Beyond diagnosis: a narrative review of the evolving therapeutic role of medical thoracoscopy in the management of pleural diseases
Introduction
Medical thoracoscopy (MT) is a minimally invasive procedure that allows for direct visualization of the pleural space and intrathoracic structures while obtaining image-guided biopsies and performing therapeutic interventions. It was first described in 1910 when Jacobaeus utilized rigid cystoscopes to evaluate the pleural space (1-3). In current practice, thoracoscopy represents one of the main endoscopic procedures, along with bronchoscopy in interventional pulmonology (4-6). Thoracoscopy can be broadly divided into minimally invasive MT/pleuroscopy and surgical thoracoscopy, also known as video-assisted thoracic surgery (VATS). VATS is performed under general anesthesia with selective bronchial intubation and is performed in an operating room (OR); on the other hand, MT can be performed under local anesthesia, mild or moderate sedation (non-operating room anesthesia; NORA) in an endoscopy suite (7). The most common use of MT is in the setting of an undiagnosed exudative pleural effusion to confirm or confute a diagnosis of malignant pleural effusion (MPE) (8-10); however, additional conditional indications of MT include pleural effusions of undetermined etiology, biopsies of pleural anomalies detected on chest computed tomography (CT), chemical and mechanical pleurodesis, and mechanical adhesiolysis (9,10). The additional benefits of performing a MT instead of just placing a chest drain are the possibility of carrying out a direct endoscopic evaluation of the pleural cavity, performing adhesiolysis and carrying out a biopsy sampling if necessary. The decision to perform MT rather than VATS should be carefully made based on patient-related factors, the underlying pathology and the operator’s experience in MT.
MT is a safe procedure with low mortality, major and minor complications reported in the current literature (10-12). Its application has also been described in patients admitted to the intensive care unit, where, in highly selected cases, it may represent a valuable therapeutic alternative in critically ill patients (13,14).
The increasing adoption of MT worldwide (15,16) is due to interventional pulmonologist’s efforts in offering less invasive procedures to reduce complications and length of hospital stay (LOS) with an overall better cost-effectiveness profile (17). We present this article in accordance with the Narrative Review reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1745/rc).
Rationale and knowledge gap
MT is an endoscopic technique with well-defined procedural standards (18). However, few randomized controlled trials (RCTs) compare MT with other procedures focusing on diagnostic yield and treatment success in pleural disease (19-23). The latest guidelines also elaborate on the role of MT in pleural disease diagnosis and treatment (24). In diagnosing MPE, its use aimed at bioptical diagnosis is considered the gold standard (24). In the context of pleurodesis for symptomatic MPE, talc slurry or talc poudrage (TP) may be equally offered to patients in order to control fluid accumulation and reduce the need for repeated invasive procedures (24). The guidelines do not currently support using MT in pneumothorax, favouring a surgical approach where necessary (24). Although in daily clinical practice MT is often used as first approach in pleural infection (PI), due to lack of supporting evidence, latest guidelines do not recommend MT as a viable treatment option for patients with infection of pleural space (24). On the contrary, in the remaining literature and daily clinical practice, MT is also applied in other contexts, including pneumothorax, PI, retained hemothorax (RH), and retrieval of intrapleural foreign bodies (FBs) (25-28).
Methods
Objective
This narrative review aims to summarize current literature, performing an overview of novel clinical contexts in which the use of MT has the potential to bring additional therapeutic alternatives to patient care. This manuscript is written as per SANRA quality scale (29). A comprehensive and systematical online literature search via Medline/PubMed and Cochrane database for the period January 2010 to December 2022 was performed for articles published using the keywords “medical thoracoscopy”, “local anesthetic thoracoscopy”, and “thoracoscopy”. The search strategy is summarized in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | 01 February 2023 |
| Databases and other sources searched | Medline/PubMed and Cochrane |
| Search terms used | “medical thoracoscopy” OR “local anesthetic thoracoscopy” OR “thoracoscopy” |
| Timeframe | From January 2010 to December 2022 |
| Inclusion and exclusion criteria | Inclusion criteria: human-based studies (study type: original article, research article, full paper available), English language |
| Exclusion criteria: animal-based studies | |
| Selection process | The selection process was conducted by A.F. and N.C. independently, then removing duplicated results. Consensus on additional papers consideration and review of the final list of references included was performed by all authors |
Equipment
In order to perform the procedure, standard equipment should be available (1,2) (Figure 1). There are two types of thoracoscopes, the rigid model being the most widespread and the novel flexi-rigid thoracoscope, produced only by Olympus Corporation (Tokyo, Japan). Each device has its characteristics, and the choice of a specific model is based on operator comfort, level of experience, and type of procedure to be carried out (see Table 2), with the aim to maximize both therapeutic results and diagnostic yields. In this regard, the rigid thoracoscope is the instrument most chosen by operators, with an external diameter between 6 and 10 mm and a large working channel, compared to the 3 mm one equipped on the flexi-rigid thoracoscope (8). A greater working channel allows for the insertion of bigger tools than the standard flexible biopsy forceps and the cryoprobe utilized within the bronchoscopy field and flexi-rigid instrumentation. The mechanical advantage of rigid forceps favors obtaining larger biopsy samples, even from very dense lesions, such as fibrous plaques, and solid malignant lesions, such as sarcomatoid mesothelioma, which may require greater tearing force (9,31). In addition, when using rigid thoracoscopes, it is possible to insert different instruments through the working channel, facilitating the work of debridement of the pleural space by using rigid biopsy forceps and management of complications such as controlling intra-procedural hemorrhage (30-32). Lastly, the rigid thoracoscope may offer more durability with less frequent need for maintenance and repair compared to flexi-rigid instrumentation when preserved appropriately (8).
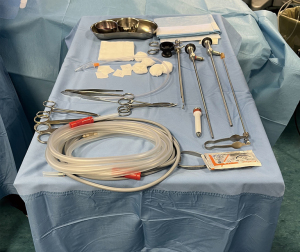
Table 2
| Type of thoracoscope | Learning curve length | Ease and completeness of exploration of the pleural space | Biopsy size | Possibility of talc instillation under direct vision | Suitability for mechanical lysis of adhesions |
|---|---|---|---|---|---|
| Rigid | + | ++ | +++ | +++ | +++ |
| Flexi-rigid | +++ | +++ | +† | +++ | + |
†, increasable by using a cryoprobe (30). +, not favourable; ++, adequately favourable; +++, optimal.
On the other hand, the flexi-rigid thoracoscope combines the flexibility of bronchoscopes with the stability of rigid thoracoscopes, thus providing increased maneuverability and flexibility, which facilitates the complete exploration of the pleural space available and homogenous insufflation of talc within the pleural space (10). Its resemblance to the familiar bronchoscope increases confidence among interventional pulmonologists approaching the procedure for the first time. Furthermore, the external smaller diameter reduces the incision size site and, consequently, post-procedural chest wall pain (9,10,33).
In a recent RCT, Bansal and colleagues compared a mini-thoracoscopy set-up with a flexirigid one, demonstrating no significant difference in diagnostic yield between the two devices (33). There is still no literature data on comparison between the various devices in the therapeutic setting of pleural diseases.
Contraindications
Absolute contraindications to performing MT include an uncorrectable bleeding disorder, significant pulmonary hypertension, cardiovascular instability, acute uncorrectable type 1 respiratory failure, ongoing type 2 respiratory failure, and absence of the possibility of generating an adequate pleural space (10,18,24). Conditions that may require changing the entry site into the pleural cavity are the presence of cutaneous infection, tract metastasis or rib fracture around the port insertion site.
Of note, if the procedure is therapeutic, such as in the setting of pleural effusion or pneumothorax, and clinical improvement may be expected, MT may be performed even in the presence of hypercapnia, considering adequate post-procedural assistance and monitoring (7,34).
Sedation
MT is performed in an endoscopy suite without the mandatory necessity of an anesthesiologist. It is typically performed under local anesthesia with conscious, moderate, or deep sedation (see Table 3) (35-41). The sedation and analgesic components should be carefully managed during the operative and postoperative phases since pain is a common symptom during pleural and chest wall manipulation. Pain may be elicited by the cutaneous incision, when the thoracoscope applies pressure on the ribs, while the operator samples the parietal pleura, and when initiating talc insufflation (1,42,43). With the increase of patient’s age and in case of infiltration of the pleura by malignant diseases, the pleura itself tends to become less tender (8,9).
