
Impact of atrial fibrillation on 1-year outcome in patients with implantable cardioverter defibrillator or cardiac resynchronization therapy with defibrillator: results from the German DEVICE Registry
Highlight box
Key findings
• Atrial fibrillation (AF) is frequently present in patients referred for implantable cardioverter defibrillators (ICDs) or cardiac resynchronization therapy with defibrillator (CRT-D) implantation.
• AF patients are older and suffer from a significantly higher rate of comorbidities than non-AF patients.
• Patients with AF at time of device implantation have a significantly higher 1-year mortality rate and 1-year major adverse cardiac and cerebrovascular events (MACCE) rate and experience significantly more electrical shocks from cardiac rhythm devices, even after adjusting for age, sex, advanced New York Heart Association (NYHA) class, severely impaired left ventricular ejection fraction (LVEF), coronary artery disease (CAD), chronic obstructive pulmonary disease (COPD), diabetes mellitus (DM), chronic renal failure (CRF), QRS duration, and type of indication for electronic device implantation.
What is known and what is new?
• AF patients are older and suffer from a significantly higher rate of comorbidities than non-AF patients.
• Presence of AF at time of device implantation have a significantly higher 1-year estimated mortality rate and 1-year MACCE rate and experience significantly more electrical shocks from cardiac rhythm devices even after adjusting for age, sex, advanced NYHA class, severely impaired LVEF, CAD, COPD, DM, CRF, QRS duration, and type of indication for electronic device implantation.
What is the implication, and what should change now?
• AF should be considered an epiphenomenon of a more complex risk profile in general. Therefore, AF patients referred for ICD/CRT implantation should be considered high risk patients.
• Cardiac rhythm devices should be programmed carefully to try to avoid inappropriate shocks. Nevertheless, more trials to further improve cardiac rhythm device discrimination of supraventricular and ventricular tachycardia is needed.
Introduction
Cardiac resynchronization therapy (CRT) and the implantable cardioverter defibrillator (ICD) are well-established therapies in heart failure (HF) management and primary and secondary prevention of sudden cardiac death (1,2).
Patients with an indication for CRT or ICD implantation often suffer from extensive comorbidities, with atrial fibrillation (AF) being one of the most common additional diagnoses (3). AF affects approximately 25% of ICD patients and 40% of CRT patients, becoming even more frequent in elderly patients with advanced HF (3-5). Through continuous rise of the populations mean age both, the incidence of AF and the number of ICD/CRT implantations increase constantly. By a reduction in biventricular pacing, the loss of atrioventricular synchrony, inappropriate ICD interventions, and unfavourable influence on adequate ICD interventions, AF can attenuate the potential benefits of CRT and ICD therapy (4-6). Furthermore, registry data suggest that AF could be a risk factor for procedural and in-hospital complications in ICD patients (7).
Current data on the impact of AF on peri-procedural and in-hospital complications, as well as clinical outcomes during follow-up after ICD/CRT implantation, remain limited. Based on this on-hand data from the prospective nationwide German DEVICE Registry, we aim to gain additional information from the implantation period and the clinical 12 months follow-up of this patient group and, in this way, to contribute to a better understanding of the influence that AF could have on this cohort. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-274/rc).
Methods
Data collection
The data on hand was collected and analysed by the German DEVICE I and II Registry. The German DEVICE Registry [“Institut für Herzinfarktforschung” (IHF), Ludwigshafen, Germany] is a prospective nationwide multicenter registry on the implantations and revision of ICDs and CRT-systems.
Over 50 cardiology centers throughout Germany voluntarily participate in the registry by integrating patient data into a web-based electronic data hub. The IHF handles data administration and management, as well as patient monitoring and telephone follow-up. Patients included in the registry all provided written informed consent to the use, for scientific purposes, of their clinical data. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of the State Medical Association of Rhineland-Palatinate approved the registry [No. 837.279.15 (10047)].
For this analysis, we collected baseline, procedural, and 12-month follow-up data from patients who received either a new ICD or CRT-D implantation or a device replacement for either battery exchange, device malfunction or device change to a different system. Patients for this analysis were enrolled between 2014 and 2019.
A baseline 12-Lead-ECG was conducted and interpreted by the enrolling center on the day of admission prior to implantation. Patients were classified into sinus rhythm (SR) or AF on the basis of these findings and prior medical history.
Additionally, each patient underwent echocardiography before implantation, also performed and reported by the enrolling center. LVEF, right ventricular function parameters, wall motion disorders and cardiac valve lesions were determined as a minimum requirement in every echocardiography.
