
Triglyceride-glucose index and triglyceride to high-density lipoprotein cholesterol ratio predict the prognosis in patients with type B aortic dissection receiving thoracic endovascular aortic repair
Highlight box
Key findings
• The triglyceride to high-density lipoprotein cholesterol (TG/HDL-c) ratio is significantly related to the risk of 1-year all-cause mortality among type B aortic dissection (TBAD) patients undergoing thoracic endovascular aortic repair (TEVAR).
What is known and what is new?
• Continuous TG/HDL-c ratio and a higher or an overly low TG/HDL-c ratio are independent risk factors for 1-year all-cause mortality.
• Insulin resistance, represented by TG/HDL-c ratio, may be a novel mechanism influencing aortic remodeling after TEVAR.
What is the implication, and what should change now?
• The evaluation of TG/HDL-c ratio before TEVAR is necessary for TBAD patients for additional risk stratification, refined management and postoperative monitoring.
Introduction
Type B aortic dissection (TBAD) is one of the most fatal cardiovascular diseases (CVDs); it is classified as the entry tear distal to the left subclavian artery with sparing of the ascending aorta (1). The incidence of aortic dissection (AD) is the highest in age group of 65 to 75 years, with 35 cases per 100,000 people per year in this population (2). Hypertension, dyslipidemia and genetic disorders are considered as other contributing factors (3). Patients with TBAD are typically treated with medication and thoracic endovascular aortic repair (TEVAR) (4), which has been found to lower the incidence of aortic-related adverse events (ARAEs) and mortality and led to favorable aortic remodeling in TBAD patients (5,6). However, ARAEs such as retrograde type A dissection (RTAD), aortic rupture, aortic dilatation, etc., after TEVAR may undermine its advantage (7). Therefore, it is crucial to have better risk stratification of patients, which is also a challenge for the facilitation of the management of patients with TBAD.
A number of factors including D-dimer, platelets, neutrophil-to-lymphocyte ratio (NLR), etc. have been proposed to be possibly related with the outcomes of TBAD patients who undergo TEVAR (8-10). Our previous study found that peripheral eosinophil count was associated with 1-year all-cause mortality in TBAD patients (11). Additional indicators mediated by novel mechanisms may improve the effectiveness of the current prognostic model for TBAD patients undergoing TEVAR.
Insulin resistance (IR) refers to diminished or impaired reactivity to endogenous and exogenous insulin in the tissues and organs that are dependent on insulin (12). IR has been proven to be a significant risk factor for the development of CVD and type 2 diabetes mellitus (T2DM) (13). Triglyceride-glucose (TyG) index and triglyceride to high-density lipoprotein cholesterol (TG/HDL-c) ratio were also suggested as straightforward and reliable surrogate indicators of IR as a result of strongly correlating with the euglycemic hyperinsulinemia clamp and are appropriate for clinical use and large epidemiological studies (14,15). TyG index has been found to be positively correlated with coronary heart disease risk and reflect coronary atherosclerosis severity (16). An analysis of UK Biobank data also revealed that TyG index and TG/HDL-c ratio were potential CVD risk factors (17).
However, very few studies focused on the influence of the TG/HDL-c ratio and TyG index on the prognosis of TBAD patients who undergo TEVAR. Consequently, our study aims to explore the effect of the baseline TG/HDL-c ratio and TyG index on 30-day and 1-year outcomes in TBAD patients. We present this article in accordance with the TRIPOD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1411/rc).
Methods
Study cohort and design
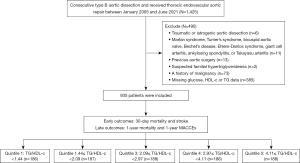
This is a retrospective cohort study. From January 2005 to June 2021, a total of 1,425 consecutive TBAD patients underwent TEVAR at The First Affiliated Hospital of the Navy Medical University, Shanghai, China. The exclusion criteria included: (I) traumatic AD; (II) Marfan syndrome, Turner’s syndrome, bicuspid aortic valve, Bechet’s disease, Ehlers-Danlos syndrome, giant cell arteritis, ankylosing spondylitis, or Takayasu arteritis; (III) previous aortic surgery; (IV) suspected familial hypertriglyceridemia (plasma TGs ≥500 mg/dL); (V) a history of malignancy; (VI) and missing admission glucose or TG measurement (Figure 1). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The research protocol was approved by The First Affiliated Hospital of the Navy Medical University central ethics committee (CHEC-Y2021, March 1, 2021). Informed consent was waived due to the retrospective nature of the study.
Data collection and definitions
The electronic medical records were used to gather information on sociodemographic characteristics, medical history, smoking status, comorbidities, laboratory testing, and intra-operative details. The imaging department provided the image information. TBAD was classified as an AD with an entry tear in zone 3 or 4 (18). AD was categorized as acute (1–14 days), subacute (15–90 days), and chronic (>90 days) according to Society for Vascular Surgery (SVS)/Society of Thoracic Surgeons (STS) recommendations (18). ARAEs included aortic rupture, malperfusion, RTAD, aortic dilation, and type I/III endoleak (7). The blood samples of patients who received surgery that are not performed in urgent/emergency setting were collected at 6 a.m. on the day of surgery. If it was an emergency surgery, the blood samples were obtained in the emergency room or during surgery. Standard biochemical methods were used at the Clinical Laboratory of The First Affiliated Hospital of the Navy Medical University to assess laboratory parameters such as D-dimer, creatinine, glucose, TG, total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and uric acid. The following equation was used to construct the TyG index: ln [TG (mg/dL) × glucose (mg/dL)/2] (19). The TG/HDL-c ratio was computed by dividing the TG (mg/dL) by the HDL-C (mg/dL) levels (17). Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2.
