The value of high-risk clinicopathologic features for chemotherapy in stage I non-small cell lung cancer: a propensity score-matched study
Highlight box
Key findings
• This study identified visceral pleural invasion (VPI) as the primary predictor, and the examination of an insufficient number of lymph nodes (LNs) as the secondary predictor. Additionally, a high histologic grade may be a potential factor to consider when deciding whether or not to include chemotherapy in the management of stage I non-small cell lung cancer (NSCLC).
What is known, and what is new?
• High-risk clinicopathologic features, such as a high histologic grade, the presence of VPI, the examination of an insufficient number of LNs, and limited resection, were independent risk factors for a poor prognosis in stage I NSCLC.
• This study evaluates the association between chemotherapy and survival in stage I NSCLC patients, stratified by the pathologic stage and high-risk clinicopathologic features. The benefit of chemotherapy varied based on specific factors, which challenges the notion that chemotherapy has a universal benefit for all stage I NSCLC patients. This study emphasizes the importance of considering individual patient characteristics and high-risk features in making informed decisions about the inclusion of chemotherapy in treatment plans for early stage NSCLC.
What is the implication, and what should change now?
• This study identified VPI as the primary indicator for whether or not to include chemotherapy, the examination of an insufficient number of LNs as a secondary indicator, and a high histologic grade as a potential factor signaling that chemotherapy should be included in the management of stage I NSCLC.
Introduction
Surgical resection is the main method of treating early stage non-small cell lung cancer (NSCLC). However, NSCLC patients, including stage I patients, still experience recurrence and death after surgery (1,2). Several studies have reported that adjuvant chemotherapy significantly improves survival in patients with resected stage II and III NSCLC (3-7). Early randomized controlled trials have reported that adjuvant chemotherapy prolongs disease-free survival and overall survival (OS) in patients with completely resected early stage NSCLC (4,8). An increasing number of studies have expressed different opinions on whether adjuvant chemotherapy is necessary for stage I lung cancer (9-13). Therefore, the debate continues as to whether adjuvant chemotherapy can prolong survival in stage I NSCLC patients (9,14-16).
Several high-risk clinicopathologic features are considered risk factors for survival and indicators of adjuvant chemotherapy in patients with NSCLC, including a larger tumor size, a high pathological stage, the presence of visceral pleural invasion (VPI), a high grade histological subtype, the presence of vascular invasion, limited resection and the examination of an insufficient number of lymph nodes (LNs) (11,13,17). The latest version of the National Comprehensive Cancer Network (NCCN) guidelines recommends that chemotherapy be considered for stage IB wand IIA high-risk patients after R0 resection (17). However, the indicative role and reference value status of these risk factors in decision making for chemotherapy patients have not yet been determined. Thus, research needs to be conducted to determine which specific patients may benefit from additional treatment in early-stage NSCLC after surgery (18).
In this study, we assessed the association between chemotherapy and survival in patients with stage I NSCLC stratified by pathologic stage and high-risk clinicopathologic features. The value of high-risk clinicopathologic features in making decisions to administer chemotherapy to treat stage I NSCLC was investigated. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-305/rc).
Methods
Data collection
The Surveillance, Epidemiology, and End Results (SEER) program was used to collect research data using the SEER*Stat software (version 8.3.6, National Cancer Institute) (https://seer.cancer.gov/). Collaborative stage (CS) data collection system coding has been used since 2004, in which the column “CS site-specific factor 2” represents pleural invasion. A cohort of 666,689 patients diagnosed with lung cancer from 2004 to 2016 was exported (site recode: lung and bronchus, year of diagnosis: 2004–2016). We filled in the missing information of patients as much as possible by referring to other relevant information. For example, we filled in information on N stage by referring to regional node positivity, CS LNs and CS extension, and we filled in information on VPI by referring to CS extension and CS site-specific factor 2. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Because this research is the study of a public database, it does not involve ethical issues.
Patient screening
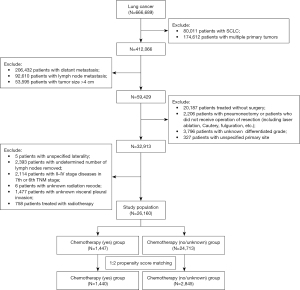
In this study, the inclusion criteria for the study cohort were as follows: (I) patients presenting with primary lung cancer only; (II) patients with stage I NSCLC with tumor size ≤4 cm; (III) tumors resected surgically by limited resection or lobectomy; and (IV) patients with complete information. The exclusion criteria for the study cohort were as follows: (I) patients with small cell lung cancer (SCLC); (II) patients with multiple lung cancers; (III) patients with stage II–IV disease; (IV) patients receiving radiotherapy; (V) tumors that were not resected by surgery or pneumonectomy; and/or (VI) patients with incomplete information. The data selection process is shown in Figure 1. Ultimately, a cohort of 26,160 patients diagnosed with stage I NSCLC was included in this study.
Variables studied
We collected the basic demographic information, basic clinicopathological information, treatment information and survival data of the study cohort, including sex, age at time of diagnosis, race, marital status, primary site, laterality, surgery type, histologic type, tumor size, histologic grading, VPI, number of LNs examined, chemotherapy, survival classification, and survival time. The stage of the tumor was readjusted according to the eighth edition of the TNM classification system (International Union Against Cancer staging system) (1). Dai et al. reported that 8 to 11 nodes were optimal for LN evaluation at the curative resection of stage I lung cancer (8, 9, 10, and 11 nodes for T1a, T1b, T1c, and T2a tumors, respectively) (13). The patients were divided into four groups according to the number of LNs examined. In the subgroup survival analysis of this study, the optimal boundary for the LN evaluation was 10 nodes for stage IA and 11 nodes for stage IB. The survival classifications were divided into lung cancer-specific survival (LCSS) and OS. LCSS was defined as the survival time from lung cancer diagnosis to death specific to lung cancer-related death. In this study, institutional review board approval was not required because the cohort data were anonymous and publicly available.
Statistical analysis
The screened cohort was divided into the following two groups according to whether or not chemotherapy was administered: the chemotherapy (yes) group versus the chemotherapy (no/unknown) group. The propensity score matching (PSM) method was used to reduce the differences among the baseline variables between groups. The propensity score model included sex, age at time of diagnosis, race, marital status, primary site, laterality, surgery type, histologic type, tumor size, histologic grading, VPI, and the number of LNs examined. Patients were matched based on propensity scores using a 1:2 nearest neighbor method. The caliper of the matching algorithm was set to 0.05. The absolute standardized mean difference (ASMD) was used to assess the balance of the covariate’s distributions before and after the matching procedure between the groups. An ASMD <0.1, which indicated a balance in the covariate distributions between the two groups, was acceptable (19). Before and after matching, comparisons of two continuous variables were evaluated by the student’s t-test, and comparisons of categorical variables were evaluated by Fisher’s exact test or the chi-squared test. A matched cohort was used to investigate the association between chemotherapy and survival outcomes, including LCSS and OS. Univariate and multivariate survival analyses were evaluated by a Cox proportional hazards regression model. The survival curves of LCSS and OS were generated using the Kaplan-Meier method, and non-parametric group comparisons were performed using the log-rank test. Statistical analyses were performed with IBM SPSS Statistics version 25.0 (IBM Corp., Armonk, New York, USA), GraphPad Prism version 7.04, and R version 4.0.2 (R Development Core Team, Austria, Vienna), including the “foreign”, “survival” and “MatchIt” packages. A two-sided P value <0.05 was considered statistically significant.