Follow-up
Enrolled patients were contacted by telephone 12 months after device implantation or replacement by the IHF. Information on complications, hospitalizations, cardiac events (e.g., myocardial infarction, ischemic events, arrhythmic events), resuscitation, interventional ablation or revascularisation procedures, syncopal events, heart failure status, change of medication, general symptoms and quality of life were reviewed with the patient. Cases of death were documented as well. In case of hospitalization or other inpatient treatment in a medical facility, patients were asked to provide relevant documentation and records to review the course of disease since device implantation. The available medical records were analysed and used for follow-up.
Statistical analysis
Metric data are displayed as mean ± standard deviation. Categorical data are presented as absolute counts and percentage values. Chi-square test or Kruskal-Wallis test was used to compare categorical variables. All statistical tests were performed two-tailed and P values ≤0.05 were considered statistically significant. Kaplan-Meier estimator was used to calculate 1-year mortality. Analysis was performed using SPSS version 28 (IBM, Armonk, NY, USA).
Results
Data from 4,929 patients implanted with an ICD or CRT system were reviewed and analysed for this study. Table 1 summarizes demographic data and type of implanted device.
Table 1
| Parameter | Atrial fibrillation | Sinus rhythm | P value |
|---|---|---|---|
| Total patients | 946 (19.2%) | 3,982 (80.8%) | |
| Age (years), median [IQR] | 72 [66–77] | 66 [56–73] | <0.001 |
| Male | 85.5% (809/946) | 79.9% (3,181/3,983) | <0.001 |
| BMI (kg/m2), median [IQR] | 27.5 [24.7–31.0] | 26.8 [24.1–30.0] | 0.083 |
| Device-systems | |||
| ICD (single lead) | 57.9% (548/946) | 51.6% (2,057/3,983) | <0,001 |
| ICD (dual lead) | 13.4% (127/946) | 21.1% (840/3,983) | <0.001 |
| CRT-D | 28.6% (271/946) | 27.3% (1,086/3,983) | 0.39 |
| Implantation procedure duration (min), median [IQR] | 58 [40–104] | 60 [40–105] | 0.887 |
| Implantation of a new device | 87.2% (821/942) | 87.3% (3,467/3,973) | 0.93 |
| Revision of an existing device | 12.8% (121/942) | 12.7% (506/3,972) | 0.93 |
IQR, interquartile range; BMI, body mass index; ICD, implantable cardioverter defibrillator; CRT-D, cardiac resynchronization therapy with defibrillator.
AF was documented at time of implantation in 946 (19.2%) of patients and the remaining 3,982 (80.8%) were implanted in SR.
Biological sex was male in 80.9% of the patients with in unequal distribution of 85.5% in the AF vs. 79.9% in the SR group (P<0.001). Mean BMI was 27.5 kg/m2 in AF patients and 26.8 kg/m2 in SR patients (P=0.083). Proportional implantation of ICD and CRT was similar in both groups.
87.2% of the patients underwent implantation of a new device, while 12.8% had revision of an existing device for battery exchange, device malfunction or device change with no difference in distribution between AF and SR patients (87.2% vs. 87.3%; P=0.93, 12.8% vs. 12.7%; P=0.93).
Table 2 summarizes comorbidity profile data.
Table 2
| Pre-existing condition | Atrial fibrillation | Sinus rhythm | P value |
|---|---|---|---|
| Coronary artery disease | 59.2% (560/946) | 61.5% (2,449/3,983) | 0.19 |
| Post MI | 30.5% (289/946) | 36% (1,434/3,983) | 0.002 |
| Dilatative cardiomyopathy | 40.2% (380/946) | 31.6% (1,260/3,982) | <0.001 |
| Hypertrophic cardiomyopathy | 1.9% (18/946) | 3.7% (146/3,982) | <0.001 |
| Previous stroke | 4.9% (46/946) | 3.7% (149/3,982) | 0.11 |
| Peripheral artery disease | 4.5% (43/946) | 2.7% (106/3,982) | 0.002 |
| Diabetes | 33.6% (318/946) | 25.5% (1,014/3,982) | <0.001 |
| Hypertension | 58.4% (552/946) | 51.1% (2,035/3,982) | <0.001 |
| COPD | 4.2% (40/946) | 3.6% (144/3,982) | 0.37 |
| Renal failure | 22.6% (214/946) | 15.3% (609/3,982) | <0.001 |
| EF ≤30% | 68.2% (628/921) | 61.0% (2,334/3,828) | <0.001 |
| NYHA classification | |||
| NYHA III | 45.5% (394/866) | 38.9% (1,409/3,624) | <0.001 |
| NYHA IV | 3.9% (34/866) | 2.7% (99/3,624) | 0.082 |
MI, myocardial infarction; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; NYHA, New York Heart Association.
