0.38], a comparable median duration of surgery and no difference in the pain score or morphine consumption after 8 hours is not sufficient to draw any conclusions (Table 2). Additionally, after a period of observed difference in pain score while coughing and morphine consumption at 24 and 48 hours this was no longer present at the follow-up.
Comparison with similar researches
When planning this trial, the current 2021 recommendation guidelines for intravenous lidocaine for postoperative pain and recovery (initial dose: no more than 1.5 mg/kg, infusion of no more than 1.5 mg/kg/hour, not longer than 24 hours) were not available (9). There is tremendous variation in dosage (1.5–5 mg/kg/hour) and duration of continuous infusions (1 to 48 hours after surgery) (6). In a recently published trial in which lidocaine (bolus of 1.5 mg/kg, followed by an infusion of 2 mg/kg/hour) was administered systemically to patients only during VATS, this showed no effect (12). Slovack et al. showed no effect of intravenous lidocaine in thoracic surgery, however, the number of patients was limited. In addition, the authors did not specify the exact time frame of lidocaine infusion or its local application, pain intensity was not reported and morphine consumption in the control group was lower than expected (13). A recently published meta-analysis in patients undergoing colorectal surgery suggested that the analgesic effect of intravenous lidocaine might depend on the duration of infusion, with better effects when lidocaine infusion is administered for at least 24 hours (14).
Explanations of findings
We believe that we selected an appropriate and safe dose of lidocaine (3 mg/kg/hour after an induction with 1.5 mg/kg) and an infusion time of up to 2 hours after skin closure providing the desired outcome. Since in our hospital, it is standard procedure to monitor patients for at least 2 hours after general anesthesia, the safety of our study population was ensured during study drug infusion, as it is recommended in the above-mentioned guidelines (9).
Since pain is a subjective feeling and difficult to measure precisely, we assessed pain scores while coughing. In the first 24 hours, patients experienced over VAS 5 in the placebo group and over VAS 4 in the lidocaine group. This reduction of 20% in VAS is statistically relevant and worthy of a report, although, on the lower limit of the clinical relevancy definition (15). Morphine on demand was provided to both groups over a 48-hour period and patients made more use of it than in a normal clinical setting. In an Asian study population, Cui et al. used less intravenous lidocaine (app 2 mg/kg/hour) when performing a thoracotomy and longer lasting more extensive surgeries. Nevertheless, patients needed less opiates during the perioperative period (study group: 12 mg morphine/48 hours; placebo group 30 mg morphine/48 hours) (16). This indicates the extreme influence of cultural and personal pain perception.
Intravenous lidocaine did not affect any of the other secondary parameters, which may mainly be due to the small study size. For instance, we observed some statistically insignificant trends in favor of intravenous lidocaine, such as the improved time to defecation. Guinot et al. demonstrated the effect of lidocaine on postoperative complications after cardiac surgery while decreasing administered opioids (17) and Zhang et al. lowered only intraoperative opiate consumption after pancreatic surgery (18), both with larger study populations.
Implications and actions needed
It is generally believed that thoracic surgery has a high incidence of chronic (>3 months) pain (19). According to the meta-analyses data this incidence is lower after VATS, 25% vs. 40% when compared to open surgery (19). In our trial, this incidence was even lower: 9 of 52 patients (17%) experienced low intensity pain (mean VAS score <2) when coughing after 14 days (Figure 3). After 180 days, only 4 of the 52 patients studied (8%), had pain while coughing with a VAS intensity level of 3 or less. We therefore conclude that intravenous lidocaine appears to have an effect on acute but not on chronic pain, persisting for more than 3 months (20). The temporal profile of the analgesic effect can be explained by its relatively short half-life.
The durations for simple VATS procedures are usually short. We evaluated the influence of surgery duration by the number of ports used and a pain score as part of the regression analysis. The majority of surgeries were performed by two surgeons, one using a classical 3-port approach, the other using one Alexis-retractor and one camera-port. We found no difference between both approaches. The strengths of our trial include that results are derived from a single institution, prospective, double-blind, randomized trial. We used a strict anesthesia and postoperative pain protocol, and similar surgery. We have no observer bias to report. We did not perform a general standardized screening and measuring of lidocaine plasma levels since this was calculated using actual body weight of every patient before infusion. Finally, very high doses of remifentanil might induce short-term hyperalgesia after surgery, and the use of regional techniques such as serratus anterior plane or erector spinae plane blocks for pain relief in thoracotomy might result in improved pain relief after thoracotomy and VATS.
The small number of included patients resulted in the confirmation of the primary hypothesis, but not of the other clinically important secondary endpoints. Further prospective randomized trials are warranted.
Conclusions
Our results suggest that perioperative intravenous administration of lidocaine reduces pain scores after VATS surgery. However, intravenous lidocaine might be an option as parts of a multimodal pain management for patients, in whom thoracic epidural analgesia and other types of local pain control are not possible or contraindicated, such as patients with chest trauma, hematologic disorders and infectious diseases.
Acknowledgments
The authors would like to thank Marielle Rutquist and Gilles Dutilh of the Department for Clinical Research at University Hospital Basel for data management and statistical analysis, and Cecile Buenter for editorial and language assistance. The study was presented at the annual meeting of the German Society for Thoracic Surgery, the abstract was published in Zentralblatt für Chirurgie 2023;148(S 01):S91.