Results
Patient characteristics
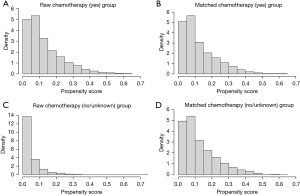
The data of 666,689 patients diagnosed with lung cancer were collected from the SEER database. Through the screening procedure shown in Figure 1, 26,160 patients diagnosed with stage I NSCLC were identified. Ultimately, 4,285 patients diagnosed with stage I NSCLC were included in this study using PSM. The clinicopathological characteristics of the patients based on the status of chemotherapy before and after matching are summarized in Table 1. In the cohort before matching, 1,447 (5.5%) patients were treated with chemotherapy. An ASMD >0.1 indicated an imbalance among the variables. After matching, 1,440 (33.6%) patients treated with chemotherapy were enrolled. An ASMD <0.1 indicated a balance among all the variables after matching. The distributions of the propensity scores before and after matching are displayed by histograms (Figure 2).
Table 1
| Characteristic | Chemotherapy | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Before matching (N=26,160) | After matching (N=4,285) | ||||||||
| No/unknown (N=24,713) | Yes (N=1,447) | ASMD | P value | No/unknown (N=2,845) | Yes (N=1,440) | ASMD | P value | ||
| Sex | 0.0305 | 0.26 | 0.0056 | 0.91 | |||||
| Female | 14,055 (56.9) | 801 (55.4) | 1,578 (55.5) | 796 (55.3) | |||||
| Male | 10,658 (43.1) | 646 (44.6) | 1,267 (44.5) | 644 (44.7) | |||||
| Age, years | 67.50±10.298 | 63.49±9.371 | 0.4280 | <0.001 | 63.79±11.170 | 63.56±9.318 | 0.0124 | 0.49 | |
| ≤65 | 9,647 (39.0) | 811 (56.0) | 1,504 (52.9) | 804 (55.8) | |||||
| >65 | 15,066 (61.0) | 636 (44.0) | 1,341 (47.1) | 636 (44.2) | |||||
| Race | 0.0298 | 0.22 | 0.0371 | 0.18 | |||||
| White | 20,817 (84.2) | 1,196 (82.7) | 2,397 (84.3) | 1,190 (82.6) | |||||
| Black | 1,969 (8.0) | 131 (9.1) | 240 (8.4) | 130 (9.0) | |||||
| Asian or Pacific Islander | 1,732 (7.0) | 112 (7.7) | 182 (6.4) | 112 (7.8) | |||||
| Others | 195 (0.8) | 8 (0.6) | 26 (0.9) | 8 (0.6) | |||||
| Marital status | 0.1150 | <0.001 | 0.0197 | 0.56 | |||||
| Married | 13,720 (55.5) | 872 (60.3) | 1,673 (58.8) | 868 (60.3) | |||||
| Unmarried | 2,722 (11.0) | 169 (11.7) | 358 (12.6) | 167 (11.6) | |||||
| Others | 8,271 (33.5) | 406 (28.1) | 814 (28.6) | 405 (28.1) | |||||
| Primary site | 0.0209 | 0.003 | 0.0378 | 0.28 | |||||
| Main bronchus | 63 (0.3) | 0 | 8 (0.3) | 0 | |||||
| Upper lobe | 15,059 (60.9) | 896 (61.9) | 1,789 (62.9) | 890 (61.8) | |||||
| Middle lobe | 1,505 (6.1) | 85 (5.9) | 165 (5.8) | 85 (5.9) | |||||
| Lower lobe | 7,962 (32.2) | 449 (31.0) | 858 (30.2) | 449 (31.2) | |||||
| Overlapping lesion | 124 (0.5) | 17 (1.2) | 25 (0.9) | 16 (1.1) | |||||
| Laterality | 0.0192 | 0.48 | 0.0050 | 0.95 | |||||
| Right | 14,643 (59.3) | 871 (60.2) | 1,718 (60.4) | 868 (60.3) | |||||
| Left | 10,070 (40.7) | 576 (39.8) | 1,127 (39.6) | 572 (39.7) | |||||
| Surgery type | 0.1583 | <0.001 | 0.0208 | 0.49 | |||||
| Limited resection | 5,398 (21.8) | 232 (16.0) | 480 (16.9) | 231 (16.0) | |||||
| Lobectomy | 19,315 (78.2) | 1,215 (84.0) | 2,365 (83.1) | 1,209 (84.0) | |||||
| Histologic type | 0.0763 | <0.001 | 0.0347 | 0.004 | |||||
| Adenocarcinoma | 15,840 (64.1) | 912 (63.0) | 1,700 (59.8) | 909 (63.1) | |||||
| Squamous cell carcinoma | 5,606 (22.7) | 273 (18.9) | 663 (23.3) | 272 (18.9) | |||||
| Other | 3,267 (13.2) | 262 (18.1) | 482 (16.9) | 259 (18.0) | |||||
| Tumor size, mm | 23.20±43.136 | 29.22±44.807 | 0.6386 | <0.001 | 29.07±48.597 | 29.19±44.933 | 0.0079 | 0.94 | |
| >0 to 10 | 2,177 (8.8) | 44 (3.0) | 57 (2.0) | 44 (3.1) | |||||
| >10 to 20 | 10,803 (43.7) | 361 (24.9) | 653 (23.0) | 361 (25.1) | |||||
| >20 to 30 | 8,147 (33.0) | 463 (32.0) | 1,131 (39.8) | 462 (32.1) | |||||
| >30 to 40 | 3,586 (14.5) | 579 (40.1) | 1,004 (35.3) | 573 (39.8) | |||||
| Histologic grading | 0.4906 | <0.001 | 0.0125 | 0.49 | |||||
| G1 | 6,044 (24.5) | 151 (10.4) | 330 (11.6) | 151 (10.5) | |||||
| G2 | 11,494 (46.5) | 596 (41.2) | 1,120 (39.4) | 595 (41.3) | |||||
| G3 | 6,816 (27.6) | 648 (44.8) | 1,285 (45.2) | 644 (44.7) | |||||
| G4 | 359 (1.4) | 52 (3.6) | 110 (3.9) | 50 (3.5) | |||||
| VPI | 0.5878 | <0.001 | 0.0014 | 0.70 | |||||
| Absence | 20,302 (82.2) | 764 (52.8) | 1,527 (53.7) | 764 (53.1) | |||||
| Presence | 4,411 (17.8) | 683 (47.2) | 1,318 (46.3) | 676 (46.9) | |||||
| No. of LNs examined | 7.82±7.268 | 8.06±7.524 | 0.0322 | 0.22 | 8.06±7.056 | 8.05±7.498 | 0.0024 | 0.96 | |
| 0 | 2,710 (11.0) | 146 (10.1) | 235 (8.3) | 145 (10.1) | |||||
| 1–7 | 11,807 (47.8) | 683 (47.2) | 1,375 (48.3) | 679 (47.2) | |||||
| 8–11 | 4,626 (18.7) | 265 (18.3) | 572 (20.1) | 265 (18.4) | |||||
| >11 | 5,570 (22.5) | 353 (24.4) | 663 (23.3) | 351 (24.4) | |||||
Data are presented as n (%) or mean ± SD. NSCLC, non-small cell lung cancer; ASMD, absolute standardized mean difference; SD, standardized difference; VPI, visceral pleural invasion; LN, lymph node; G1, well differentiated: G2, moderately differentiated; G3, poorly differentiated; G4, undifferentiated.