Follow-up and endpoints
Research was conducted with qualified researchers by phone survey or by reviewing the medical records of all patients. Furthermore, adverse events were assessed in the comprehensive clinical files of patients requiring re-admission or reviewed in clinic. Study endpoints included the following 30-day outcomes: 30-day all-cause death; 30-day stroke and 1-year outcomes: 1-year all-cause death and 1-year major adverse cardiovascular and cerebrovascular events (MACCEs). ARAEs, which were traditionally believed to be only associated with technical failure, were also depicted in this study. Two clinicians experienced in the diagnosis and treatment of TBAD blindly evaluated the adverse events and patients’ outcome.
Statistical analysis
The participants were classified into five groups [Quintile 1 (Q1) (n=186, TG/HDL-c ratio <1.44), Quintile 2 (Q2) (n=187, 1.44≤ TG/HDL-c ratio <2.09), Quintile 3 (Q3) (n=188, 2.09≤ TG/HDL-c ratio <2.97), Quintile 4 (Q4) (n=186, 2.97≤ TG/HDL-c ratio <4.11), and Quintile 5 (Q5) (n=188, TG/HDL-c ratio ≥4.11)], by the quintiles of TG/HDL-c ratio, and the Q4 group was used as the reference group, and the characteristics were depicted. Variables with continuous distributions were compared using a student t-test or Mann-Whitney test based on the means, standard deviations or medians (quintiles 1 through 5). Categorical variables were reported as percentages and tested with Chi-squared or Fisher’s exact tests. TG/HDL-c ratio was originally input as a continuous variable and then modeled as a categorical variable. We calculated cumulative survival curves using Kaplan-Meier (KM) methods and used log-rank tests to differentiate between groups.
Univariable and multivariable Cox regression models for 1-year all-cause mortality, as well as Cox regression models for 1-year ARAEs, were applied to investigate the relationship between the preoperative TG/HDL-c ratio and 1-year outcomes. Variables with a P value of 0.1 in the univariable analysis were introduced into the multivariable models using a stepwise forward approach. Moreover, we employed general additive models (GAMs) with restricted cubic splines (RCS) (giving 4 degrees of freedom) for visual analysis to evaluate the nonlinear correlations between continuous variables and outcomes. The statistical analyses were performed using R version 3.6.3 and EmpowerStats software (www.empowerstats.com). A P value of 0.05 was chosen as the statistical significance level.
Results
Clinical characteristics
Table 1 demonstrates the participant characteristics both overall and stratified by the quintiles of the TG/HDL-c ratio. The mean age of the included 935 patients was 59.8±13.3 years, with 759 (81.2%) men (P=0.001). Q1 group was significantly older in the all groups (P<0.001). Smoking history and sex were statistically different among the groups (P=0.001). The body mass index (BMI) of the five quintiles were 23.5±3.7, 24.0±3.9, 24.4±3.3, 24.9±3.1, and 25.4±3.7 kg/m2, respectively, which was significantly different (P<0.001). Systolic blood pressure (SBP), and diastolic blood pressure (DBP) did not differ significantly among the groups (all P>0.05). There was no significant difference in comorbidities among groups (all P>0.05). In terms of laboratory tests, the difference in platelet (P=0.002), creatinine (P=0.012), total cholesterol (P<0.001) and LDL-C (P=0.028) was statistically significant among groups. The uric acid was remarkably higher in Q5 group (231.9±2,323.6 mg/dL, P<0.001) compared with the other groups. The difference in other laboratory tests [white blood cell (WBC), hemoglobin, D-dimer and glucose] was not significant (all P>0.05). Branch, adjunct, and hybrid technique utilization and the timing of TEVAR among groups were not statistically different (all P>0.05). Besides, the anatomical characteristics of the five groups were comparable (all P>0.05) except for the extent of proximal thrombosis of the false lumen (P<0.001).
Table 1
| Variables | Total | TG/HDL-c | P value | ||||
|---|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |||
| N | 935 | 186 | 187 | 188 | 186 | 188 | – |
| Age (years) | 59.8±13.3 | 62.7±14.0 | 60.3±13.3 | 60.7±12.7 | 59.8±13.2 | 55.6±12.1 | <0.001 |
| Male | 759 (81.2) | 140 (75.3) | 142 (75.9) | 151 (80.3) | 158 (84.9) | 168 (89.4) | 0.001 |