Table 3
| Level of sedation | Responsiveness | Airway | Ventilation |
|---|---|---|---|
| Minimal sedation | Normal response | Unaffected | Unaffected |
| Moderate sedation | Response to verbal stimuli | No intervention required | Adequate |
| Deep sedation | No response to verbal stimuli | Intervention may be required | May be inadequate |
| General anesthesia | Unarousable | Must be supported via airway device | Inadequate, must be artificially supported |
Modified from Kochhar et al. 2016 (35).
When performing MT, local anesthesia, e.g., lidocaine 1%, is typically injected at the entry site to anesthetize the skin and the parietal pleura while being careful not to exceed the 4.5 mg/kg dose (300 mg maximum total dose) in order to avoid systemic toxicity (1,24,36).
Koulelidis et al. performed a RCT comparing MT with local lidocaine alone and with intravenous midazolam, enrolling 80 patients, finding no significant difference in hypoxemia measured by peripheral oxygen saturation SpO2 at the beginning of the procedure, at 15 minutes, and at the end of the procedure. However, midazolam was associated with reduced periprocedural and post-procedural cough and pain (44).
The most common agents used for sedation include a combination of midazolam and fentanyl. However, there is currently no consensus regarding the choice of sedative agents while performing MT regarding safety and reducing the risk of respiratory side effects such as hypoxemia and cardiovascular events (12,44).
Different prospective and pilot studies evaluating propofol (45,46) and dexmedetomidine have been published (40). Tschopp et al. published a prospective cohort study evaluating pulmonologist-administered propofol for MT in 53 patients. They achieved a high safety profile, with only four patients requiring pharmacological intervention due to the appearance of side effects but with a rapid discharge of all patients from the recovery unit after a median dose of 145 mg of propofol (41). A noninferiority trial by Grendelmeier et al. compared propofol with midazolam, reporting a higher incidence of hypoxemia and hypotension when using the former (46). In a pilot RCT, Sirohiya et al. compared dexmedetomidine with midazolam sedation in 60 patients. Post-procedural satisfaction was greater in patients sedated with dexmedetomidine, and patients treated with midazolam had a higher proportion of needs for concomitant administration of fentanyl (40).
While most cases of MT can be performed with conscious sedation, deep sedation is preferred for uncooperative patients, children, foreseeably prolonged or technically advanced procedures, and patients with demonstrated allergy to the active ingredients for local anesthesia (1,47).
More recently, the literature explored the possibility of nerve blocks as intercostal and erector spinae (ESP) blocks (48,49). McPherson et al. published a pilot study on ESP blocks for day-case MT. They enrolled five patients, associating propofol and remifentanil analgosedation with 20–35 mL of 0.25% l-bupivacaine injection. They concluded that ESP blocks provide a sufficient level of intraprocedural analgesia. However, 78% of patients required oral analgesia on day zero post-discharge, and 55% required oral analgesia on postoperative day one (49).
Monitoring
Patient monitoring should be continuous until 15 minutes post-procedure (18). Afterward, parameters monitoring should be done every 30 minutes for 1 hour, followed by four hourly observations (18). Current guidelines do not suggest monitoring hypoventilation phenomena; therefore, skin carbon dioxide tension measurement is optional (50). Tschopp et al. demonstrated the possibility of adjusting the depth of sedation guided by the Bispectral index (BIS). In their study, the level of sedation was individually optimized by titrating the propofol infusion according to BIS and clinical assessment (41).
Complications
MT is a safe procedure with an overall reported mortality of 0.34%, which nears 0% when performing a diagnostic procedure (10). Mortality in MT has primarily been attributed to using non-graded talc for TP in MPE, which is associated with respiratory failure and acute respiratory distress syndrome (ARDS), resulting in a 0.69% mortality rate. However, this problem has been resolved with widespread implementation of graded talc (51-54).
Complications in MT can be divided in major and minor adverse events. Significant complications include empyema, hemorrhage, prolonged air leak, periprocedural pneumothorax, pneumonia, and port site tumor growth, and have a cumulative rate of 1.8% (52,53,55). Minor complications include subcutaneous emphysema, minor hemorrhage, operative skin site infection, procedural hypotension, atrial fibrillation, and increased body temperature, with a cumulative rate of 7.3% (10,55). While these rates refer to MT performed typically in an endoscopy suite, similar numbers were reported in its use in the intensive care unit and when performed at the patient’s bedside (14).
Procedural risks are influenced by the underlying pathology with visceral pleural tears more frequently reported when obtaining biopsy samples of honey-comb lung in end-stage pulmonary fibrosis and lung laceration during trocar insertion in the presence of extensive pleural adhesions (53). Precautions may be considered to reduce the risk of complications, such as postponing MT in the presence of severe cough, maintenance of chest tube until no air leakage is detected, and gradual lung re-expansion to prevent re-expansion pulmonary edema. In addition, in the setting of suspected mesothelioma, it is possible to administer radiation therapy to the incision area to prevent port site tumor growth (56,57).
Pleurodesis
MPE
The most common application of MT is in the diagnostic and therapeutic pathway of MPE, which typically presents unilaterally as an exudative effusion (58-60). In these patients, the initially performed procedure is often an ultrasound-guided thoracentesis for pleural fluid analysis (59,61-63). The general diagnostic yield rate for pleural fluid cytology is about 60%, with better results for primary adenocarcinoma of the lung, breast, and ovarian neoplasms (64). However, mesothelioma and squamous cell lung cancer are typically less exfoliating, resulting in a lower diagnostic yield (64-66). When negative cytology results are obtained, diagnosis may be attempted by ultrasound-guided or CT-guided pleural sampling (67). However, a diagnosis of malignancy will be commonly followed by a medical or surgical additional procedure such as pleurodesis to improve symptoms and reduce the risk of MPE recurrence. Current emphasis is placed on obtaining a timely diagnosis while minimizing manipulation of the pleural space and the chest wall tract (24). In this regard, MT maximizes diagnostic yield allowing both diagnostic and therapeutic interventions to be performed during a single session, using a single thoracic port.
TP is the primary therapeutic procedure carried out during MT (Figure 2). The procedural workflow has been described in different scientific works that propose different methods of talc deposition during MT (Figure 3, Table 4) (70). Upon completion, uniform distribution of the talc can be confirmed by direct visualization during thoracoscopy, and a chest tube is placed inside the pleural space to aspirate residual air and allow lung re-expansion and pleural adhesion (1). Only one study has been performed directly comparing MT and TP with VATS in the setting of MPE: a retrospective study by McDonald et al. found that MT with TP was associated with a significantly lower hospital length of stay (0 vs. 3 days, P<0.001) and per-procedure cost (17).
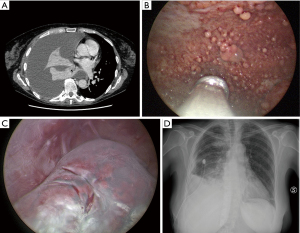
Table 4
| Delivery system | Technique | Description | Manufacturer | Talc dose | Advantages |
|---|---|---|---|---|---|
| Through the thoracoscope | Talc insufflation | Instillation via dedicated operating channel with vials loaded with talc | Storz | 3–4 g | Direct observation and orientation in talc deposition |
| Through other devices | Talc insufflation | Deposition using a cannula connected to an insufflator | Novatech | 3–4 g | Does not require a large trocar lumen |
| Talc spray | Instillation via a vaporizer/spray | Novatech, Sciarra Laboratories Inc., experimental models (68,69) | 3–5 g | High level of vaporization and distribution of talc in the pleural cavity |
When a diagnosis of malignancy is made in symptomatic pleural effusion, further intervention is generally required either with chest drain insertion and medical pleurodesis or by either talc slurry or TP by MT. A RCT on 330 patients comparing TP with talc slurry yielded no statistically significant difference in terms of pleurodesis failure at 90 days (22% and 24%, respectively) defined by a need for insertion of chest tube, indwelling pleural catheter or use of MT for persistent pleural effusion (21). These findings align with a previous prospective randomized trial by Dresler et al. comparing TP with talc slurry, which found no significant difference in success rate at 30 days (78% vs. 71%, respectively) (71). However, the results from Dresler et al. suggested that TP may have higher success rates in subgroups of patients with primary lung or breast cancer compared to talc slurry (82% vs. 67%, respectively). In addition, the distribution of talc powder is more uniform at the parietal and visceral pleura levels with TP compared to talc slurry and can also be carried out under direct endoscopic vision (see Video 1).