Survival analysis for all patients after matching
In the univariate survival analyses, a Cox proportional hazards regression model was used to investigate the prognostic risk factors for LCSS and OS. Sex, age, surgery type, histologic type, tumor size, histologic grading, VPI, the number of LNs examined, and a high pathologic stage were identified as significant predictors for LCSS and OS (Table 2).
Table 2
| Characteristic | Univariate analyses | Multivariate analyses | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LCSS | OS | LCSS | OS | ||||||||||||
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | ||||
| Sex | |||||||||||||||
| Female | Ref. | Ref. | Ref. | Ref. | |||||||||||
| Male | 1.371 | 1.220–1.541 | <0.001 | 1.459 | 1.326–1.606 | <0.001 | 1.280 | 1.137–1.441 | <0.001 | 1.359 | 1.234–1.498 | <0.001 | |||
| Age | 1.028 | 1.022–1.034 | <0.001 | 1.039 | 1.034–1.044 | <0.001 | 1.020 | 1.014-1.027 | <0.001 | 1.032 | 1.027–1.037 | <0.001 | |||
| Race | |||||||||||||||
| White | Ref. | Ref. | |||||||||||||
| Black | 0.883 | 0.710–1.097 | 0.26 | 0.839 | 0.700–1.005 | 0.06 | |||||||||
| Asian or Pacific Islander | 0.866 | 0.681–1.102 | 0.24 | 0.711 | 0.573–0.881 | 0.002 | |||||||||
| Other | 0.120 | 0.017–0.854 | 0.03 | 0.395 | 0.164–0.950 | 0.04 | |||||||||
| Marital status | |||||||||||||||
| Married | Ref. | Ref. | |||||||||||||
| Unmarried | 0.995 | 0.822–1.204 | 0.96 | 0.952 | 0.811–1.118 | 0.55 | |||||||||
| Others | 1.165 | 1.022–1.328 | 0.02 | 1.254 | 1.128–1.394 | <0.001 | |||||||||
| Primary site | |||||||||||||||
| Main bronchus† | 0.413 | 0.058–2.930 | 0.38 | ||||||||||||
| Upper lobe | Ref. | Ref. | |||||||||||||
| Middle lobe | 1.094 | 0.854–1.401 | 0.48 | 0.984 | 0.797–1.215 | 0.88 | |||||||||
| Lower lobe | 1.113 | 0.980–1.265 | 0.10 | 1.066 | 0.960–1.184 | 0.23 | |||||||||
| Overlapping lesion | 1.195 | 0.691–2.070 | 0.52 | 1.030 | 0.638–1.663 | 0.91 | |||||||||
| Laterality | |||||||||||||||
| Right | Ref. | Ref. | |||||||||||||
| Left | 0.956 | 0.848–1.078 | 0.47 | 0.969 | 0.879–1.069 | 0.53 | |||||||||
| Surgery type | |||||||||||||||
| Limited resection | 1.796 | 1.563–2.065 | <0.001 | 1.759 | 1.567–1.974 | <0.001 | 1.482 | 1.252–1.755 | <0.001 | 1.432 | 1.245–1.647 | <0.001 | |||
| Lobectomy | Ref. | Ref. | Ref. | Ref. | |||||||||||
| Histologic type | |||||||||||||||
| Adenocarcinoma | Ref. | Ref. | Ref. | Ref. | |||||||||||
| Squamous cell carcinoma | 1.446 | 1.255–1.666 | <0.001 | 1.701 | 1.521–1.903 | <0.001 | 1.158 | 0.999–1.344 | 0.05 | 1.295 | 1.151–1.456 | <0.001 | |||
| Other | 1.440 | 1.237–1.677 | <0.001 | 1.393 | 1.225–1.584 | <0.001 | 1.327 | 1.129–1.559 | 0.001 | 1.228 | 1.071–1.408 | 0.003 | |||
| Tumor size | 1.206 | 1.122–1.297 | <0.001 | 1.204 | 1.135–1.278 | <0.001 | 1.262 | 1.137–1.400 | <0.001 | 1.190 | 1.092–1.298 | <0.001 | |||
| Histologic grading | |||||||||||||||
| G1 | Ref. | Ref. | Ref. | Ref. | |||||||||||
| G2 | 1.917 | 1.482–2.481 | <0.001 | 1.837 | 1.493–2.261 | <0.001 | 1.655 | 1.276–2.146 | <0.001 | 1.533 | 1.243–1.890 | <0.001 | |||
| G3 | 2.507 | 1.947–3.229 | <0.001 | 2.439 | 1.990–2.989 | <0.001 | 1.969 | 1.520–2.551 | <0.001 | 1.832 | 1.486–2.258 | <0.001 | |||
| G4 | 2.108 | 1.441–3.084 | <0.001 | 2.221 | 1.664–2.999 | <0.001 | 1.491 | 1.002–2.219 | 0.049 | 1.564 | 1.140–2.145 | 0.006 | |||
| VPI | |||||||||||||||
| Absence | Ref. | Ref. | Ref. | Ref. | |||||||||||
| Presence | 1.356 | 1.206–1.524 | <0.001 | 1.213 | 1.102–1.335 | <0.001 | 1.530 | 1.280–1.828 | <0.001 | 1.217 | 1.055–1.404 | 0.007 | |||
| No. of LNs examined | |||||||||||||||
| 0 | 2.243 | 1.814–2.775 | <0.001 | 2.047 | 1.718–2.439 | <0.001 | 1.686 | 1.320–2.152 | <0.001 | 1.492 | 1.219–1.826 | <0.001 | |||
| 1–7 | 1.298 | 1.099–1.533 | 0.002 | 1.240 | 1.085–1.418 | 0.002 | 1.266 | 1.071–1.497 | 0.006 | 1.208 | 1.056–1.383 | 0.006 | |||
| 8–11 | Ref. | Ref. | Ref. | Ref. | |||||||||||
| >11 | 1.013 | 0.831–1.236 | 0.90 | 0.983 | 0.838–1.154 | 0.84 | 0.978 | 0.801–1.193 | 0.82 | 0.947 | 0.807–1.112 | 0.51 | |||
| Pathologic TNM stage | |||||||||||||||
| IA1 | Ref. | Ref. | Ref. | Ref. | |||||||||||
| IA2 | 1.237 | 0.789–1.939 | 0.35 | 1.128 | 0.806–1.579 | 0.48 | 1.229 | 0.783–1.929 | 0.37 | 1.132 | 0.807–1.586 | 0.47 | |||
| IA3 | 1.668 | 1.083–2.569 | 0.02 | 1.363 | 0.986–1.885 | 0.06 | 1.330 | 0.851–2.080 | 0.21 | 1.136 | 0.810–1.594 | 0.46 | |||
| IB | 1.861 | 1.229–2.818 | 0.003 | 1.558 | 1.145–2.120 | 0.005 | 0.945 | 0.585–1.529 | 0.82 | 0.989 | 0.685–1.427 | 0.95 | |||
| Chemotherapy | |||||||||||||||
| No/unknown | Ref. | Ref. | Ref. | Ref. | |||||||||||
| Yes | 1.172 | 1.039–1.321 | 0.01 | 0.935 | 0.846–1.035 | 0.19 | 1.218 | 1.079–1.375 | 0.001 | 0.974 | 0.879–1.079 | 0.61 | |||
†, there was no lung cancer-related death in this group with tumors located in the main trachea. NSCLC, non-small cell lung cancer; LCSS, lung cancer-specific survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; VPI, visceral pleural invasion; LN, lymph node; G1, well differentiated; G2, moderately differentiated; G3, poorly differentiated; G4, undifferentiated.