| BMI (kg/m2) | 24.4±3.6 | 23.5±3.7 | 24.0±3.9 | 24.4±3.3 | 24.9±3.1 | 25.4±3.7 | <0.001 |
| Smoking | 475 (50.8) | 72 (38.7) | 90 (48.1) | 99 (52.7) | 108 (58.1) | 106 (56.4) | 0.001 |
| SBP at admission (mmHg) | 137.3±21.8 | 139.9±24.0 | 137.6±22.0 | 136.1±20.3 | 135.9±22.2 | 137.3±20.6 | 0.415 |
| DBP at admission (mmHg) | 81.7±11.6 | 82.2±11.9 | 80.7±12.1 | 81.5±11.0 | 81.3±11.3 | 82.6±11.4 | 0.538 |
| Comorbidities | |||||||
| Hypertension | 705 (75.4) | 134 (72.0) | 137 (73.3) | 140 (74.5) | 149 (80.1) | 145 (77.1) | 0.379 |
| Diabetes mellitus | 87 (9.3) | 9 (4.8) | 17 (9.1) | 17 (9.0) | 23 (12.4) | 21 (11.2) | 0.123 |
| Stroke | 57 (6.1) | 7 (3.8) | 11 (5.9) | 14 (7.4) | 17 (9.1) | 8 (4.3) | 0.164 |
| CAD | 60 (6.4) | 12 (6.5) | 7 (3.7) | 9 (4.8) | 18 (9.7) | 14 (7.4) | 0.154 |
| COPD | 124 (13.3) | 28 (15.1) | 24 (12.8) | 30 (16.0) | 27 (14.5) | 15 (8.0) | 0.162 |
| CKD | 56 (6.0) | 15 (8.1) | 6 (3.2) | 10 (5.3) | 16 (8.6) | 9 (4.8) | 0.142 |
| Pericardial effusion | 69 (7.4) | 19 (10.2) | 12 (6.4) | 19 (10.1) | 9 (4.8) | 10 (5.3) | 0.116 |
| Pleural effusion | 316 (33.8) | 67 (36.0) | 72 (38.5) | 61 (32.4) | 66 (35.5) | 50 (26.6) | 0.135 |
| Laboratory tests | |||||||
| WBC (×109/L) | 8.6±3.6 | 8.6±3.6 | 8.9±4.1 | 8.1±3.0 | 8.4±3.7 | 8.9±3.7 | 0.167 |
| Platelet (×109/L) | 210.6±87.6 | 183.8±76.1 | 220.1±86.5 | 210.1±97.2 | 218.8±83.3 | 218.3±88.3 | 0.002 |
| Hemoglobin (g/L) | 128.0±19.9 | 125.4±21.0 | 128.0±17.3 | 127.3±20.2 | 129.1±19.7 | 130.0±21.2 | 0.288 |
| D-dimer (mg/L) | 3.4±4.6 | 4.0±6.1 | 3.5±4.2 | 3.1±3.8 | 2.9±3.7 | 3.5±4.8 | 0.346 |
| Creatinine (μmol/L) | 103.4±111.7 | 114.0±140.2 | 90.7±62.9 | 99.9±92.2 | 112.8±139.3 | 100.9±108.0 | 0.012 |
| Glucose (mg/dL) | 120.7±41.3 | 116.4±33.0 | 124.6±44.4 | 115.4±35.7 | 122.3±48.7 | 124.6±42.0 | 0.065 |
| Total cholesterol (mg/dL) | 170.9±42.7 | 162.1±36.4 | 168.7±35.4 | 167.9±35.4 | 174.4±38.6 | 181.2±60.2 | <0.001 |
| Triglycerides (mg/dL) | 123.7±85.7 | 58.7±16.7 | 86.0±18.4 | 107.4±22.3 | 136.2±31.6 | 229.4±131.5 | <0.001 |
| LDL-C (mg/dL) | 53.9±179.3 | 29.5±58.4 | 41.4±116.1 | 38.8±34.1 | 80.4±269.7 | 78.9±262.4 | 0.028 |
| HDL-C (mg/dL) | 44.7±13.7 | 58.9±15.8 | 49.0±9.9 | 43.2±8.4 | 39.0±8.3 | 33.3±8.8 | <0.001 |
| Uric acid (mg/dL) | 89.6±1045.1 | 50.2±16.7 | 51.9±17.0 | 56.7±20.6 | 55.2±20.3 | 231.9±2,323.6 | <0.001 |
| Intra-operative details | |||||||
| Timing of operation | 0.03 | ||||||
| Acute | 537 (57.4) | 113 (60.8) | 119 (63.6) | 105 (55.9) | 114 (61.3) | 86 (45.7) | |
| Sub-acute | 285 (30.5) | 50 (26.9) | 49 (26.2) | 58 (30.9) | 55 (29.6) | 71 (37.8) | |
| Chronic | 113 (12.1) | 23 (12.4) | 19 (10.2) | 25 (13.3) | 17 (9.1) | 31 (16.5) | |
| Branch | 153 (16.4) | 29 (15.6) | 34 (18.2) | 27 (14.4) | 29 (15.6) | 34 (18.1) | 0.814 |
| Adjunct | 135 (14.4) | 18 (9.7) | 35 (18.7) | 27 (14.4) | 27 (14.5) | 28 (14.9) | 0.184 |
| Hybrid | 10 (1.1) | 2 (1.1) | 2 (1.1) | 1 (0.5) | 3 (1.6) | 2 (1.1) | 0.905 |
| Anatomical characteristics | |||||||
| Dissection length (mm) | 419.7±135.9 | 379.2±110.3 | 451.4±105.9 | 411.0±159.7 | 383.8±174.3 | 456.1±120.8 | 0.175 |
| Proximal thrombosis of FL | <0.001 | ||||||
| Patent | 387 (41.4) | 66 (35.5) | 70 (37.4) | 77 (41.0) | 97 (52.2) | 76 (40.4) | |
| Partial | 298 (31.9) | 71 (38.2) | 77 (41.2) | 60 (31.9) | 35 (18.8) | 56 (29.8) | |
| Complete | 146 (15.6) | 32 (17.2) | 24 (12.8) | 28 (14.9) | 25 (13.4) | 36 (19.1) | |
| ULP | 104 (11.1) | 17 (9.1) | 16 (8.6) | 23 (12.2) | 29 (15.6) | 20 (10.6) | |
| Superior mesenteric arteries | 5 (0.5) | 0 (0.0) | 1 (0.5) | 1 (0.5) | 2 (1.1) | 1 (0.5) | 0.732 |
| Renal arteries | 33 (3.5) | 4 (2.2) | 10 (5.3) | 8 (4.3) | 4 (2.2) | 7 (3.7) | 0.379 |
| Common hepatic arteries | 1 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 0.402 |
| Lower-extremity arteries | 9 (1.0) | 0 (0.0) | 3 (1.6) | 0 (0.0) | 4 (2.2) | 2 (1.1) | 0.125 |
Values are presented as n (%) or mean ± standard deviation. Quintile 1: TG/HDL-c ratio <1.44. Quintile 2: 1.44≤ TG/HDL-c ratio <2.09. Quintile 3: 2.09≤ TG/HDL-c ratio <2.97. Quintile 4: 2.97≤ TG/HDL-c ratio <4.11. Quintile 5: TG/HDL-c ratio ≥4.11. TBAD, type B aortic dissection; TG/HDL-c, triglyceride to high-density lipoprotein cholesterol; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; WBC, white blood cell; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; FL, false lumen; ULP, ulcer-like projection.