Several studies have been carried out in pre-clinical settings focusing on using talc-based foams, but the evidence on humans is still insufficient for their application (72,73).
There are still few reports comparing the therapeutical efficacy between TP and indwelling tunneled pleural catheters (IPCs) in the setting of MPE pleurodesis attempts. Because IPC requires regular maintenance and MT, if not carried out as a day case procedure, is associated with a greater LOS than IPC placement alone (74-78), the debate on which of the two procedures to prefer for pleurodesis in MPE remains open. In order to propose the benefits of both treatments, Foo et al. published a retrospective chart-based study, enrolling 45 patients, performing TP and concomitant IPC placement into a single day-case procedure, describing pleurodesis success rates of 71.1% at 3 months and 78.8% at 6 months, with 86.7% of patients being discharged on the same day (74). Based on the current evidence, IPC should be considered an alternative or complementary treatment to TP in patients with suspected non-expandable lung (NEL) or recurrent pleural effusion (79-81). The ongoing TACTIC RCT aims to evaluate whether TP associated with IPC placement is the optimal treatment for this group of patients (22). All these data may prove vital if we consider that successful pleurodesis in MPE, as demonstrated by the systematic review of 15 pooled studies, has been associated with increased survival (3.5–5.8 months) (58).
Pneumothorax
Pneumothorax is a highly prevalent disease, classified into two main categories, namely primary spontaneous pneumothorax (PSP), in the absence of underlying lung disease, and secondary spontaneous pneumothorax (SSP), when a documented underlying lung condition exists (Figure 4) (82-89). Recurrence is common, ranging between 17–54% in PSP (90-93) and 13–50% in SSP within 5 years, and performing a simple aspiration in the latter is unsuccessful in 15–62% of cases (94-96). In addition, for SSP, a 3.5-fold increase in mortality is associated with each secondary pneumothorax occurrence for patients affected by chronic obstructive pulmonary disease (COPD) (94,97). The current guidelines recommend different workflows for the first episodes of PSP and SSP (24).
Patients with COPD who develop SSP commonly possess a significantly decreased lung function, making them poor surgical candidates; therefore, determining the best treatment for this group of patients is challenging, and MT with TP might be a practical alternative (Video 2). A case series by Lee et al. reported a 95% success rate for TP in SSP associated with COPD during a 3-year follow-up, with four deaths (9.8%) reported within 30 days (95). These results align with the findings described in a case series by Tschopp et al. in which TP performed for persistent air leak or recurrent pneumothorax was successful in 95% of cases over a 5-year follow-up (90).
Recently, in the literature, the use of argon plasma coagulation (APC) during MT has been proposed in the setting of SSP. Guo et al. enrolled 70 patients with refractory pneumothorax to compare the prognosis of APC treatment performed during MT (MT/APC) with VATS and surgical pleurodesis (SP) (98). Compared with the MT/APC and SP groups, patients managed with VATS demonstrated poor short-term prognosis and higher hospitalization costs. Zhang et al. described a case series of patients with long-lasting SSPs treated with APC and autologous blood pleurodesis, resulting in complete resolution without further treatment in all patients considered (99).
In the context of PSP, the scientific debate is certainly more heated, given the young age of the subjects and the possibility of withstanding surgical stress. While pleurodesis is not typically performed during the first episode of PSP, a randomized study performed by Tschopp et al. comparing TP with intercostal tube drainage alone for the initial treatment of PSP reported superiority of TP both in terms of preventing recurrence at 5 years (5% vs. 34%, respectively) and increasing cost-effectiveness (100). Several systematic reviews have highlighted how a primary surgical approach to patients at the first episode of PSP seems to guarantee a lower recurrence rate than that of a primary approach consisting of chest drain placement, aspiration, or observation treatment (101-103), the latter being the lowest cost alternative (104). However, it is unclear which patients have a higher recurrence rate after the first episode and, therefore, who should immediately be a candidate for a more invasive procedure (105). The guidelines support VATS or thoracotomy as the gold standard for recurrent PSP (24). The authors’ current practice (see Figure 5) is to consider MT as a feasible therapeutic option in specific settings, after a multidisciplinary discussion between the thoracic surgical team and the interventional pulmonology team, as an alternative or a first therapeutic proposal for relapsing PSP in patients unwilling to undergo surgery or not fit enough despite a young age (91,106).
In both PSP and SSP, differently than MPE, the parietal pleura innervation is typically preserved; as a result, the use of talc pleurodesis is likely to evoke intense pain and autonomic nervous activity, which may require deep sedation and pain relief therapy in order to improve tolerability (95,97).
Chylothorax
No further evidence has been published from the first article proposing MT as a therapeutic alternative for chylothorax (107). The authors believe MT may be considered a pleurodesis technique if there are no surgical indications (e.g., thoracic duct ligation) (108).
Hepatic hydrothorax
Patients suffering from an advanced liver disease with transudative pleural effusion are characterized by an unfavourable prognosis and are fragile from a procedural point of view. Both MT and VATS in these patients have favourable results on symptoms in the short term but are burdened by high periprocedural morbidity and mortality (109,110). There are currently no evidence-supported therapeutic roles for MT in the setting of hepatic hydrothorax outside of overlapping complications such as PI (111).
Lysis of septations and debridement of the pleural cavity
PI
About 85% of parapneumonic effusions completely resolve with medical treatment alone, the remaining 15% develop empyema (112). The early stages of a parapneumonic effusion (see Table 5) usually do not require invasive intervention (113) stages III and IV require further procedures to reduce the risk of severe sepsis, septic shock, and mortality (114,115).
Table 5
| Stage/category | Pleural space appearance | Pleural fluid bacteriology | Pleural fluid chemistry | Invasive intervention needed |
|---|---|---|---|---|
| I | Minimal, free-flowing effusion (<10 mm on lateral decubitus) | Culture and Gram stain results unknown | pH unknown | No |
| II | Small to moderate free-flowing effusion (>10 mm and, <1⁄2 hemithorax) | Negative culture and Gram stain | pH ≥7.20 | Tailored therapy |
| III | Large, free-flowing effusion (≥1⁄2 hemithorax), loculated effusion, or effusion with thickened parietal pleura | One positive culture or Gram stain | pH <7.20 | Yes (chest drain, fibrinolytics, MT, surgery) |
| IV | Frank purulent appearance | Yes (chest drain, fibrinolytics, MT, surgery) |
Modified from the American College of Chest Physicians guidelines on medical and surgical treatment of parapneumonic effusions (113). MT, medical thoracoscopy.
Treatment of complicated parapneumonic effusions (CPPEs) and empyema relies on pleural fluid removal and immediate definition of the next course of action. While surgery has always been a cornerstone for treating PI (116), early pathological stages respond to chest tube drainage and administration of intrapleural fibrinolysis, improving fluid drainage and reducing surgical referral and LOS, as described in the MIST-2 trial (117). MIST-3, a prospective multicenter phase III RCT, was recently published and evaluated the outcomes of standard of care, early VATS, and intrapleural enzyme therapy (IET) in PI. The study randomized 60 patients and demonstrated that early VATS may reduce LOS, while IET was associated with reduced recovery time and earlier pain relief (118,119).
The role of MT in PI is currently supported by weak evidences. However, in clinical practice it remains a useful alternative treatment approach for PI, even if current guidelines do not recommend its use in this scenario (24). Indications of MT include mechanical clearing of septations with the thoracoscope, draining of pleural fluid, irrigation of the pleural space with saline (14,120-122) or with fibrinolytics (123,124), and insertion of chest tubes under direct endoscopic visualization (125).
While few studies are available, a retrospective case series reporting on MT in CPPE and tuberculous pleural effusion described a success rate of 97.5%, with only 1.2% of treated patients subsequently requiring surgical intervention (126). An Italian cohort described by Ravaglia et al. demonstrated a 75.6% success rate, with the need to proceed subsequently to surgery in 7.8% of patients (127). The same study demonstrated a success rate of 100% for MT in stage I PI, 83.3% in stage II, and 58.1% in stage III. The authors of the aforementioned paper recommended MT as the first-line treatment, especially in fragile patients, encouraging the initiation of therapeutic measures early in the disease process (127).