Further, a multivariate analysis was performed by the Cox proportional hazards regression model. The results showed that sex (male) (HR: 1.280, 95% CI: 1.137–1.441, P<0.001), age (HR: 1.020, 95% CI: 1.014–1.027, P<0.001), limited resection (HR: 1.482, 95% CI: 1.252–1.755, P<0.001), a large tumor size (HR: 1.262, 95% CI: 1.137–1.400, P<0.001), histologic type (other: HR: 1.327, 95% CI: 1.129–1.559, P=0.001), a high histologic grade (G3: HR: 1.969, 95% CI: 1.520–2.551, P<0.001), the presence of VPI (HR: 1.530, 95% CI: 1.280–1.828, P<0.001), the examination of a small number of LNs (LNs =0: HR: 1.686, 95% CI: 1.320–2.152 P<0.001), and treatment with chemotherapy (HR: 1.218, 95% CI: 1.079–1.375, P=0.001) were independent prognostic factors for poor LCSS.
Further, sex (male) (HR: 1.359, 95% CI: 1.234–1.498, P<0.001), age (HR: 1.032, 95% CI: 1.027–1.037, P<0.001), limited resection (HR: 1.432, 95% CI: 1.245–1.647, P<0.001), a large tumor size (HR: 1.190, 95% CI: 1.092–1.298, P<0.001), histologic type (squamous cell carcinoma: HR: 1.295, 95% CI: 1.151–1.456, P<0.001), a high histologic grade (G3: HR: 1.832, 95% CI: 1.486–2.258, P<0.001), the presence of VPI (HR: 1.217, 95% CI: 1.055–1.404, P=0.007), and the examination of a small number of LNs (LNs =0: HR: 1.492, 95% CI: 1.219–1.826, P<0.001) were independent prognostic factors for poor OS, however, chemotherapy was not an independent prognostic factor for poor OS.
Thus, high-risk clinicopathologic features, including a high histologic grade, the presence of VPI, the examination of an insufficient number of LNs, and limited resection, were independent risk factors for poor prognosis.
Subgroup survival analysis
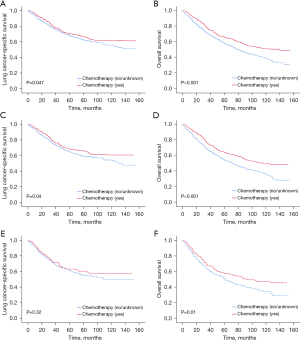
In this study, all patients with stage I NSCLC after matching were stratified by pathologic stage and high-risk clinicopathologic features to assess the association between chemotherapy and survival. After matching, the patients were divided into subgroups based on their pathologic stage status, histologic grading, VPI status, the number of LNs examined, and the surgery type. Interestingly, a statistically significant correlation between administering chemotherapy and improved LCSS was only observed in patients with VPI stage IB disease (HR: 0.839, 95% CI: 0.706–0.998, P=0.047). These patients showed an absolute increase in LCSS of 2% at 5 years and 5.8% at 10 years (Figure 3 and Table 3). Moreover, patients with either stage IB disease, a high histologic grade, fewer than eight LNs examined, or limited resection did not significantly benefit from chemotherapy in terms of LCSS.
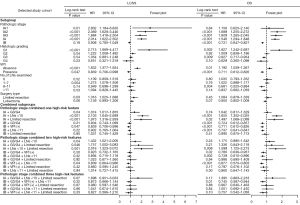
Table 3
| Selected study cohort | LCSS (%) | OS (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-year | 10-year | 5-year | 10-year | ||||||||||||
| Chemotherapy | Diff. | Chemotherapy | Diff. | Chemotherapy | Diff. | Chemotherapy | Diff. | ||||||||
| No/unknown | Yes | No/unknown | Yes | No/unknown | Yes | No/unknown | Yes | ||||||||
| Pathologic TNM stage | |||||||||||||||
| IA | 81.6 | 66.9 | −14.7 | 74.5 | 52.5 | −22 | 72.6 | 61.5 | −11.1 | 57.1 | 40.4 | −16.7 | |||
| IA1 | 91.3 | 73.1 | –18.2 | 85.4 | 64.4 | –21 | 76.6 | 68.5 | –8.1 | 64.1 | 57.3 | –6.8 | |||
| IA2 | 83.9 | 69.4 | –14.5 | 78.8 | 55.0 | –23.8 | 73.9 | 64.3 | –9.6 | 61.9 | 40.6 | –21.3 | |||
| IA3 | 78.6 | 63.4 | –15.2 | 70.1 | 46.4 | –23.7 | 71.1 | 57.6 | –13.5 | 52.7 | 35.2 | –17.5 | |||
| IB | 70.4 | 71.8 | 1.4 | 58.7 | 60.0 | 1.3 | 58.8 | 66.6 | 7.8 | 39.8 | 49.3 | 9.5 | |||
| Histologic grading | |||||||||||||||
| G1 | 90.3 | 77.7 | –12.6 | 86.7 | 62.6 | –24.1 | 83.2 | 74.5 | –8.7 | 76.5 | 51.8 | –24.7 | |||
| G2 | 75.9 | 71.6 | –4.3 | 65.5 | 56.9 | –8.6 | 66.5 | 66.0 | –0.5 | 48.1 | 45.4 | –2.7 | |||
| G3 | 69.4 | 66.6 | –2.8 | 59.1 | 56.7 | –2.4 | 57.0 | 61.6 | 4.6 | 38.6 | 45.5 | 6.9 | |||
| G4 | 71.7 | 83.6 | 11.9 | 57.5 | 66.5 | 9 | 59.3 | 72.7 | 13.4 | 37.9 | 54.8 | 16.9 | |||
| VPI | |||||||||||||||
| Absence | 79.9 | 70.4 | –9.5 | 72.1 | 55.1 | –17 | 68.7 | 64.6 | –4.1 | 51.5 | 43.2 | –8.3 | |||
| Presence | 68.2 | 70.2 | 2 | 55.6 | 61.4 | 5.8 | 57.9 | 65.6 | 7.7 | 39.7 | 51.1 | 11.4 | |||
| No. of LNs examined | |||||||||||||||
| 0 | 58.9 | 56.9 | –2 | 42.7 | 37.2 | –5.5 | 46.9 | 50.7 | 3.8 | 27.1 | 22.0 | –5.1 | |||
| 1–7 | 73.2 | 67.2 | –6 | 64.8 | 55.1 | –9.7 | 63.0 | 62.0 | –1 | 45.8 | 42.9 | –2.9 | |||
| 8–11 | 78.6 | 77.3 | –1.3 | 69.0 | 68.6 | –0.4 | 67.1 | 73.8 | 6.7 | 50.5 | 61.3 | 10.8 | |||
| >11 | 79.6 | 77.0 | –2.6 | 67.8 | 63.9 | –3.9 | 69.1 | 70.9 | 1.8 | 50.0 | 55.7 | 5.7 | |||
| Surgery type | |||||||||||||||
| Limited resection | 64.0 | 55.7 | –8.3 | 50.1 | 37.4 | –12.7 | 50.2 | 49.3 | –0.9 | 29.7 | 25.5 | –4.2 | |||
| Lobectomy | 76.5 | 73.1 | –3.4 | 67.2 | 61.5 | –5.7 | 66.4 | 68.1 | 1.7 | 49.2 | 50.7 | 1.5 | |||
| Pathologic TNM stage combined with one high-risk feature | |||||||||||||||
| IA + G3/G4 | 75.3 | 67.9 | –7.4 | 65.7 | 55.7 | –10 | 63.4 | 62.5 | –0.9 | 43.2 | 40.8 | –2.4 | |||
| IA + LNs <10 | 80.9 | 65.0 | –15.9 | 73.0 | 48.6 | –24.4 | 71.7 | 59.6 | –12.1 | 55.9 | 35.6 | –20.3 | |||
| IA + limited resection | 74.9 | 56.4 | –18.5 | 62.9 | 40.6 | –22.3 | 63.4 | 50.0 | –13.4 | 40.5 | 27.3 | –13.2 | |||