30-day outcomes
The average length of stay in the hospital following TEVAR was 11.8±6.6 days in the Q1 group, 12.4±7.4 days in the Q2 group, 11.4±6.2 days in the Q3 group, 11.2±5.7 days in the Q4 group, and 12.8±9.5 days in the Q5 group (P=0.129). In hospitalization or 30 days after TEVAR, there were no significant differences in mortality among the five groups (3.2% vs. 1.1% vs. 1.6% vs. 1.1% vs. 2.7%, P=0.441). There was also no statistical difference in the 30-day stroke after TEVAR (1.1% vs. 0.5% vs. 0.0% vs. 0.5% vs. 1.6%, P=0.444). The 30-day outcomes are detailed in Table 2.
Table 2
| Variables | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P value |
|---|---|---|---|---|---|---|
| Hospital stays of post-TEVAR (days) | 11.8±6.6 | 12.4±7.4 | 11.4±6.2 | 11.2±5.7 | 12.8±9.5 | 0.129 |
| 30-day outcomes | ||||||
| 30-day mortality | 6 (3.2) | 2 (1.1) | 3 (1.6) | 2 (1.1) | 5 (2.7) | 0.441 |
| 30-day stroke | 2 (1.1) | 1 (0.5) | 0 (0.0) | 1 (0.5) | 3 (1.6) | 0.444 |
| 1-year outcomes | ||||||
| Cumulative incidence of 1-year all cause death (%) | 8.54 [3.91–12.95] | 4.01 [0.78–7.13] | 4.87 [1.5–8.13] | 2.71 [0–5.34] | 9.16 [4.6–13.5] | 0.052 |
| Cumulative incidence of MACCEs (%) | 1.89 [0–4] | 4.82 [1.26–8.27] | 1.53 [0–3.62] | 1.51 [0–3.59] | 3.62 [0.72–6.4] | 0.264 |
Values are presented as n (%), mean ± standard deviation or mean [95% CI]. Quintile 1: TG/HDL-c ratio <1.44. Quintile 2: 1.44≤ TG/HDL-c ratio <2.09. Quintile 3: 2.09≤ TG/HDL-c ratio <2.97. Quintile 4: 2.97≤ TG/HDL-c ratio <4.11. Quintile 5: TG/HDL-c ratio ≥4.11. TBAD, type B aortic dissection; TEVAR, thoracic endovascular aortic repair; TG/HDL-c, triglyceride to high-density lipoprotein cholesterol; MACCEs, major adverse cardiovascular and cerebrovascular events; CI, confidence interval.
1-year outcomes
The cumulative incidence rates for 1-year all-cause mortality and MACCEs are reported in Table 2. A total of 20 MACCEs and 46 fatalities were found during the 1-year follow-up. There was no significant difference in the cumulative incidence of 1-year all cause death (8.54% vs. 4.01% vs. 4.87% vs. 2.71% vs. 9.16%, P=0.052) and 1-year MACCEs (1.89% vs. 4.82% vs. 1.53% vs. 1.51% vs. 3.62%, P=0.264) among the five groups (Table 2). However, there were significant difference in the 1-year all-cause mortality between Q4 and Q1 (P=0.02), as well as Q4 and Q5 (P=0.02) (Figure 2).
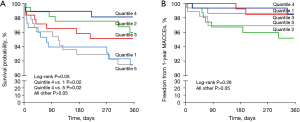
Table 3 reveals the findings of the Cox proportional hazard modeling evaluation. Multivariable Cox regression analysis indicated that TG/HDL-c ratio (modeled as a continuous variable) was strongly linked with 1-year all cause death [hazard ratio (HR) =1.07; 95% confidence interval (CI): 1.00–1.15; P=0.041] (Table 3, Table S1). Other independent predictors for 1-year all-cause mortality included stroke (HR =2.64; 95% CI: 1.09–6.41; P=0.031), CKD (HR =2.71; 95% CI: 1.08–6.82; P=0.034), and pericardial effusion (HR =0.69; 95% CI: 1.55–8.80; P=0.003) (Table S1). As a categorical variable, patients with TG/HDL-c ratio <1.44 (HR =4.67; 95% CI: 1.46–14.94; P=0.001) and TG/HDL-c ratio ≥4.11 (HR =4.84; 95% CI: 1.55–15.13; P=0.007) had higher risk of 1-year all-cause mortality (Table 3, Table S2). However, continuous TyG index was not found to be related to 1-year all-cause death and 1-year MACCEs (all P>0.05) (Table S3).