A recent RCT by Kheir et al., with LOS as the primary endpoint, compared IET with early MT for PI. Its conclusions support MT as the first therapeutic choice, being associated with a shorter LOS than IET alone (23). In addition, a pleural biopsy obtained through MT increased the microbiological diagnostic yield by 12.5%, therefore aiding in selecting personalized rather than empiric antibiotic therapy (23).
A systematic review and meta-analysis by Mondoni et al., pooling the results of eight studies, reported a pooled treatment success rate of MT of 85% for PI when used as a first-line intervention or after the failure of a chest tube insertion and a pooled complication rate of 9.0% (128). The success of the treatment was further increased by carrying out subsequent fibrinolytic therapy through the chest drain positioned at the end of the MT (128). Surgical intervention remains the primary rescue therapy in case of failure of medical therapy. In addition, when lung re-expansion is not achieved with MT alone, surgery should be considered, especially if the patient remains persistently symptomatic despite adequate infection control (129). Surgical intervention should be advised in this case because lung entrapment may be determined by the development of a pleural fibrotic peel, making the decortication procedure indispensable and preferably carried out in VATS (130,131). There is a current need for large prospective randomized trials comparing MT with surgical intervention. The pending ’studying Pleuroscopy in Routine Pleural Infection Treatment’ (SPIRIT) trial is worth mentioning, and its results are currently awaiting publication (132).
The clinical entity of pediatric empyema is also worth mentioning (133-136). About 5% of children with community-acquired pneumonia who require hospitalization develop empyema. The primary treatment consists of antibiotic therapy (136-138) and the placement of a chest tube in order to drain the pleural fluid (138,139). Refractory cases may require the use of fibrinolytic agents (140,141). There is currently no available data regarding the use of MT in treating PI in the pediatric setting. A recent case series by Zuccatosta et al. describes their experience with MT in multiloculated pediatric empyema defined by laboratory data, clinical presentation, and ultrasound imaging (142). The population consisted of six patients treated with MT; five had multiloculated empyema, with the remaining presenting with organized empyema. MT was performed in all patients with successful recovery and full lung re-expansion on chest X-rays, with microbiological diagnoses made in four cases. Despite the small sample size, it provides promising results and inspires future studies by describing a favorable safety profile for MT while providing definitive treatment without sequelae on follow-up (142).
RH
Acute hemothorax is a medical emergency most frequently caused by trauma, and its identification by chest X-ray, chest CT, and thoracentesis requires immediate treatment, commonly with chest tube placement and antibiotic prophylaxis, VATS, or thoracotomy (143,144). Quantification of blood loss can help guide medical treatment with surgical exploration with VATS or thoracotomy performed in large effusions (>1,500 mL), ongoing blood loss (>200 mL/h), or when a conservative approach is deemed insufficient (145). Thoracotomy and VATS allow clearing of the pleural cavity, quantification of blood loss, decortication, and chest tube placement for residual fluid evacuation and lung expansion (146-148). These techniques represent the gold standard of hemothorax treatment; however, thoracotomy and VATS can be challenging in fragile patients or those with multiple comorbidities (149). Current literature reporting the use of MT in hemothorax is scarce and limited to case studies in fragile patients not deemed fit enough to undergo surgery. In light of the reduced tools to deal with acute bleeding, the main indication for MT is RH (Figure 6). RH is defined as a residual hematic pleural effusion larger than 500 mL after 72 h of treatment with a chest tube (150). One case report describes the use of MT in a 76-year-old male with pleural sarcomatoid carcinoma with multiple comorbidities and high risk for surgery in whom MT was used for treating hemothorax and investigating the underlying pleural pathology (151). Bioptic samples were obtained with rigid thoracoscopy followed by APC treatment of actively bleeding nodules. The case study is promising regarding underlying MT’s utility in this setting and the implementation of APC for limiting active bleeding. A similar example was described by Srinivasan et al., reporting the case of an 82-year-old patient treated with flexi-rigid MT with a delayed presentation of hemothorax following blunt trauma resulting in RH (27). The RH proved refractory to chest drainage but the challenge was overcome with the use a cryoprobe which allowed the removal of retained adhesions and clots, resulting in a well-tolerated procedure with immediate relief of respiratory symptoms. The case highlights the usefulness of using bronchoscopic accessories in the pleural setting in order to achieve satisfactory results when dealing with complex situations in which surgery is not an option and conservative treatment is found to be ineffective.
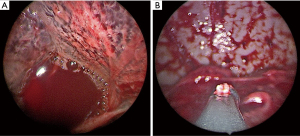
Intrapleural FB recovery
Intrapleural FBs are rare and may occur after medical manipulation of the pleural space. Retrieval is typically performed through thoracotomy and VATS under general anesthesia (152).
There is a scarcity of literature describing the retrieval of FBs through MT, and most of the documents are anecdotal case reports. MT can be used as an alternative in patients with poor lung function or those who do not tolerate surgical procedures to retrieve intrapleural FBs (14,149).
A case report describes the success of rigid thoracoscopy in the retrieval of a self-expandable metallic stent (SEMS) from the pleural cavity in a patient with right lower lobe squamous cell lung cancer that had determined endobronchial obstruction with subsequent formation of lung abscess leading to the formation of a broncho-pleural fistula and migration of the SEMS into the pleural cavity (153).
Narasimhan et al. described two cases in which MT was used to retrieve an aspiration needle dislodged within the pleural space during thoracentesis. In both cases, the procedure was conducted using a rigid thoracoscope under local anesthesia and conscious sedation, allowing for the removal en bloc of the intrapleural FB while allowing for simultaneous biopsy sampling of pleural lesions after drainage of the pleural effusion (154).
In the authors’ experience, MT was used to remove a fragment of a catheter used for extemporaneous cardiac pacing from a patient who underwent major thoracic surgery and was subsequently not deemed operable (Video 3).
While MT is useful for retrieving FBs in fragile patients, VATS remains the gold standard, particularly for large FBs, in a condition of foreseeable significant pleural adhesions or associated hemothorax.
Strengths and limitations of the review
The narrative review was designed to summarize findings from the literature on the identified topic. Despite the increase in prospective trials, RCTs, and meta-analyses developed in the field of MT, much of the inherent literature comprises retrospective studies, case series, and case reports. These may not be appropriately powered to detect significant results and are likely subject to bias and procedural errors, limiting their generalizability.
Conclusions
MT is a safe and efficient treatment with a wide range of indications for different pleural diseases. When carried out by a properly qualified operator, there are few complications even in the therapeutic setting. This procedure is one that can be performed using spontaneous ventilation and local anaesthesia with or without sedation. It should be acknowledged that there is supporting evidence that MT and VATS share common indications. Nevertheless, lung isolation and general anesthesia are frequently needed for VATS. There have been reports of VATS procedures performed under regional or epidural anesthesia and without the need for intubation (155,156), but MT continues to be the least invasive technique for visualizing the pleural space (157). The procedural boundaries may become increasingly blurred with more research on these different procedural approaches.
Future trends
Future scientific studies should assess the optimal indication for MT regarding prognostic outcomes and cost-effectiveness. The procedure should not aim to replace surgical alternatives for the pathological entities in which it is applied, but should find its role in selected cases as a low-cost, safe, and highly effective therapeutic choice in the hands of the interventional pulmonologist. The endpoints of LOS, surgical referral, short- and long-term complications and mortality must be considered to demonstrate MT’s validity as a therapeutic maneuver to be applied globally.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Avinash Aujayeb) for the series “Malignant and Benign Pleural Effusions” published in Journal of Thoracic Disease. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1745/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1745/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1745/coif). The series “Malignant and Benign Pleural Effusions” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Ethics approval was not needed for the specific document. Informed consent was obtained from all patients regarding the recording of their data in anonymized form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Loddenkemper R, Lee P, Noppen M, et al. Medical thoracoscopy/pleuroscopy: step by step. Breathe 2011;8:156-67.
- Loddenkemper R. Thoracoscopy–state of the art. Eur Respir J 1998;11:213-21. [Crossref] [PubMed]
- Loddenkemper R, Mathur PN, Lee P, et al. History and clinical use of thoracoscopy/pleuroscopy in respiratory medicine. Breathe 2011;8:144-55.