| IB + G3/G4 | 66.8 | 67.5 | 0.7 | 55.4 | 58.0 | 2.6 | 54.2 | 62.2 | 8 | 35.9 | 48.8 | 12.9 | |||
| IB + VPI (+) | 68.2 | 70.2 | 2 | 55.6 | 61.4 | 5.8 | 57.9 | 65.6 | 7.7 | 39.7 | 51.1 | 11.4 | |||
| IB + LNs <11 | 67.7 | 69.2 | 1.5 | 57.1 | 59.1 | 2 | 56.1 | 64.0 | 7.9 | 37.5 | 47.0 | 9.5 | |||
| IB + limited resection | 56.8 | 55.1 | –1.7 | 39.5 | 34.4 | –5.1 | 41.8 | 48.8 | 7 | 21.9 | 24.0 | 2.1 | |||
| Pathologic TNM stage combined with two high-risk features | |||||||||||||||
| IA + G3/G4 + LNs <10 | 73.3 | 64.4 | –8.9 | 63.5 | 51.3 | –12.2 | 62.4 | 59.0 | –3.4 | 41.7 | 35.2 | –6.5 | |||
| IA + G3/G4 + Limited resection | 71.3 | 52.5 | –18.8 | 58.3 | 39.0 | –19.3 | 57.7 | 45.1 | –12.6 | 29.8 | 22.2 | –7.6 | |||
| IA + LNs <10 + limited resection | 74.8 | 55.9 | –18.9 | 61.8 | 38.3 | –23.5 | 64.3 | 49.0 | –15.3 | 40.9 | 24.3 | –16.6 | |||
| IB + G3/G4 + VPI (+) | 64.7 | 63.0 | –1.7 | 53.0 | 57.3 | 4.3 | 53.3 | 57.9 | 4.6 | 37.6 | 48.4 | 10.8 | |||
| IB + G3/G4 + LNs <11 | 64.1 | 65.8 | 1.7 | 51.9 | 56.5 | 4.6 | 51.7 | 60.1 | 8.4 | 32.4 | 45.9 | 13.5 | |||
| IB + G3/G4 + limited resection | 53.2 | 48.5 | –4.7 | 37.1 | 31.1 | –6 | 39.5 | 42.5 | 3 | 21.1 | 17.6 | –3.5 | |||
| IB + VPI (+) + LNs <11 | 64.9 | 68.4 | 3.5 | 54.4 | 60.8 | 6.4 | 54.5 | 64.1 | 9.6 | 37.3 | 49.7 | 12.4 | |||
| IB + VPI (+) + limited resection | 52.5 | 57.9 | 5.4 | 34.1 | 35.8 | 1.7 | 40.5 | 51.6 | 11.1 | 21.7 | 25.7 | 4 | |||
| IB + LNs <11 + limited resection | 54.7 | 53.6 | –1.1 | 35.0 | 31.4 | –3.6 | 40.1 | 47.6 | 7.5 | 18.2 | 22.3 | 4.1 | |||
| Pathologic stage combined with three high-risk features | |||||||||||||||
| IA + G3/G4 + LNs <10 + limited resection | 70.8 | 52.4 | –18.4 | 56.1 | 37.6 | –18.5 | 60.0 | 44.7 | –15.3 | 29.9 | 19.8 | –10.1 | |||
| IB + G3/G4 + VPI (+) + LNs <11 | 62.0 | 63.3 | 1.3 | 50.3 | 57.6 | 7.3 | 51.1 | 58.1 | 7 | 34.3 | 47.4 | 13.1 | |||
| IB + G3/G4 + VPI (+) + limited resection | 50.6 | 47.9 | –2.7 | 31.7 | 32.6 | 0.9 | 39.3 | 41.7 | 2.4 | 19.7 | 18.7 | –1 | |||
| IB + G3/G4 + LNs <11 + limited resection | 51.5 | 46.3 | –5.2 | 32.6 | 24.8 | –7.8 | 38.0 | 41.1 | 3.1 | 17.4 | 13.9 | –3.5 | |||
| IB + VPI (+) + LNs <11 + limited resection | 49.8 | 56.8 | 7 | 28.7 | 32.3 | 3.6 | 38.0 | 51.0 | 13 | 17.3 | 22.6 | 5.3 | |||
LCSS, lung cancer-specific survival; OS, overall survival; Diff., difference; VPI, visceral pleural invasion; LN, lymph node; G1, well differentiated; G2, moderately differentiated; G3, poorly differentiated; G4, undifferentiated.
Additionally, a significant correlation between administering chemotherapy and better OS was found in patients with any of the following risk factors: stage IB (HR: 0.731, 95% CI: 0.647–0.827, P<0.001), a high histologic grade (G3: HR: 0.827, 95% CI: 0.717–0.954, P=0.009), the presence of VPI (HR: 0.711, 95% CI: 0.612–0.826, P<0.001), and 8 to 11 LNs examined (HR: 0.681, 95% CI: 0.525–0.884, P=0.004). Further, administering chemotherapy led to an absolute improvement in OS as follows: 7.8% at 5 years (from 58.8% to 66.6%) and 9.5% at 10 years (from 39.8% to 49.3%) in stage IB patients; 4.6% at 5 years (from 57.0% to 61.6%) and 6.9% at 10 years (from 38.6% to 45.5%) in patients with poorly differentiated grading; 7.7% at 5 years (from 57.9% to 65.6%), and 11.4% at 10 years (from 39.7% to 51.1%) in patients with VPI; 6.7% at 5 years (from 67.1% to 73.8%) and 10.8% at 10 years (from 50.5% to 61.3%) in patients with 8 to 11 LNs examined. Patients with limited resection did not benefit from the addition of chemotherapy in terms of LCSS or OS.
Combined subgroup survival analysis
Pathologic stage combined with one or more high-risk features was used to investigate the indicative effect of high-risk factors for chemotherapy. A statistically significant survival advantage following the addition of chemotherapy was not observed in stage IA1–IA3 patients. Therefore, we divided the pathological stage into IA and IB and then combined those patients with one or more high-risk features to evaluate the association between administering chemotherapy and survival.
Pathologic stage combined with one high-risk feature
Stage IA patients with one or even more high-risk features (i.e., histologic grade, an insufficient number of LNs examined, or limited resection) did not benefit from the addition of chemotherapy in terms of LCSS or OS. Chemotherapy significantly improved LCSS or OS in patients with VPI and stage IB disease. Further, a significant correlation was observed between the addition of chemotherapy and better OS in patients with stage IB and a high histologic grading (IB + G3/G4: HR: 0.724, 95% CI: 0.612–0.857, P<0.001) or fewer than 11 LNs examined (IB + LNs <11: HR: 0.737, 95% CI: 0.641–0.847, P<0.001). The absolute improvement in OS with the addition of chemotherapy was as follows: 8.0% at 5 years and 12.9% at 10 years in patients with stage IB and a high histologic grade; and 7.9% at 5 years and 9.5% at 10 years in patients with stage IB and fewer than 11 LNs examined. However, an improvement in LCSS from the addition of chemotherapy was observed (5-year LCSS increased 0.7% vs. 1.5%, while 10-year LCSS increased 2.6% vs. 2%), and the HR was <1 (0.904 vs. 0.902).