Funding: Financial support for the trial was provided by the Department of Thoracic Surgery and Department of Anesthesia, Intermediate Care, Prehospital Emergency Medicine and Pain Therapy, University Hospital Basel.
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1438/rc
Trial Protocol: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1438/tp
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1438/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1438/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1438/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The trial was approved by the local ethics review board EKNZ (Ethikkommission Nordwest-und Zentralschweiz) BASEC 2016-00259, 2018-07-12. Patients provided informed consent for study inclusion and study data publication.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Branson RD. The scientific basis for postoperative respiratory care. Respir Care 2013;58:1974-84. [Crossref] [PubMed]
- Warner DO. Preventing postoperative pulmonary complications: the role of the anesthesiologist. Anesthesiology 2000;92:1467-72. [Crossref] [PubMed]
- Ansari D, Gianotti L, Schröder J, et al. Fast-track surgery: procedure-specific aspects and future direction. Langenbecks Arch Surg 2013;398:29-37. [Crossref] [PubMed]
- Herroeder S, Pecher S, Schönherr ME, et al. Systemic lidocaine shortens length of hospital stay after colorectal surgery: a double-blinded, randomized, placebo-controlled trial. Ann Surg 2007;246:192-200. [Crossref] [PubMed]
- Hermanns H, Hollmann MW, Stevens MF, et al. Molecular mechanisms of action of systemic lidocaine in acute and chronic pain: a narrative review. Br J Anaesth 2019;123:335-49. [Crossref] [PubMed]
- Weibel S, Jelting Y, Pace NL, et al. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery in adults. Cochrane Database Syst Rev 2018;6:CD009642. [Crossref] [PubMed]
- Dai F, Silverman DG, Chelly JE, et al. Integration of pain score and morphine consumption in analgesic clinical studies. J Pain 2013;14:767-77.e8. [Crossref] [PubMed]
- Martin F, Cherif K, Gentili ME, et al. Lack of impact of intravenous lidocaine on analgesia, functional recovery, and nociceptive pain threshold after total hip arthroplasty. Anesthesiology 2008;109:118-23. [Crossref] [PubMed]
- Foo I, Macfarlane AJR, Srivastava D, et al. The use of intravenous lidocaine for postoperative pain and recovery: international consensus statement on efficacy and safety. Anaesthesia 2021;76:238-50. [Crossref] [PubMed]
- Beecham GB, Nessel TA, Goyal A. Lidocaine. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-.
- R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/
- Yao Y, Jiang J, Lin W, et al. Efficacy of systemic lidocaine on postoperative quality of recovery and analgesia after video-assisted thoracic surgery: A randomized controlled trial. J Clin Anesth 2021;71:110223. [Crossref] [PubMed]
- Slovack M, Taylor B, Bryce R, et al. Does intravenous lidocaine infusion during video-assisted thoracoscopic surgery reduce postoperative analgesia? A randomized controlled study. Can J Anaesth 2015;62:676-7. [Crossref] [PubMed]
- Yang W, Yan S, Yu F, et al. Appropriate Duration of Perioperative Intravenous Administration of Lidocaine to Provide Satisfactory Analgesia for Adult Patients Undergoing Colorectal Surgery: A Meta-Analysis of Randomized Controlled Trials. Anesth Analg 2023;136:494-506. [Crossref] [PubMed]
- Cooper SA, Desjardins PJ, Turk DC, et al. Research design considerations for single-dose analgesic clinical trials in acute pain: IMMPACT recommendations. Pain 2016;157:288-301. [Crossref] [PubMed]
- Cui W, Li Y, Li S, et al. Systemic administration of lidocaine reduces morphine requirements and postoperative pain of patients undergoing thoracic surgery after propofol-remifentanil-based anaesthesia. Eur J Anaesthesiol 2010;27:41-6. [Crossref] [PubMed]
- Guinot PG, Andrei S, Durand B, et al. Balanced Nonopioid General Anesthesia With Lidocaine Is Associated With Lower Postoperative Complications Compared With Balanced Opioid General Anesthesia With Sufentanil for Cardiac Surgery With Cardiopulmonary Bypass: A Propensity Matched Cohort Study. Anesth Analg 2023;136:965-74. [Crossref] [PubMed]
- Zhang H, Qu M, Guo K, et al. Intraoperative lidocaine infusion in patients undergoing pancreatectomy for pancreatic cancer: a mechanistic, multicentre randomised clinical trial. Br J Anaesth 2022;129:244-53. [Crossref] [PubMed]
- Wang L, Yang M, Meng W. Prevalence and Characteristics of Persistent Postoperative Pain After Thoracic Surgery: A Systematic Review and Meta-Analysis. Anesth Analg 2023;137:48-57. [Crossref] [PubMed]
- Bailey M, Corcoran T, Schug S, et al. Perioperative lidocaine infusions for the prevention of chronic postsurgical pain: a systematic review and meta-analysis of efficacy and safety. Pain 2018;159:1696-704. [Crossref] [PubMed]