Table 3
| Variables | Univariate Cox regression model | Multivariate Cox regression model | |||||
|---|---|---|---|---|---|---|---|
| 1-year all-cause death | P value | 1-year MACCEs | P value | 1-year all-cause death | P value | ||
| Continuous TG/HDL-c | 1.07 (1.01, 1.13) | 0.030 | 1.05 (0.95, 1.17) | 0.341 | 1.07 (1.00, 1.15) | 0.041 | |
| Continuous TyG index | 1.32 (0.84, 2.09) | 0.225 | 1.37 (0.69, 2.72) | 0.365 | NA | NA | |
| TG/HDL-c ratio groups | |||||||
| Quintile 4 | Reference | Reference | Reference | ||||
| Quintile 1 | 3.42 (1.12, 10.50) | 0.031 | 1.61 (0.27, 9.63) | 0.602 | 4.67 (1.46, 14.94) | 0.001 | |
| Quintile 2 | 1.47 (0.41, 5.21) | 0.551 | 3.46 (0.72, 16.66) | 0.122 | 1.88 (0.52, 6.82) | 0.337 | |
| Quintile 3 | 1.94 (0.58, 6.44) | 0.280 | 0.98 (0.14, 6.94) | 0.982 | 2.21 (0.65, 7.57) | 0.206 | |
| Quintile 5 | 3.55 (1.18, 10.69) | 0.024 | 2.91 (0.59, 14.44) | 0.190 | 4.84 (1.55, 15.13) | 0.007 | |
Values are presented as HR (95% CI). Covariates for the multivariable model include age, gender, body mass index, smoking, systolic blood pressure, diastolic blood pressure, hypertension, diabetes, stroke, chronic obstructive pulmonary disease, chronic kidney disease, coronary artery disease, timing of operation, pericardial effusion, pleura effusion, white blood cell counts, platelet, creatinine, LDL-C, HDL-C, uric acid. Variables with a P value <0.1 in univariable analysis were entered in the multivariable models (details in the Tables S1-S4). Quintile 1: TG/HDL-c ratio <1.44. Quintile 2: 1.44≤ TG/HDL-c ratio <2.09. Quintile 3: 2.09≤ TG/HDL-c ratio <2.97. Quintile 4: 2.97≤ TG/HDL-c ratio <4.11. Quintile 5: TG/HDL-c ratio ≥4.11. TyG, triglyceride-glucose; TG/HDL-c, triglyceride to high-density lipoprotein cholesterol; MACCEs, major adverse cardiovascular and cerebrovascular events; NA, not applicable; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio; CI, confidence interval.
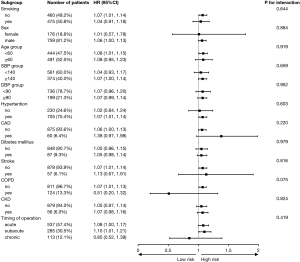
Additionally, we performed subgroup analyses to investigate the impacts of various potential confounding factors, including smoking history, gender, age, SBP, DBP, high blood pressure (HBP), coronary artery disease (CAD), diabetes mellitus, stroke, chronic obstructive pulmonary disease (COPD), CKD and timing of operation (Figure 3). There was no interaction in the all subgroups (all P for interaction >0.05). Moreover, neither TG/HDL-c nor TyG index were found to be related to 1-year MACCEs in the univariate Cox proportional hazard modeling (Table 3); the details are presented in Table S4.
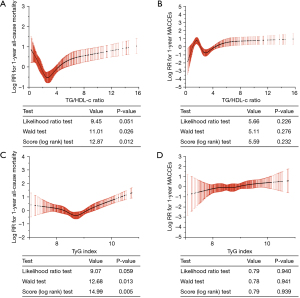
The RCS identified non-linear relationships between TG/HDL-c, TyG index and 1-year outcomes (Figure 4). Unexpectedly, there seemed to be a U-shaped correlation between TG/HDL-c and 1-year all-cause outcomes. The log relative risk (RR) for 1-year all-cause mortality was higher when the TG/HDL-c ratio was below 2.72 and when it was above 2.72. There were no obvious associations between TyG and 1-year MACCEs, nor between TG/HDL-c and 1-year MACCEs.
A discriminant analysis (Wilks’ Lambda) was also performed to investigate the potential influences of 1-year ARAEs (Tables S5,S6).
Discussion
IR is a systemic condition that affects insulin-regulated pathways and multiple organs and is characterized by diminished tissue sensitivity or reaction to circulating insulin, which may be related to obesity, genetic abnormalities, or anti-insulin receptor (20,21). Euglycemic clamp is the gold standard for measuring IR; however, due to the sophisticated procedures, it is not ideal to utilize it as a routine screening item for admission and inappropriate for large-scale investigations (22). Previous studies have shown that TyG index and TG/HDL-c ratio are simple and reliable surrogate markers for IR, that are applicable to both clinical practice and large epidemiological studies (23,24). It has been shown that TG/HDL-c ratio and TyG index were associated with adverse cardiovascular events such as acute coronary syndrome, chronic coronary syndrome, myocardial infarction, ischemic stroke, and cardiac death (17,25). The study has been designed specifically to analyse a potential relationship between TG/HDL-c ratio and the 1-year prognosis of TBAD patients undergoing TEVAR, which may have a role to play in the management of the residual disease. We found a strong association between the TG/HDL-c ratio and 1-year all-cause mortality. The independent association remained statistically significant after adjustment for the well-established AD risk factors and potential confounding risk factors.