Pathologic stage combined with two high-risk features
Similarly, stage IA patients with any two high-risk features did not benefit from the addition of chemotherapy in terms of LCSS or OS. There was a significant association between the addition of chemotherapy and better LCSS and OS in patients with stage IB combined with the presence of VPI and fewer than 11 LNs examined (LCSS: HR: 0.809, 95% CI: 0.664–0.986, P=0.04; OS: HR: 0.677, 95% CI: 0.570–0.803, P<0.001, Figure 4) with an absolute increase in LCSS of 3.5% at 5 years (from 64.9% to 68.4%), and 6.4% at 10 years (from 54.4% to 60.8%), and an absolute increase in OS of 9.6% at 5 years (from 54.5% to 64.1%) and 12.4% at 10 years (from 37.3% to 49.7%). Additionally, chemotherapy contributed to statistically significant better OS in stage IB patients with a high histologic grade combined with the presence of VPI (HR: 0.780, 95% CI: 0.636–0.957, P=0.02), with an absolute increase in OS of 4.6% at 5 years (from 53.3% to 57.9%), and 10.8% at 10 years (from 37.6% to 48.4%). Similarly, chemotherapy also statistically contributed to better OS in stage IB patients with a high histologic grade and fewer than 11 LNs examined (HR: 0.739, 95% CI: 0.610–0.896, P=0.002) with an absolute increase in OS of 8.4% at 5 years (from 51.7% to 60.1%) and 13.5% at 10 years (from 32.4% to 45.9%).
Pathologic stage combined with three high-risk features
There was a statistically significant relationship between the addition of chemotherapy and better OS in patients who met the following conditions at the same time, including stage IB, a high histological grade, the presence of VPI, and fewer than 11 LNs examined (HR: 0.745, 95% CI: 0.587–0.944, P=0.01) with an absolute increase in OS of 7.0% at 5 years (from 51.1% to 58.1%) and 13.1% at 10 years (from 34.3% to 47.4%). The patients in this group did not benefit significantly from the addition of chemotherapy in terms of LCSS, but statistically non-significant improvement in LCSS with the addition of chemotherapy was observed.
Discussion
In our study, the association between chemotherapy and survival was assessed in stage I patients stratified by pathologic stage and several high-risk clinicopathologic features, including the presence of VPI, a high histological grade, the examination of an insufficient number of LNs, and limited resection. The purpose of this study was to evaluate the value of high-risk clinicopathologic features in decisions to administer chemotherapy for stage I NSCLC.
Adjuvant chemotherapy is an important traditional method to improve survival in NSCLC patients, even stage I NSCLC patients, after resection (3-7). However, the debate continues as to whether adjuvant chemotherapy can prolong survival in stage I NSCLC patients (9,14-16). The latest NCCN guidelines recommend chemotherapy for stage IB or IIA patients with high-risk factors, including poorly differentiated tumors, vascular invasion, wedge resection, a tumor size >4 cm, visceral pleural involvement and unknown LN status after R0 resection (17); however, the indicative role and reference value of these risk factors in chemotherapy decision making have not yet been determined. Among these high-risk factors, a poorly differentiated grade, also named high histological grading, indicates a poor prognosis. Notably, solid and micropapillary adenocarcinomas are considered high grade in the 2015 World Health Organization classification of lung tumors (20).
Studies have increasingly reported that patients with solid or micropapillary patterns have a poorer prognosis even if their patterns are not predominant (21-24). Wedge resection is one type of limited resection or sublobar resection that may affect the survival of patients due to an insufficient resection range caused by tumor spread through air spaces (STAS) (25-27). VPI is widely recognized as a risk factor for the prognosis of lung cancer patients and is included in the TNM classification (1). The presence of VPI is a significant predictive factor in pathologic stage I NSCLC after resection (28-30).
Unknown LN status is a risk factor for prognosis; however, the examination of an insufficient number of LNs also indicates a poor prognosis (13,29,31-33). A recent study showed that eight to 11 nodes were optimal for LN evaluation following curative resection in stage I lung cancer patients (13). In this study, the high-risk clinicopathologic features included a high histologic grade, the presence of VPI, the examination of an insufficient number of LNs, and limited resection. To investigate the value of high-risk clinicopathologic features in decisions to administer chemotherapy for stage I NSCLC, we assessed the association between chemotherapy and survival in patients with stage I NSCLC stratified by pathologic stage and high-risk clinicopathologic features.
In the present study, all the variables were balanced using the PSM method between the two groups based on the status of chemotherapy. An ASMD less than 0.1 and no significant correlation between risk factors and chemotherapy indicated successful matching. The univariate and multivariate Cox regression survival analyses showed that several clinicopathologic features, including limited resection, a larger tumor size, a high histologic grade, the presence of VPI, and the examination of an insufficient number of LNs, were significant independent risk factors for LCSS and OS in stage I NSCLC, which is consistent with the findings of most previous studies (10,11,13,16,28-30,34). It is worth noting that chemotherapy was a risk factor for poor survival in all the patients after matching in both the univariate and multivariate survival analyses. This suggests that patients need to be stratified according to high-risk factors to assess the relationship between chemotherapy and survival.
TNM stage classification comprehensively considers tumor size and some risk factors and is an important reference for predicting the survival and guiding the treatment plan of lung cancer patients (1). Therefore, we stratified all patients after matching according to the high-risk factors and pathologic TNM stage. After rigorous matching and a subgroup survival analysis, chemotherapy only showed a significant survival improvement in both LCSS and OS in patients with the presence of VPI, with an absolute increase in survival of almost 4.9% at 5 years (2% of 5-year LCSS versus 7.7% of 5-year OS) and 8.6% at 10 years (5.8% of 10-year LCSS versus 11.4% of 10-year OS). Since VPI is an ascending factor of T stage, tumors less than 3 cm in diameter with VPI are classified as T2a stage (1). Similarly, patients with VPI had tumor sizes ranging from 0 to 4 cm in this study. This means that if the VPI is positive, chemotherapy should be considered for patients with node-negative NSCLC less than 4 cm, regardless of tumor size. Additionally, stage IB patients did not benefit significantly in terms of LCSS from the addition of chemotherapy, but there was still improvement in LCSS at 5 years and 10 years (1.4% versus 1.3%). Thus, chemotherapy may not contribute to survival in all patients with stage IB NSCLC (i.e., chemotherapy may only need to be considered for VPI-positive stage IB patients).
In this study, the optimal boundary of the LN evaluation was 10 nodes for stage IA and 11 nodes for stage IB (13). A significant association was observed between the addition of chemotherapy and better LCSS and OS in stage IB patients with VPI and fewer than 11 LNs examined, with an absolute increase in survival of almost 6.6% at 5 years (3.5% of 5-year LCSS versus 9.6% of 5-year OS) and 9.4% at 10 years (6.4% of 10-year LCSS versus 12.4% of 10-year OS). After combining the high-risk factors for the examination of an insufficient number of LNs, the benefit of chemotherapy further improved the survival of stage IB patients with the presence of VPI. Stage IB patients with fewer than 11 LNs examined did not significantly benefit from the addition of chemotherapy in terms of LCSS; however, there was still an improvement in LCSS at 5 years and 10 years (1.5% versus 2.0%). One meta-analysis reported that the examination of an insufficient number of LNs was associated with inferior survival rates in patients with early stage NSCLC (31). Similarly, research has shown that chemotherapy is beneficial for stage IB patients who had suboptimal nodal staging (13,35). Thus, the examination of an insufficient number of LNs may be an indicator for administering chemotherapy in stage IB NSCLC. It is recommended to perform adequate LNs dissection. And the adjuvant chemotherapy may be required for the patients with an insufficient number of LNs removed.