- Seijo LM, Sterman DH. Interventional pulmonology. N Engl J Med 2001;344:740-9. [Crossref] [PubMed]
- Hooper CE, Lee YC, Maskell NA. Setting up a specialist pleural disease service. Respirology 2010;15:1028-36. [Crossref] [PubMed]
- Alraiyes AH, Dhillon SS, Harris K, et al. Medical Thoracoscopy: Technique and Application. PLEURA 2016;3:2373997516632752.
- Sikachi RR, Chaddha U, Agrawal A. Anesthetic considerations for medical pleuroscopy. Respir Med 2023;213:107225. [Crossref] [PubMed]
- Murthy V, Bessich JL. Medical thoracoscopy and its evolving role in the diagnosis and treatment of pleural disease. J Thorac Dis 2017;9:S1011-21. [Crossref] [PubMed]
- Rodriguez-Panadero F, Janssen JP, Astoul P. Thoracoscopy: general overview and place in the diagnosis and management of pleural effusion. Eur Respir J 2006;28:409-22. [Crossref] [PubMed]
- Rahman NM, Ali NJ, Brown G, et al. Local anaesthetic thoracoscopy: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii54-60. [Crossref] [PubMed]
- Boutin C, Loddenkemper R, Astoul P. Diagnostic and therapeutic thoracoscopy: techniques and indications in pulmonary medicine. Tuber Lung Dis 1993;74:225-39. [Crossref] [PubMed]
- Oldenburg FA Jr, Newhouse MT. Thoracoscopy. A safe, accurate diagnostic procedure using the rigid thoracoscope and local anesthesia. Chest 1979;75:45-50. [Crossref] [PubMed]
- Ooi H. Bedside pleuroscopy in the Intensive Care Unit. Ci Ji Yi Xue Za Zhi 2018;30:97-101. [Crossref] [PubMed]
- Thakore S, Alraiyes AH, Kheir F. Medical thoracoscopy in intensive care unit. J Thorac Dis 2021;13:5232-41. [Crossref] [PubMed]
- de Fonseka D, Bhatnagar R, Maskell NA. Local Anaesthetic (Medical) Thoracoscopy Services in the UK. Respiration 2018;96:560-3. [Crossref] [PubMed]
- Madan K, Tiwari P, Thankgakunam B, et al. A survey of medical thoracoscopy practices in India. Lung India 2021;38:23-30. [Crossref] [PubMed]
- McDonald CM, Pierre C, de Perrot M, et al. Efficacy and Cost of Awake Thoracoscopy and Video-Assisted Thoracoscopic Surgery in the Undiagnosed Pleural Effusion. Ann Thorac Surg 2018;106:361-7. [Crossref] [PubMed]
- Asciak R, Bedawi EO, Bhatnagar R, et al. British Thoracic Society Clinical Statement on pleural procedures. Thorax 2023;78:s43-68. [Crossref] [PubMed]
- Dhooria S, Singh N, Aggarwal AN, et al. A randomized trial comparing the diagnostic yield of rigid and semirigid thoracoscopy in undiagnosed pleural effusions. Respir Care 2014;59:756-64. [Crossref] [PubMed]
- Haridas N. Medical Thoracoscopy vs Closed Pleural Biopsy in Pleural Effusions: A Randomized Controlled Study. J Clin Diagn Res 2014;8:MC01-4. [Crossref] [PubMed]
- Bhatnagar R, Piotrowska HEG, Laskawiec-Szkonter M, et al. Effect of Thoracoscopic Talc Poudrage vs Talc Slurry via Chest Tube on Pleurodesis Failure Rate Among Patients With Malignant Pleural Effusions: A Randomized Clinical Trial. JAMA 2020;323:60-9. [Crossref] [PubMed]
- Dipper A, Sundaralingam A, Hedley E, et al. The andomized thoracoscopic talc poudrage+indwelling pleural catheters versus thoracoscopic talc poudrage only in malignant pleural effusion trial (TACTIC): study protocol for a andomized controlled trial. BMJ Open Respir Res 2023;10:e001682. [Crossref] [PubMed]
- Kheir F, Thakore S, Mehta H, et al. Intrapleural Fibrinolytic Therapy versus Early Medical Thoracoscopy for Treatment of Pleural Infection. Randomized Controlled Clinical Trial. Ann Am Thorac Soc 2020;17:958-64. [Crossref] [PubMed]
- Roberts ME, Rahman NM, Maskell NA, et al. British Thoracic Society Guideline for pleural disease. Thorax 2023;78:s1-42. [Crossref] [PubMed]
- Fantin A, Castaldo N, Vailati P, et al. Full medical treatment of COVID-19 associated large pneumothorax – A case report. Monaldi Arch Chest Dis 2021;92: [Crossref] [PubMed]
- Sumalani KK, Rizvi NA, Asghar A. Role of medical Thoracoscopy in the Management of Multiloculated Empyema. BMC Pulm Med 2018;18:179. [Crossref] [PubMed]
- Srinivasan A, Sivaramakrishnan M, Pattabhiraman VR, et al. Medical thoracoscopic cryoevacuation: A novel technique to manage retained hemothorax. Lung India 2019;36:356-9. [Crossref] [PubMed]
- Gupta R, James P, Thangakunam B, et al. Medical thoracoscopic removal of a metal needle from the pleural space. BMJ Case Rep 2014;2014:bcr2014207035. [Crossref] [PubMed]
- Baethge C, Goldbeck-Wood S, Mertens S. SANRA-a scale for the quality assessment of narrative review articles. Res Integr Peer Rev 2019;4:5. [Crossref] [PubMed]
- Nakai T, Matsumoto Y, Sasada S, et al. Cryobiopsy during flex-rigid pleuroscopy: an emerging alternative biopsy method in malignant pleural mesothelioma. A comparative study of pathology. Jpn J Clin Oncol 2019;49:559-66. [Crossref] [PubMed]
- Yap KH, Phillips MJ, Lee YC. Medical thoracoscopy: rigid thoracoscopy or flexi-rigid pleuroscopy? Curr Opin Pulm Med 2014;20:358-65. [Crossref] [PubMed]
- Sabath BF, Lin J, Salahuddin M, et al. Control of bleeding from intercostal artery laceration. Respir Med Case Rep 2022;40:101783. [Crossref] [PubMed]
- Bansal S, Mittal S, Tiwari P, et al. Rigid Mini-Thoracoscopy Versus Semirigid Thoracoscopy in Undiagnosed Exudative Pleural Effusion: The MINT Randomized Controlled Trial. J Bronchology Interv Pulmonol 2020;27:163-71. [Crossref] [PubMed]
- Conacher ID. Anesthesia for thoracoscopic surgery. J Minim Access Surg 2007;3:127-31. [Crossref] [PubMed]
- Kochhar GS, Gill A, Vargo JJ. On the Horizon: The Future of Procedural Sedation. Gastrointest Endosc Clin N Am 2016;26:577-92. [Crossref] [PubMed]
- Avasarala SK, Lentz RJ, Maldonado F. Medical Thoracoscopy. Clin Chest Med 2021;42:751-66. [Crossref] [PubMed]
- Skalski JH, Astoul PJ, Maldonado F. Medical thoracoscopy. Semin Respir Crit Care Med 2014;35:732-43. [Crossref] [PubMed]
- Kostroglou A, Kapetanakis EI, Rougeris L, et al. Review of the Physiology and Anesthetic Considerations for Pleuroscopy/Medical Thoracoscopy. Respiration 2022;101:195-209. [Crossref] [PubMed]
- Kumar V, Sirohiya P, Gupta N, et al. Use of fentanyl-dexmedetomidine in conscious sedation for thoracoscopy. Lung India 2018;35:277-8. [Crossref] [PubMed]
- Sirohiya P, Kumar V, Mittal S, et al. Dexmedetomidine Versus Midazolam for Sedation During Medical Thoracoscopy: A Pilot Randomized-Controlled Trial (RCT). J Bronchology Interv Pulmonol 2022;29:248-54. [Crossref] [PubMed]
- Tschopp JM, Purek L, Frey JG, et al. Titrated sedation with propofol for medical thoracoscopy: a feasibility and safety study. Respiration 2011;82:451-7. [Crossref] [PubMed]
- Kuroda H, Sakao Y. Analgesic management after thoracoscopic surgery: recent studies and our experience. J Thorac Dis 2018;10:S1050-4. [Crossref] [PubMed]
- Abdelhady AM, Gadallah M, Shaheen M, et al. Intracavitary anaesthesia for medical thoracoscopy procedural pain: the CAMP andomized trial. The Egyptian Journal of Bronchology 2021;15:43.