The impact of the TG/HDL-c ratio on the outcomes of TBAD patients may be mediated by inflammation. AD is recognized as an inflammatory-related vascular disease (26,27). Local neutrophils recruitment and activation has been observed to trigger aortic rupture while disruption of IL-6 significantly decreases dilatation of the dissected aorta (28). Inflammatory factors may influence the progression and outcome of AD patients. A previous study found that perioperative NLR and systemic inflammatory response index were associated with re-intervention and 1-year outcomes of TBAD patients (29,30). IR is a prominent comorbidity of obesity, arising from a combination of fat excess-triggered abnormalities, including lipotoxicity and meta-inflammation (31). Perry et al. revealed a critical role for inflammation as a divulged etiology for IR (32). Patients with IR may have higher levels of inflammatory cytokine release, leading to adverse events of TBAD patients. Besides, a study found that mitophagy-mediated adipose inflammation leads to type 2 diabetes with hepatic IR (33). Perivascular adipose tissue (PVAT) has been reported to undergo inflammatory changes in response to vascular injury (34). Adachi et al. showed that the beiging (brown adipose tissue-like phenotype change) of PVAT fine-tunes inflammatory response and thus regulates the vascular remodeling (35). PVAT density, which measures vessel inflammation, has been found to be independently associated with the progression of coronary atherosclerosis (36). TG/HDL-c ratio has been used to predict outcomes of numerous cardiovascular inflammatory disorders (37,38).
In addition, IR may have a profound effect on aortic structural cells. The progression of thoracic AD is directly correlated with the breakdown of extracellular matrix and the switch from contractile to synthetic vascular smooth muscle cells (VSMCs) (39). Zheng et al. found that IR promotes the formation of AD by inducing the phenotypic switch of VSMCs (40). Except for VSMCs in the media, endothelial cells are a key component of the intima of the aorta. It has been recently shown that the disruption of endothelial tight junctions’ function is an early event prior to TAAD formation (41). Insulin stimulates the phosphatidylinositol 3-kinase pathway, resulting in endothelial nitric oxide generation and vasodilation, while IR compromises this pathway and leads to vascular dysfunction (42). According to the available research, IR is a secondary cause of progressive endothelial dysfunction, disorders of glucolipid metabolism, vascular damage brought on by lipid deposition and oxidative stress in the vascular wall, an inflammatory response, and the release of chemoattractants and cytokines, which worsen IR and endothelial dysfunction (43).
Other factors may underpin the potential association between the TG/HDL-c ratio and prognosis of TBAD patients. The indicator is not closely related to various comorbidities, including hypertension, diabetes mellitus, stroke, etc. It has been reported that dyslipidemia, diabetes mellitus, and hypertension explained 45.8%, 27.0%, and 15.0% of TyG index’s association with CVD in mediation analyses, respectively (17). However, after adjustment of the confounding factors in the multivariate Cox analyses, TG/HDL-c ratio is still independent risk factor for 1-year outcomes (Table 3). In stratified analysis (Figure 3), we discovered that the covariates did not substantially modify the correlation of TG/HDL-c ratio with the prognosis of TBAD patients treated with TEVAR, suggesting that the results of this study are relevant to most of the general population. Furthermore, Tripolino et al. revealed that a high TyG index is associated with increased wall shear stress in the common carotid artery, which may explain the higher risk of 1-year aortic dilation after TEVAR (44).
Moreover, we discovered a fascinating phenomenon in our study: the relationship between TG/HDL-c ratio and 1-year prognosis showed a slight U-shaped relationship. A lower TG/HDL-c ratio also led to an increased risk of adverse events and mortality. According to a cohort study, people with CAD have a paradoxically increased mortality risk when their HDL-C levels are quite high (45). It has also been indicated that men and women in the general population with extremely high HDL-C paradoxically have high all-cause mortality (46). This may be an important reason for the association between low TG/HDL-c ratio and poor prognosis.
Overall, our findings reinforce the predictive value of TG/HDL-c ratio, which may represent new mechanisms affecting the prognosis of patients with TBAD. Compared with other detection methods, TG/HDL-c ratio detection is simple, rapid and reproducible, and they are an ideal clinical monitoring index that has high clinical relevance for preoperative evaluation, postoperative monitoring and prevention of complications. Implementing the TG/HDL-c ratio into routine clinical assessments requires more standardized protocols for measuring fasting triglyceride and glucose levels. Establishing threshold values indicating increased risk and developing guidelines for clinical decision-making based on these values would be essential. Large-scale prospective studies are needed to confirm its reliability and reproducibility across diverse patient populations. Patients with a higher TG/HDL-c ratio than the threshold might be considered for more intensive monitoring post-TEVAR or could receive more aggressive management of cardiovascular risk factors, such as stricter blood pressure control and the utilization of antihypertensive drugs. Follow-up criteria for TBAD patients who have undergone TEVAR includes regular imaging studies, which are essential to evaluate the aortic repair site; regular clinical examinations to assess symptoms, including chest or back pain, signs of organ malperfusion; strict blood pressure control that is vital in preventing aortic expansion and reducing the risk of further dissection or rupture; periodic blood tests which can help assess renal function, monitor for end-organ damage, and evaluate metabolic parameters; medication adherence that ensure patients are compliant with prescribed medications, including antihypertensives, antiplatelet agents, or anticoagulants, if indicated.
Finally, TyG index and the TG/HDL-c ratio, representing the IR mechanism which is often neglected in the treatment of TBAD patients, may be used to improve the precision of traditional predication models. Combination indexes, such as TyG-platelets index, may also be made by integrating the TyG index and other traditional risk factors such as NLR, platelets, and D-dimer etc.
Limitations
This study has several limitations. (I) This study was a retrospective study. Data records for certain patients may be inaccurate, resulting in increased margins of error. (II) The role of TG/HDL-c ratio and TyG index in the diagnosis of TBAD needs further study, including in combination with other biomarkers. (III) The study was not originally planned for the TG/HDL-c ratio and TyG index, but is a retrospective assessment of computer-collected data, including the TyG index.
Conclusions
The findings of this study indicated that continuous TG/HDL-c ratio, TG/HDL-c ratio <1.44, and TG/HDL-c ratio ≥4.11 were independent risk factors related to increased risks of 1-year all-cause death for TBAD patients receiving TEVAR. Special attention should be paid to TBAD patients with continuous TG/HDL-c and too low or too high TG/HDL-c ratio.