In terms of OS, the addition of chemotherapy was only observed to have a benefit in stage IB patients with a high histologic grade when combined with either or both of the following high-risk factors: the presence of VPI and/or fewer than 11 LNs examined. However, chemotherapy did not significantly contribute to better LCSS in patients with a higher histologic grade, even if other high-risk factors were combined; however, some increase in survival was observed. This study included all types of NSCLC, such as adenocarcinoma, squamous cell carcinoma, and all other types except SCLC. In addition, studies have frequently reported that adjuvant chemotherapy contributes to survival benefits in stage I patients with high-grade histological subtypes (10,12,16,36,37). Further accurate stratification of patients with a high histologic grade is needed to investigate the benefits of chemotherapy. Thus, a high histologic grade is a potential indicator for administering chemotherapy in stage I NSCLC.
In the present study, stage I patients with limited resection did not significantly benefit from the addition of chemotherapy in terms of LCSS or OS, regardless of the combination with any high-risk factors. However, recent studies showed that adjuvant chemotherapy was associated with a survival benefit in patients with tumors larger than 3 to 4 cm who underwent sublobar surgery (11,38). A larger wedge volume and smaller tumor volume are related to better survival in wedge resection (39). Meanwhile, high-quality limited resection, such as segmentectomy, is suitable for clinical stage IA lung adenocarcinoma, with a survival equivalent to that of standard lobectomy (40,41). Segmentectomy is significantly better than lobectomy in terms of OS and pulmonary function (42,43). Therefore, the quality of the limited resection may affect the survival of patients and determine whether adjuvant chemotherapy should be considered.
For stage IA NSCLC, patients receiving adjuvant chemotherapy had worse survival in isolation and when stratified by high-risk features. However, chemotherapy did not contribute to survival benefits in stage IA patients, regardless of the combination of any of the high-risk factors. Thus, patients with stage IA NSCLC need to be further stratified according to other risk factors, such as lymphovascular invasion (44-46) or tumor STAS (47,48).
This study had several limitations. First, it was a retrospective study based on data from the SEER database. Several risk factors were not available, such as lymphovascular invasion, histologic subtype, and tumor STAS, which still need further exploration. Second, patients in the control cohort did not receive chemotherapy and had an unknown chemotherapy status. This may not have affected our conclusions, but prospective studies with large samples at multiple centers are still needed to further confirm our findings. Third, there are no specific data on chemotherapy in the SEER database, such as the chemotherapy treatment plan, drug composition, treatment cycle, toxicity, and side effects. Thus, we could not assess the differences between different chemotherapy regimens, and careful randomized controlled trials need to be conducted in this area. Finally, the quality of chemotherapy data in the SEER database was unknown. Previous investigations comparing SEER data with Medicare claims data found that the sensitivity of SEER data to identify individuals who received chemotherapy was only 68% (49). Although we have conducted an in-depth analysis based on the available data set, it is undeniable that the limitation of the database may affect the robustness of the results of this study. Therefore, we expect that more real-world studies or prospective clinical trials can improve the above data and conduct a more comprehensive analysis to further verify the results of this article.
Conclusions
In conclusion, we found that the presence of VPI was the dominant indicator, the examination of an insufficient number of LNs was the secondary indicator, and a high histologic grade was a potential indicator for the need to administer chemotherapy in the treatment of stage I NSCLC. More multi-center prospective clinical trials and real-world studies with large samples are needed to further verify the results in this study.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-305/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-305/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-305/coif). N.S. obtained research grants from Eli Lilly, Chugai Pharmaceutical, Taiho Pharmaceutical, Pfizer, Ono Pharmaceutical, Nippon Kayaku, Takeda Pharmaceutical, Eisai, Shionogi, Daiichi Sankyo, and Boehringer Ingelheim and received speaking honoraria from Eli Lilly, AstraZeneca, MSD, Chugai Pharmaceutical, Taiho Pharmaceutical, Pfizer, Ono Pharmaceutical, Nippon Kayaku, Takeda Pharmaceutical, Daiichi Sankyo, Boehringer Ingelheim, Novartis, Kyowa Kirin, and Bristol Myers Squibb. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Because this research is the study of a public database, it does not involve ethical issues.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Fedor D, Johnson WR, Singhal S. Local recurrence following lung cancer surgery: incidence, risk factors, and outcomes. Surg Oncol 2013;22:156-61. [Crossref] [PubMed]
- Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351-60. [Crossref] [PubMed]
- Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 2005;352:2589-97. [Crossref] [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- NSCLC Meta-analyses Collaborative Group. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet 2010;375:1267-77. [Crossref] [PubMed]
- Ahmad U, Crabtree TD, Patel AP, et al. Adjuvant Chemotherapy Is Associated With Improved Survival in Locally Invasive Node Negative Non-Small Cell Lung Cancer. Ann Thorac Surg 2017;104:303-7. [Crossref] [PubMed]
- Wakelee H, Liberman M, Kato T, et al. Perioperative Pembrolizumab for Early-Stage Non-Small-Cell Lung Cancer. N Engl J Med 2023;389:491-503. [Crossref] [PubMed]
- Li X, Zhang C, Sun Z, et al. Propensity-matched analysis of adjuvant chemotherapy for completely resected Stage IB non-small-cell lung cancer patients. Lung Cancer 2019;133:75-82. [Crossref] [PubMed]
- Qian F, Yang W, Wang R, et al. Prognostic significance and adjuvant chemotherapy survival benefits of a solid or micropapillary pattern in patients with resected stage IB lung adenocarcinoma. J Thorac Cardiovasc Surg 2018;155:1227-1235.e2. [Crossref] [PubMed]
- Pathak R, Goldberg SB, Canavan M, et al. Association of Survival With Adjuvant Chemotherapy Among Patients With Early-Stage Non-Small Cell Lung Cancer With vs Without High-Risk Clinicopathologic Features. JAMA Oncol 2020;6:1741-50. [Crossref] [PubMed]
- Morgensztern D, Du L, Waqar SN, et al. Adjuvant Chemotherapy for Patients with T2N0M0 NSCLC. J Thorac Oncol 2016;11:1729-35. [Crossref] [PubMed]
- Dai J, Liu M, Yang Y, et al. Optimal Lymph Node Examination and Adjuvant Chemotherapy for Stage I Lung Cancer. J Thorac Oncol 2019;14:1277-85. [Crossref] [PubMed]
- Wang J, Wu N, Lv C, et al. Should patients with stage IB non-small cell lung cancer receive adjuvant chemotherapy? A comparison of survival between the 8th and 7th editions of the AJCC TNM staging system for stage IB patients. J Cancer Res Clin Oncol 2019;145:463-9.