- Koulelidis A, Anevlavis S, Nikitidis N, et al. Local Anesthesia Thoracoscopy with versus without Midazolam: A Randomized Controlled Trial. Respiration 2020;99:789-99. [Crossref] [PubMed]
- Vorster MJ, Bruwer JW, Frank W, et al. The use of propofol for sedation in medical thoracoscopy. Respiration 2015;89:435. [Crossref] [PubMed]
- Grendelmeier P, Tamm M, Jahn K, et al. Propofol versus midazolam in medical thoracoscopy: a randomized, noninferiority trial. Respiration 2014;88:126-36. [Crossref] [PubMed]
- Spyropoulos G, Kontakiotis T, Spyratos D, et al. AB 66. One-year experience of the pulmonary department of Aristotle University of Thessaloniki in thoracoscopy with local anaesthesia (medical thoracoscopy). J Thorac Dis 2012;4:AB66.
- Ajmal S, Johnstone S, Tufail M, et al. The Role of Multilevel Intercostal Nerve Block in Local Anesthetic Thoracoscopy. J Bronchology Interv Pulmonol 2023; Epub ahead of print. [Crossref]
- McPherson J, Halvey E, Aujayeb A. Erector spinae plane blocks for day-case medical thoracoscopy: a pilot clinical study. Pleura Peritoneum 2022;7:187-90. [Crossref] [PubMed]
- Chhajed PN, Kaegi B, Rajasekaran R, et al. Detection of hypoventilation during thoracoscopy: combined cutaneous carbon dioxide tension and oximetry monitoring with a new digital sensor. Chest 2005;127:585-8. [Crossref] [PubMed]
- Bridevaux PO, Tschopp JM, Cardillo G, et al. Short-term safety of thoracoscopic talc pleurodesis for recurrent primary spontaneous pneumothorax: a prospective European multicentre study. Eur Respir J 2011;38:770-3. [Crossref] [PubMed]
- Zhang W, Zhao YL, Li SJ, et al. Complications of thoracoscopic talc insufflation for the treatment of malignant pleural effusions: a meta-analysis. J Cardiothorac Surg 2021;16:125. [Crossref] [PubMed]
- Wan YY, Zhai CC, Lin XS, et al. Safety and complications of medical thoracoscopy in the management of pleural diseases. BMC Pulm Med 2019;19:125. [Crossref] [PubMed]
- Azzopardi M, Porcel JM, Koegelenberg CF, et al. Current controversies in the management of malignant pleural effusions. Semin Respir Crit Care Med 2014;35:723-31. [Crossref] [PubMed]
- Nour Moursi Ahmed S, Saka H, Mohammadien HA, et al. Safety and Complications of Medical Thoracoscopy. Adv Med 2016;2016:3794791. [Crossref] [PubMed]
- Boutin C, Rey F, Viallat JR. Prevention of malignant seeding after invasive diagnostic procedures in patients with pleural mesothelioma. A randomized trial of local radiotherapy. Chest 1995;108:754-8. [Crossref] [PubMed]
- Shojaee S, Lee HJ. Thoracoscopy: medical versus surgical-in the management of pleural diseases. J Thorac Dis 2015;7:S339-51. [Crossref] [PubMed]
- Hassan M, Harriss E, Mercer RM, et al. Survival and pleurodesis outcome in patients with malignant pleural effusion – a systematic review. Pleura Peritoneum 2021;6:1-5. [Crossref] [PubMed]
- Aelony Y, King RR, Boutin C. Thoracoscopic talc poudrage in malignant pleural effusions: effective pleurodesis despite low pleural Ph. Chest 1998;113:1007-12. [Crossref] [PubMed]
- Fantin A, Castaldo N, Vailati P, et al. Pleural effusion aetiology, presentation, treatment and outcome in haematological diseases: a review. Acta Biomed 2021;92:e2021268. [Crossref] [PubMed]
- Feller-Kopman D, Light R. Pleural Disease. N Engl J Med 2018;378:740-51. [Crossref] [PubMed]
- Taghizadeh N, Fortin M, Shieh B, et al. Prevalence and Etiology of Pleural Effusions in Hospitalized Patients: An Analysis of HCUP-NIS 2012. Chest 2017;152:A519.
- Naylor B, Schmidt RW. The case for exfoliative cytology of serous effusions. Lancet 1964;1:711-2. [Crossref] [PubMed]
- Assawasaksakul T, Boonsarngsuk V, Incharoen P. A comparative study of conventional cytology and cell block method in the diagnosis of pleural effusion. J Thorac Dis 2017;9:3161-7. [Crossref] [PubMed]
- Wu H, Khosla R, Rohatgi PK, et al. The minimum volume of pleural fluid required to diagnose malignant pleural effusion: A retrospective study. Lung India 2017;34:34-7. [Crossref] [PubMed]
- Khan SL, Haris M, Munavvar M. Diagnostic accuracy of pleural fluid cytology compared to pleural biopsy histology obtained via thoracoscopy. Eur Respir J 2014;44:2775.
- Mei F, Bonifazi M, Rota M, et al. Diagnostic Yield and Safety of Image-Guided Pleural Biopsy: A Systematic Review and Meta-Analysis. Respiration 2021;100:77-87. [Crossref] [PubMed]
- Colt HG, Dumon JF. Development of a disposable spray canister for talc pleurodesis. A preliminary report. Chest 1994;106:1776-80. [Crossref] [PubMed]
- Boonsarngsuk V, Juthakarn S, Boonsarngsuk W. Homemade talc spray atomizer dedicated to flexible-rigid pleuroscope. Clin Respir J 2012;6:40-5. [Crossref] [PubMed]
- Jutley RS, Waqar S, Raha N, et al. Simple technique of talc delivery for video-assisted talc pleurodesis. Gen Thorac Cardiovasc Surg 2009;57:116-7. [Crossref] [PubMed]
- Dresler CM, Olak J, Herndon JE 2nd, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest 2005;127:909-15. [Crossref] [PubMed]
- Beck TN, Deneka AY, Chai L, et al. An improved method of delivering a sclerosing agent for the treatment of malignant pleural effusion. BMC Cancer 2019;19:614. [Crossref] [PubMed]
- Baxter J, Lima TA, Huneke R, et al. The efficacy of hydrogel foams in talc Pleurodesis. J Cardiothorac Surg 2020;15:58. [Crossref] [PubMed]
- Foo CT, Pulimood T, Knolle M, et al. Ambulatory Thoracoscopic Pleurodesis Combined With Indwelling Pleural Catheter in Malignant Pleural Effusion. Front Surg 2021;8:738719. [Crossref] [PubMed]
- Dipper A, Welch H, Maskell N. Multimodal Approaches Toward Management of Malignant Pleural Effusion: Establishing Treatment Goals is Paramount. Arch Bronconeumol 2022;58:640-1. [Crossref] [PubMed]
- Turner M, Craighead F, Mackenzie J, et al. P99 Day case thoracoscopy with IPC insertion- experience from 2 district general hospitals. Thorax 2022;77:A134-5.
- Turner M, Craighead F, MacKenzie JD, et al. Day Case Local Anaesthetic Thoracoscopy: Experience from 2 District General Hospitals in the United Kingdom. Med Sci (Basel) 2023;11:23. [Crossref] [PubMed]
- Reddy C, Ernst A, Lamb C, et al. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest 2011;139:1419-23. [Crossref] [PubMed]
- Bertolaccini L, Viti A, Terzi A. Management of malignant pleural effusions in patients with trapped lung with indwelling pleural catheter: how to do it. J Vis Surg 2016;2:44. [Crossref] [PubMed]
- Reddy CB, DeCamp MM, Diekemper RL, et al. Summary for Clinicians: Clinical Practice Guideline for Management of Malignant Pleural Effusions. Ann Am Thorac Soc 2019;16:17-21. [Crossref] [PubMed]
- Dipper A, Jones HE, Bhatnagar R, et al. Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst Rev 2020;4:CD010529. [Crossref] [PubMed]
- Menga G, Girbal MS, Montoto Piazza L, et al. Alpha-1 antitrypsin deficiency and spontaneous pneumothorax. Just a coincidence? Medicina (B Aires) 2020;80:473-8.