Acknowledgments
The authors thank all members of the Department of Vascular Surgery at The First Affiliated Hospital of the Navy Medical University, Shanghai, China, for their assistance in the data investigation.
Funding: The study, and collection, analysis, interpretation of data, and preparation of the manuscript were supported by the source of funding as follows:
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1411/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1411/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1411/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1411/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The research protocol was approved by The First Affiliated Hospital of the Navy Medical University central ethics committee (CHEC-Y2021, March 1, 2021). Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lombardi JV, Hughes GC, Appoo JJ, et al. Society for Vascular Surgery (SVS) and Society of Thoracic Surgeons (STS) reporting standards for type B aortic dissections. J Vasc Surg 2020;71:723-47. [Crossref] [PubMed]
- Nienaber CA, Clough RE, Sakalihasan N, et al. Aortic dissection. Nat Rev Dis Primers 2016;2:16053. [Crossref] [PubMed]
- Evangelista A, Isselbacher EM, Bossone E, et al. Insights From the International Registry of Acute Aortic Dissection: A 20-Year Experience of Collaborative Clinical Research. Circulation 2018;137:1846-60. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. [Crossref] [PubMed]
- Qin YL, Wang F, Li TX, et al. Endovascular Repair Compared With Medical Management of Patients With Uncomplicated Type B Acute Aortic Dissection. J Am Coll Cardiol 2016;67:2835-42. [Crossref] [PubMed]
- Ahmad W, Brunkwall J, Bunck AC, et al. Favorable Remodeling After TEVAR in Uncomplicated Acute and Subacute Type B Aortic Dissection in Comparison to Conservative Treatment: A Midterm Analysis. J Endovasc Ther 2023; Epub ahead of print. [Crossref]
- Zhang L, Zhao Z, Chen Y, et al. Reintervention after endovascular repair for aortic dissection: A systematic review and meta-analysis. J Thorac Cardiovasc Surg 2016;152:1279-1288.e3. [Crossref] [PubMed]
- Xie E, Liu J, Liu Y, et al. Association between platelet counts and morbidity and mortality after endovascular repair for type B aortic dissection. Platelets 2022;33:73-81. [Crossref] [PubMed]
- Wen D, Du X, Dong JZ, et al. Value of D-dimer and C reactive protein in predicting inhospital death in acute aortic dissection. Heart 2013;99:1192-7. [Crossref] [PubMed]
- Zhu H, Zhang L, Liang T, et al. Elevated preoperative neutrophil-to-lymphocyte ratio predicts early adverse outcomes in uncomplicated type B aortic dissection undergoing TEVAR. BMC Cardiovasc Disord 2021;21:95. [Crossref] [PubMed]
- Zhao K, Zhu H, Ma J, et al. Peripheral Eosinophil Count Is Associated With the Prognosis of Patients With Type B Aortic Dissection Undergoing Endovascular Aortic Repair: A Retrospective Cohort Study. J Am Heart Assoc 2022;11:e027339. [Crossref] [PubMed]
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006;444:840-6. [Crossref] [PubMed]
- Wang T, Li M, Zeng T, et al. Association Between Insulin Resistance and Cardiovascular Disease Risk Varies According to Glucose Tolerance Status: A Nationwide Prospective Cohort Study. Diabetes Care 2022;45:1863-72. [Crossref] [PubMed]
- Brito ADM, Hermsdorff HHM, Filgueiras MS, et al. Predictive capacity of triglyceride-glucose (TyG) index for insulin resistance and cardiometabolic risk in children and adolescents: a systematic review. Crit Rev Food Sci Nutr 2021;61:2783-92. [Crossref] [PubMed]
- Giannini C, Santoro N, Caprio S, et al. The triglyceride-to-HDL cholesterol ratio: association with insulin resistance in obese youths of different ethnic backgrounds. Diabetes Care 2011;34:1869-74. [Crossref] [PubMed]
- Zhao J, Fan H, Wang T, et al. TyG index is positively associated with risk of CHD and coronary atherosclerosis severity among NAFLD patients. Cardiovasc Diabetol 2022;21:123. [Crossref] [PubMed]
- Che B, Zhong C, Zhang R, et al. Triglyceride-glucose index and triglyceride to high-density lipoprotein cholesterol ratio as potential cardiovascular disease risk factors: an analysis of UK biobank data. Cardiovasc Diabetol 2023;22:34. [Crossref] [PubMed]
- MacGillivray TE, Gleason TG, Patel HJ, et al. The Society of Thoracic Surgeons/American Association for Thoracic Surgery clinical practice guidelines on the management of type B aortic dissection. J Thorac Cardiovasc Surg 2022;163:1231-49. [Crossref] [PubMed]
- Fritz J, Bjørge T, Nagel G, et al. The triglyceride-glucose index as a measure of insulin resistance and risk of obesity-related cancers. Int J Epidemiol 2020;49:193-204. [Crossref] [PubMed]
- James DE, Stöckli J, Birnbaum MJ. The aetiology and molecular landscape of insulin resistance. Nat Rev Mol Cell Biol 2021;22:751-71. [Crossref] [PubMed]
- Wu H, Ballantyne CM. Metabolic Inflammation and Insulin Resistance in Obesity. Circ Res 2020;126:1549-64. [Crossref] [PubMed]