- Xie J, Zhang X, Hu S, et al. Effects of adjuvant chemotherapy on survival of patients with stage IB non-small cell lung cancer with visceral pleural invasion. J Cancer Res Clin Oncol 2020;146:2231-9. [Crossref] [PubMed]
- Wang C, Yang J, Lu M. Micropapillary Predominant Lung Adenocarcinoma in Stage IA Benefits from Adjuvant Chemotherapy. Ann Surg Oncol 2020;27:2051-60. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aisner DL, et al. NCCN Guidelines® Insights: Non-Small Cell Lung Cancer, Version 2.2023. J Natl Compr Canc Netw 2023;21:340-50. [Crossref] [PubMed]
- Kris MG, Gaspar LE, Chaft JE, et al. Adjuvant Systemic Therapy and Adjuvant Radiation Therapy for Stage I to IIIA Completely Resected Non-Small-Cell Lung Cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. J Clin Oncol 2017;35:2960-74. [Crossref] [PubMed]
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083-107. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Yanagawa N, Shiono S, Abiko M, et al. The Clinical Impact of Solid and Micropapillary Patterns in Resected Lung Adenocarcinoma. J Thorac Oncol 2016;11:1976-83. [Crossref] [PubMed]
- Hung JJ, Yeh YC, Wu YC, et al. Prognostic Factors in Completely Resected Node-Negative Lung Adenocarcinoma of 3 cm or Smaller. J Thorac Oncol 2017;12:1824-33. [Crossref] [PubMed]
- Lee G, Lee HY, Jeong JY, et al. Clinical impact of minimal micropapillary pattern in invasive lung adenocarcinoma: prognostic significance and survival outcomes. Am J Surg Pathol 2015;39:660-6. [Crossref] [PubMed]
- Ujiie H, Kadota K, Chaft JE, et al. Solid Predominant Histologic Subtype in Resected Stage I Lung Adenocarcinoma Is an Independent Predictor of Early, Extrathoracic, Multisite Recurrence and of Poor Postrecurrence Survival. J Clin Oncol 2015;33:2877-84. [Crossref] [PubMed]
- Kadota K, Nitadori JI, Sima CS, et al. Tumor Spread through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. J Thorac Oncol 2015;10:806-14. [Crossref] [PubMed]
- Eguchi T, Kameda K, Lu S, et al. Lobectomy Is Associated with Better Outcomes than Sublobar Resection in Spread through Air Spaces (STAS)-Positive T1 Lung Adenocarcinoma: A Propensity Score-Matched Analysis. J Thorac Oncol 2019;14:87-98. [Crossref] [PubMed]
- Masai K, Sakurai H, Sukeda A, et al. Prognostic Impact of Margin Distance and Tumor Spread Through Air Spaces in Limited Resection for Primary Lung Cancer. J Thorac Oncol 2017;12:1788-97. [Crossref] [PubMed]
- Jiwangga D, Cho S, Kim K, et al. Recurrence Pattern of Pathologic Stage I Lung Adenocarcinoma With Visceral Pleural Invasion. Ann Thorac Surg 2017;103:1126-31. [Crossref] [PubMed]
- Jiang L, Liang W, Shen J, et al. The impact of visceral pleural invasion in node-negative non-small cell lung cancer: a systematic review and meta-analysis. Chest 2015;148:903-11. [Crossref] [PubMed]
- Tsutani Y, Suzuki K, Koike T, et al. High-Risk Factors for Recurrence of Stage I Lung Adenocarcinoma: Follow-up Data From JCOG0201. Ann Thorac Surg 2019;108:1484-90. [Crossref] [PubMed]
- Meng D, Zhou Z, Wang Y, et al. Lymphadenectomy for clinical early-stage non-small-cell lung cancer: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2016;50:597-604. [Crossref] [PubMed]
- Saji H, Tsuboi M, Yoshida K, et al. Prognostic impact of number of resected and involved lymph nodes at complete resection on survival in non-small cell lung cancer. J Thorac Oncol 2011;6:1865-71. [Crossref] [PubMed]
- Samayoa AX, Pezzi TA, Pezzi CM, et al. Rationale for a Minimum Number of Lymph Nodes Removed with Non-Small Cell Lung Cancer Resection: Correlating the Number of Nodes Removed with Survival in 98,970 Patients. Ann Surg Oncol 2016;23:1005-11. [Crossref] [PubMed]
- Qian J, Xu J, Wang S, et al. Adjuvant Chemotherapy Candidates in Stage I Lung Adenocarcinomas Following Complete Lobectomy. Ann Surg Oncol 2019;26:2392-400. [Crossref] [PubMed]
- John AO, Ramnath N. Neoadjuvant Versus Adjuvant Systemic Therapy for Early-Stage Non-Small Cell Lung Cancer: The Changing Landscape Due to Immunotherapy. Oncologist 2023;28:752-64. [Crossref] [PubMed]
- Hung JJ, Wu YC, Chou TY, et al. Adjuvant Chemotherapy Improves the Probability of Freedom From Recurrence in Patients With Resected Stage IB Lung Adenocarcinoma. Ann Thorac Surg 2016;101:1346-53. [Crossref] [PubMed]
- Burdett S, Pignon JP, Tierney J, et al. Adjuvant chemotherapy for resected early-stage non-small cell lung cancer. Cochrane Database Syst Rev 2015;2015:CD011430. [Crossref] [PubMed]
- Lee PH, Chiang CJ, Tseng JS, et al. Adjuvant chemotherapy compared with observation in patients with T2aN0 stage IB lung adenocarcinoma. Front Oncol 2023;13:1096683. [Crossref] [PubMed]
- Wang Y, Wang R, Zheng D, et al. Predicting the recurrence risk factors and clinical outcomes of peripheral pulmonary adenocarcinoma ≤3 cm with wedge resection. J Cancer Res Clin Oncol 2017;143:1043-51. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Oncologic outcomes of segmentectomy compared with lobectomy for clinical stage IA lung adenocarcinoma: propensity score-matched analysis in a multicenter study. J Thorac Cardiovasc Surg 2013;146:358-64. [Crossref] [PubMed]
- Smith CB, Swanson SJ, Mhango G, et al. Survival after segmentectomy and wedge resection in stage I non-small-cell lung cancer. J Thorac Oncol 2013;8:73-8. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Altorki N, Wang X, Kozono D, et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N Engl J Med 2023;388:489-98. [Crossref] [PubMed]
- Yun JK, Lee GD, Choi S, et al. Comparison of prognostic impact of lymphovascular invasion in stage IA non-small cell lung cancer after lobectomy versus sublobar resection: A propensity score-matched analysis. Lung Cancer 2020;146:105-11. [Crossref] [PubMed]
- Tsutani Y, Miyata Y, Kushitani K, et al. Propensity score-matched analysis of adjuvant chemotherapy for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2014;148:1179-85. [Crossref] [PubMed]
- Chen K, Chen W, Cai J, et al. Favorable prognosis and high discrepancy of genetic features in surgical patients with multiple primary lung cancers. J Thorac Cardiovasc Surg 2018;155:371-379.e1. [Crossref] [PubMed]
- Bains S, Eguchi T, Warth A, et al. Procedure-Specific Risk Prediction for Recurrence in Patients Undergoing Lobectomy or Sublobar Resection for Small (≤2 cm) Lung Adenocarcinoma: An International Cohort Analysis. J Thorac Oncol 2019;14:72-86. [Crossref] [PubMed]
- Vaghjiani RG, Takahashi Y, Eguchi T, et al. Tumor Spread Through Air Spaces Is a Predictor of Occult Lymph Node Metastasis in Clinical Stage IA Lung Adenocarcinoma. J Thorac Oncol 2020;15:792-802. [Crossref] [PubMed]
- Noone A-M, Lund JL, Mariotto A, et al. Comparison of SEER Treatment Data With Medicare Claims. Med Care 2016;54:e55-e64. [Crossref] [PubMed]