- Hallifax R. Aetiology of Primary Spontaneous Pneumothorax. J Clin Med 2022;11:490. [Crossref] [PubMed]
- Noppen M. Spontaneous pneumothorax: epidemiology, pathophysiology and cause. Eur Respir Rev 2010;19:217-9. [Crossref] [PubMed]
- Zarogoulidis P, Kioumis I, Pitsiou G, et al. Pneumothorax: from definition to diagnosis and treatment. J Thorac Dis 2014;6:S372-6. [Crossref] [PubMed]
- Nava GW, Walker SP. Management of the Secondary Spontaneous Pneumothorax: Current Guidance, Controversies, and Recent Advances. J Clin Med 2022;11:1173. [Crossref] [PubMed]
- Vetrugno L, Castaldo N, Fantin A, et al. Ventilatory associated barotrauma in COVID-19 patients: A multicenter observational case control study (COVI-MIX-study). Pulmonology 2023;29:457-68. [Crossref] [PubMed]
- Aiello M, Fantin A, Longo C, et al. Clinical manifestations in patients with PI*MM(Malton) genotypes. A matter still unsolved in alpha-1 antitrypsin deficiency. Respirol Case Rep 2020;8:e00528. [Crossref] [PubMed]
- Serapinas D, Obrikyte V, Vaicius D, et al. Alpha-1 antitrypsin deficiency and spontaneous pneumothorax: possible causal relationship. Pneumologia 2014;63:32-5.
- Tschopp JM, Brutsche M, Frey JG. Treatment of complicated spontaneous pneumothorax by simple talc pleurodesis under thoracoscopy and local anaesthesia. Thorax 1997;52:329-32. [Crossref] [PubMed]
- Tschopp JM, Schnyder JM, Froudarakis M, et al. VATS or simple talc poudrage under medical thoracoscopy for recurrent spontaneous pneumothorax. Eur Respir J 2009;33:442-3. [Crossref] [PubMed]
- Tschopp JM, Bintcliffe O, Astoul P, et al. ERS task force statement: diagnosis and treatment of primary spontaneous pneumothorax. Eur Respir J 2015;46:321-35. [Crossref] [PubMed]
- Walker SP, Bibby AC, Halford P, et al. Recurrence rates in primary spontaneous pneumothorax: a systematic review and meta-analysis. Eur Respir J 2018;52:1800864. [Crossref] [PubMed]
- Hallifax RJ, Yousuf A, Jones HE, et al. Effectiveness of chemical pleurodesis in spontaneous pneumothorax recurrence prevention: a systematic review. Thorax 2017;72:1121-31. [Crossref] [PubMed]
- Lee P, Yap WS, Pek WY, et al. An Audit of medical thoracoscopy and talc poudrage for pneumothorax prevention in advanced COPD. Chest 2004;125:1315-20. [Crossref] [PubMed]
- Hallifax RJ, Goldacre R, Landray MJ, et al. Trends in the Incidence and Recurrence of Inpatient-Treated Spontaneous Pneumothorax, 1968-2016. JAMA 2018;320:1471-80. [Crossref] [PubMed]
- Videm V, Pillgram-Larsen J, Ellingsen O, et al. Spontaneous pneumothorax in chronic obstructive pulmonary disease: complications, treatment and recurrences. Eur J Respir Dis 1987;71:365-71.
- Guo HY, Pan XQ, Hu M, et al. Medical Thoracoscopy-Assisted Argon Plasma Coagulation Combined with Electrosurgical Unit for the Treatment of Refractory Pneumothorax in Elderly Patients. Ann Thorac Cardiovasc Surg 2019;25:237-45. [Crossref] [PubMed]
- Zhang L, Xie T, Fu Y, et al. Assessment and review of treatment for secondary spontaneous pneumothorax using medical thoracoscopy-assisted argon plasma coagulation in association with autologous blood pleurodesis. Ther Adv Respir Dis 2021;15:1753466620986390. [Crossref] [PubMed]
- Tschopp JM, Boutin C, Astoul P, et al. Talcage by medical thoracoscopy for primary spontaneous pneumothorax is more cost-effective than drainage: a andomized study. Eur Respir J 2002;20:1003-9. [Crossref] [PubMed]
- Vuong NL, Elshafay A, Thao LP, et al. Efficacy of treatments in primary spontaneous pneumothorax: A systematic review and network meta-analysis of randomized clinical trials. Respir Med 2018;137:152-66. [Crossref] [PubMed]
- Mendogni P, Vannucci J, Ghisalberti M, et al. Epidemiology and management of primary spontaneous pneumothorax: a systematic review. Interact Cardiovasc Thorac Surg 2020;30:337-45. [Crossref] [PubMed]
- Muhetaer M, Paerhati K, Sun Q, et al. Effects of Different Treatment Regimens on Primary Spontaneous Pneumothorax: A Systematic Review and Network Meta-Analysis. Ann Thorac Cardiovasc Surg 2022;28:389-402. [Crossref] [PubMed]
- Eamer G, Povolo CA, Petropoulos JA, et al. Observation, Aspiration, or Tube Thoracostomy for Primary Spontaneous Pneumothorax: A Systematic Review, Meta-Analysis, and Cost-Utility Analysis. Chest 2023;164:1007-18. [Crossref] [PubMed]
- Speck KE, Kulaylat AN, Baerg JE, et al. Evaluation and Management of Primary Spontaneous Pneumothorax in Adolescents and Young Adults: A Systematic Review From the APSA Outcomes & Evidence-Based Practice Committee. J Pediatr Surg 2023;58:1873-85. [Crossref] [PubMed]
- Parrish S, Browning RF, Turner JF Jr, et al. The role for medical thoracoscopy in pneumothorax. J Thorac Dis 2014;6:S383-91. [Crossref] [PubMed]
- Mares DC, Mathur PN. Medical thoracoscopic talc pleurodesis for chylothorax due to lymphoma: a case series. Chest 1998;114:731-5. [Crossref] [PubMed]
- Agrawal A, Chaddha U, Kaul V, et al. Multidisciplinary Management of Chylothorax. Chest 2022;162:1402-12. [Crossref] [PubMed]
- Lee WJ, Kim HJ, Park JH, et al. Chemical pleurodesis for the management of refractory hepatic hydrothorax in patients with decompensated liver cirrhosis. Korean J Hepatol 2011;17:292-8. [Crossref] [PubMed]
- Milanez de Campos JR, Filho LO, de Campos Werebe E, et al. Thoracoscopy and talc poudrage in the management of hepatic hydrothorax. Chest 2000;118:13-7. [Crossref] [PubMed]
- Papakonstantinou NA, Hardavella G, Papavasileiou G, et al. Medical thoracoscopy for the treatment of complicated hepatic hydrothorax. J Surg Case Rep 2012;2012:2. [Crossref] [PubMed]
- Bobbio A, Bouam S, Frenkiel J, et al. Epidemiology and prognostic factors of pleural empyema. Thorax 2021;76:1117-23. [Crossref] [PubMed]
- Colice GL, Curtis A, Deslauriers J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest 2000;118:1158-71. [Crossref] [PubMed]
- Cassina PC, Hauser M, Hillejan L, et al. Video-assisted thoracoscopy in the treatment of pleural empyema: stage-based management and outcome. J Thorac Cardiovasc Surg 1999;117:234-8. [Crossref] [PubMed]
- Wozniak CJ, Paull DE, Moezzi JE, et al. Choice of first intervention is related to outcomes in the management of empyema. Ann Thorac Surg 2009;87:1525-30; discussion 1530-1. [Crossref] [PubMed]
- Shiroshita A, Kimura Y, Yamada A, et al. Effectiveness of Immediate Video-Assisted Thoracoscopic Surgery for Empyema: A Multicentre, Retrospective Cohort Study. Respiration 2023;102:821-32. [Crossref] [PubMed]
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and Dnase in pleural infection. N Engl J Med 2011;365:518-26. [Crossref] [PubMed]
- Bedawi EO, Sundaralingam A, Rahman NM. “The Cold Steel of a Surgeon or Some Fool of a Physician?”: The Debate Continues. Ann Am Thorac Soc 2022;19:1801-3. [Crossref] [PubMed]