- Elahi D. In praise of the hyperglycemic clamp. A method for assessment of beta-cell sensitivity and insulin resistance. Diabetes Care 1996;19:278-86. [Crossref] [PubMed]
- Abbasi F, Reaven GM. Comparison of two methods using plasma triglyceride concentration as a surrogate estimate of insulin action in nondiabetic subjects: triglycerides × glucose versus triglyceride/high-density lipoprotein cholesterol. Metabolism 2011;60:1673-6. [Crossref] [PubMed]
- Salazar MR, Carbajal HA, Espeche WG, et al. Comparison of two surrogate estimates of insulin resistance to predict cardiovascular disease in apparently healthy individuals. Nutr Metab Cardiovasc Dis 2017;27:366-73. [Crossref] [PubMed]
- Shi W, Xing L, Jing L, et al. Value of triglyceride-glucose index for the estimation of ischemic stroke risk: Insights from a general population. Nutr Metab Cardiovasc Dis 2020;30:245-53. [Crossref] [PubMed]
- Son BK, Sawaki D, Tomida S, et al. Granulocyte macrophage colony-stimulating factor is required for aortic dissection/intramural haematoma. Nat Commun 2015;6:6994. [Crossref] [PubMed]
- Liu R, Lo L, Lay AJ, et al. ARHGAP18 Protects Against Thoracic Aortic Aneurysm Formation by Mitigating the Synthetic and Proinflammatory Smooth Muscle Cell Phenotype. Circ Res 2017;121:512-24. [Crossref] [PubMed]
- Anzai A, Shimoda M, Endo J, et al. Adventitial CXCL1/G-CSF expression in response to acute aortic dissection triggers local neutrophil recruitment and activation leading to aortic rupture. Circ Res 2015;116:612-23. [Crossref] [PubMed]
- Yang F, Liu J, Chen L, et al. Impact of Lymphocyte-Related Blood Parameters on Short- and Long-Term Outcomes of Patients Undergoing Thoracic Endovascular Aortic Repair. Angiology 2021;72:953-60. [Crossref] [PubMed]
- Zhao Y, Hong X, Xie X, et al. Preoperative systemic inflammatory response index predicts long-term outcomes in type B aortic dissection after endovascular repair. Front Immunol 2022;13:992463. [Crossref] [PubMed]
- Paccoud R, Saint-Laurent C, Piccolo E, et al. SHP2 drives inflammation-triggered insulin resistance by reshaping tissue macrophage populations. Sci Transl Med 2021;13:eabe2587. [Crossref] [PubMed]
- Perry BI, Burgess S, Jones HJ, et al. The potential shared role of inflammation in insulin resistance and schizophrenia: A bidirectional two-sample mendelian randomization study. PLoS Med 2021;18:e1003455. [Crossref] [PubMed]
- He F, Huang Y, Song Z, et al. Mitophagy-mediated adipose inflammation contributes to type 2 diabetes with hepatic insulin resistance. J Exp Med 2021;218:e20201416. [Crossref] [PubMed]
- Koenen M, Hill MA, Cohen P, et al. Obesity, Adipose Tissue and Vascular Dysfunction. Circ Res 2021;128:951-68. [Crossref] [PubMed]
- Adachi Y, Ueda K, Nomura S, et al. Beiging of perivascular adipose tissue regulates its inflammation and vascular remodeling. Nat Commun 2022;13:5117. [Crossref] [PubMed]
- Lee SE, Sung JM, Andreini D, et al. Association Between Changes in Perivascular Adipose Tissue Density and Plaque Progression. JACC Cardiovasc Imaging 2022;15:1760-7. [Crossref] [PubMed]
- Hoshino T, Mizuno T, Ishizuka K, et al. Triglyceride-glucose index as a prognostic marker after ischemic stroke or transient ischemic attack: a prospective observational study. Cardiovasc Diabetol 2022;21:264. [Crossref] [PubMed]
- Huang R, Lin Y, Ye X, et al. Triglyceride-glucose index in the development of heart failure and left ventricular dysfunction: analysis of the ARIC study. Eur J Prev Cardiol 2022;29:1531-41. [Crossref] [PubMed]
- Pan L, Bai P, Weng X, et al. Legumain Is an Endogenous Modulator of Integrin αvβ3 Triggering Vascular Degeneration, Dissection, and Rupture. Circulation 2022;145:659-74. [Crossref] [PubMed]
- Zheng H, Qiu Z, Chai T, et al. Insulin Resistance Promotes the Formation of Aortic Dissection by Inducing the Phenotypic Switch of Vascular Smooth Muscle Cells. Front Cardiovasc Med 2021;8:732122. [Crossref] [PubMed]
- Yang X, Xu C, Yao F, et al. Targeting endothelial tight junctions to predict and protect thoracic aortic aneurysm and dissection. Eur Heart J 2023;44:1248-61. [Crossref] [PubMed]
- Artunc F, Schleicher E, Weigert C, et al. The impact of insulin resistance on the kidney and vasculature. Nat Rev Nephrol 2016;12:721-37. [Crossref] [PubMed]
- Cersosimo E, DeFronzo RA. Insulin resistance and endothelial dysfunction: the road map to cardiovascular diseases. Diabetes Metab Res Rev 2006;22:423-36. [Crossref] [PubMed]
- Tripolino C, Irace C, Scavelli FB, et al. Triglyceride glucose index and common carotid wall shear stress. J Investig Med 2014;62:340-4. [Crossref] [PubMed]
- Liu C, Dhindsa D, Almuwaqqat Z, et al. Association Between High-Density Lipoprotein Cholesterol Levels and Adverse Cardiovascular Outcomes in High-risk Populations. JAMA Cardiol 2022;7:672-80. [Crossref] [PubMed]
- Madsen CM, Varbo A, Nordestgaard BG. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J 2017;38:2478-86. [Crossref] [PubMed]