- Bedawi EO, Stavroulias D, Hedley E, et al. Early Video-assisted Thoracoscopic Surgery or Intrapleural Enzyme Therapy in Pleural Infection: A Feasibility Randomized Controlled Trial. The Third Multicenter Intrapleural Sepsis Trial-MIST-3. Am J Respir Crit Care Med 2023;208:1305-15. [Crossref] [PubMed]
- Brutsche MH, Tassi GF, Györik S, et al. Treatment of sonographically stratified multiloculated thoracic empyema by medical thoracoscopy. Chest 2005;128:3303-9. [Crossref] [PubMed]
- Waller DA. Thoracoscopy in management of postpneumonic pleural infections. Curr Opin Pulm Med 2002;8:323-6. [Crossref] [PubMed]
- Tassi GF, Marchetti GP, Aliprandi PL. Advanced medical thoracoscopy. Monaldi Arch Chest Dis 2011;75:99-101. [Crossref] [PubMed]
- Terashita S, Kawachi H, Tajiri T, et al. Intrapleural urokinase directly under medical thoracoscopy for the diagnosis of tuberculous pleurisy. Respirol Case Rep 2020;8:e00498. [Crossref] [PubMed]
- Stirpe E, Bardaro F, Köhl J. Intracavitary fibrinolysis directly under vision during medical thoracoscopy: a case report. Monaldi Arch Chest Dis 2022;
- Zgoda M, Lunn W, Ernst A, et al. Direct Visual Guidance for Chest Tube Placement After Single-port Thoracoscopy: A Novel Technique. Chest 2004;126:820S. [Crossref] [PubMed]
- Ranganatha R, Tousheed SZ. Role of medical thoracoscopy in the treatment of complicated parapneumonic effusions. Lung India 2021;38:149-53. [Crossref] [PubMed]
- Ravaglia C, Ghirotti C, Puglisi S, et al. Medical Thoracoscopy and Intrapleural Fibrinolytic Therapy for the Management of Pleural Empyema: A Cohort Study. Respiration 2023;102:46-54. [Crossref] [PubMed]
- Mondoni M, Saderi L, Trogu F, et al. Medical thoracoscopy treatment for pleural infections: a systematic review and meta-analysis. BMC Pulm Med 2021;21:127. [Crossref] [PubMed]
- Ricciardi S, Giovanniello D, Carleo F, et al. Which Surgery for Stage II-III Empyema Patients? Observational Single-Center Cohort Study of 719 Consecutive Patients. J Clin Med 2022;12:136. [Crossref] [PubMed]
- Shin JA, Chang YS, Kim TH, et al. Surgical decortication as the first-line treatment for pleural empyema. J Thorac Cardiovasc Surg 2013;145:933-939.e1. [Crossref] [PubMed]
- Chung JH, Lee SH, Kim KT, et al. Optimal timing of thoracoscopic drainage and decortication for empyema. Ann Thorac Surg 2014;97:224-9. [Crossref] [PubMed]
- Sundaralingam A, Banka R, Rahman NM. Management of Pleural Infection. Pulm Ther 2021;7:59-74. [Crossref] [PubMed]
- Balfour-Lynn IM, Abrahamson E, Cohen G, et al. BTS guidelines for the management of pleural infection in children. Thorax 2005;60:i1-21. [Crossref] [PubMed]
- Mandal KC, Mandal G, Halder P, et al. Empyema Thoracis in Children: A 5-Year Experience in a Tertiary Care Institute. J Indian Assoc Pediatr Surg 2019;24:197-202. [Crossref] [PubMed]
- Givan DC, Eigen H. Common pleural effusions in children. Clin Chest Med 1998;19:363-71. [Crossref] [PubMed]
- Islam S, Calkins CM, Goldin AB, et al. The diagnosis and management of empyema in children: a comprehensive review from the APSA Outcomes and Clinical Trials Committee. J Pediatr Surg 2012;47:2101-10. [Crossref] [PubMed]
- Stockmann C, Ampofo K, Pavia AT, et al. Comparative Effectiveness of Oral Versus Outpatient Parenteral Antibiotic Therapy for Empyema. Hosp Pediatr 2015;5:605-12. [Crossref] [PubMed]
- Carter E, Waldhausen J, Zhang W, et al. Management of children with empyema: Pleural drainage is not always necessary. Pediatr Pulmonol 2010;45:475-80. [Crossref] [PubMed]
- Lin CH, Lin WC, Chang JS. Comparison of pigtail catheter with chest tube for drainage of parapneumonic effusion in children. Pediatr Neonatol 2011;52:337-41. [Crossref] [PubMed]
- Sonnappa S, Cohen G, Owens CM, et al. Comparison of urokinase and video-assisted thoracoscopic surgery for treatment of childhood empyema. Am J Respir Crit Care Med 2006;174:221-7. [Crossref] [PubMed]
- St Peter SD, Tsao K, Spilde TL, et al. Thoracoscopic decortication vs tube thoracostomy with fibrinolysis for empyema in children: a prospective, randomized trial. J Pediatr Surg 2009;44:106-11; discussion 111. [Crossref] [PubMed]
- Zuccatosta L, Piciucchi S, Martinello S, et al. Is there any role for medical thoracoscopy in the treatment of empyema in children? Clin Respir J 2023;17:105-8. [Crossref] [PubMed]
- Bauman ZM, Kulvatunyou N, Joseph B, et al. Randomized Clinical Trial of 14-French (14F) Pigtail Catheters versus 28-32F Chest Tubes in the Management of Patients with Traumatic Hemothorax and Hemopneumothorax. World J Surg 2021;45:880-6. [Crossref] [PubMed]
- Choi J, Villarreal J, Andersen W, et al. Scoping review of traumatic hemothorax: Evidence and knowledge gaps, from diagnosis to chest tube removal. Surgery 2021;170:1260-7. [Crossref] [PubMed]
- Boersma WG, Stigt JA, Smit HJ. Treatment of haemothorax. Respir Med 2010;104:1583-7. [Crossref] [PubMed]
- Chou YP, Lin HL, Wu TC. Video-assisted thoracoscopic surgery for retained hemothorax in blunt chest trauma. Curr Opin Pulm Med 2015;21:393-8. [Crossref] [PubMed]
- Wilson JM, Boren CH Jr, Peterson SR, et al. Traumatic hemothorax: is decortication necessary? J Thorac Cardiovasc Surg 1979;77:489-95.
- Zambetti BR, Lewis RH Jr, Chintalapani SR, et al. Optimal time to thoracoscopy for trauma patients with retained hemothorax. Surgery 2022;172:1265-9. [Crossref] [PubMed]
- Mondoni M, Radovanovic D, Sotgiu G, et al. Interventional pulmonology techniques in elderly patients with comorbidities. Eur J Intern Med 2019;59:14-20. [Crossref] [PubMed]
- Zeiler J, Idell S, Norwood S, et al. Hemothorax: A Review of the Literature. Clin Pulm Med 2020;27:1-12. [Crossref] [PubMed]
- Liu QH, Lin DJ. Utility of medical thoracoscopy in diagnosis and treatment of hemothorax due to carcinoma: A case report. J Cancer Res Ther 2020;16:933-4. [Crossref] [PubMed]
- Yu PS, Chan HH, Lau RW, et al. Penetrating thoracic injury with retained foreign body: can video-assisted thoracic surgery take up the leading role in acute management? J Thorac Dis 2016;8:2247-51. [Crossref] [PubMed]
- Cheng WC, Wu BR, Chen CH, et al. Medical thoracoscopy removal of a self-expandable metallic stent migration into pleural cavity. J Thorac Dis 2018;10:3054-8. [Crossref] [PubMed]
- Narasimhan RL, Sehgal IS, Dhooria S, et al. Removal of Intrapleural Foreign Body by Medical Thoracoscopy: Report of Two Cases and a Systematic Review of the Literature. J Bronchology Interv Pulmonol 2017;24:244-9.
- Gonzalez-Rivas D, Bonome C, Fieira E, et al. Non-intubated video-assisted thoracoscopic lung resections: the future of thoracic surgery? Eur J Cardiothorac Surg 2016;49:721-31. [Crossref] [PubMed]
- AlGhamdi ZM, Ahn S, Kim KC, et al. Non-intubated uniportal VATS surgery is feasible approach. J Thorac Dis 2020;12:1147-50. [Crossref] [PubMed]
- Ali MS, Light RW, Maldonado F. Pleuroscopy or video-assisted thoracoscopic surgery for exudative pleural effusion: a comparative overview. J Thorac Dis 2019;11:3207-16. [Crossref] [PubMed]