Primary prophylaxis was the leading reason for device implantation with 62.4% in AF patients and 59.6% in SR patients with no statistical difference between the groups. Most common cause for secondary prophylactic device implantation was sustained ventricular tachycardia with 48.6% vs. 44.1% respectively (P=0.13).
Left ventricular ejection fraction (LVEF) was differently distributed between the AF and SR patients. Patients in AF had a significantly more comorbid profile including older age {72 [interquartile range (IQR), 66–77] vs. 66 (IQR, 56–73) years; P<0.001}, and higher rate of patients with LVEF <30% (68.2% vs. 61.0%; P<0.001). A significantly higher number of patients with LVEF >55% was present in the SR group (3.4% vs. 9.4%; P<0.001). Furthermore, the prevalence of peripheral artery disease (4.5% vs. 2.7%; P=0.002), diabetes mellitus (DM) (33.6% vs. 25.5%; P<0.001), arterial hypertension (58.4% vs. 51.1%; P<0.001) and renal failure (22.6% vs. 15.3%; P<0.001) was considerably higher in the AF group. Coronary artery disease (CAD) was the most common underlying disease, equally distributed between both groups (59.2% vs. 61.5%; P=0.19). Cases of dilatative cardiomyopathy (DCM) were significantly more common in AF patients, while there were significantly more cases of hypertrophic cardiomyopathy in the SR group (40.2% vs. 31.6%; P<0.001, 1.9% vs. 3.7%; P<0.001). In addition, AF patients were more symptomatic at baseline, reporting NYHA class III or higher symptoms more frequently than those in SR (49.4% vs. 41.6%; P<0.001). NYHA class I or less symptoms were significantly more frequent in the SR group with 11.2% vs. 18.9% respectively (P<0.001).
Detailed demographic and medical baseline data is depicted in Tables 1-3.
Table 3
| Drug | Total | AF | SR | P value | OR (95% CI) |
|---|---|---|---|---|---|
| ACE-I | 86.2% (4,234/4,914) | 89.3% (842/943) | 85.4% (3,392/3,971) | 0.002 | 1.42 (1.14–1.78) |
| Betablocker | 90.1% (4,427/4,914) | 92.5% (872/943) | 89.5% (3,555/3,971) | 0.006 | 1.44 (1.11–1.87) |
| Aldosterone-antagonist | 39.7% (1,951/4,914) | 43.7% (412/943) | 38.8% (1,539/3,971) | 0.005 | 1.23 (1.06–1.42) |
| Diuretics | 71.3% (3,504/4,912) | 84.3% (795/943) | 68.3% (2709/3,969) | <0.001 | 2.50 (2.01–3.01) |
| Cardiac glycosides | 16.8% (825/4,913) | 33.1% (312/943) | 12.9% (513/3,970) | <0.001 | 3.33 (2.83–3.93) |
| AAD (Class I, III or IV) | 9.5% (465/4,913) | 22.5% (212/943) | 6.4% (253/3,970) | <0.001 | 3.12 (2.06–6.40) |
| OAC | 34.0% (1,670/4,913) | 76.7% (723/943) | 23.9% (947/3,970) | <0.001 | 11.32 (8.05–15.91) |
| Statins | 60.9% (2,991/4,914) | 58.3% (550/943) | 61.5% (2,441/3,971) | 0.075 | 0.88 (0.76–1.01) |
AF, atrial fibrillation; SR, sinus rhythm; OR, odds ratio; CI, confidence interval; ACE-I, angiotensin-converting-enzyme inhibitor; AAD, anti-arrhythmic drug; OAC, oral anticoagulation.
Perioperative complications
During implantation, perioperative death (0.6% vs. 0.2%; P=0.016) and major adverse cardiac and cerebrovascular events (MACCE) (0.8% vs. 0.2%; P=0.014) both occurred more commonly in the AF group. Other major complications requiring surgical or interventional treatment were equally distributed between both groups (2.3% vs. 1.7%; P=0.22) and included pericardial effusion (0.1%), haematothorax (0.1%), pneumothorax (0.4%), and pocket haematoma (1.1%), making the latter the most common observed. Patients from AF group suffered from a higher number of pocket haematoma (1.9% vs. 1.0%; P=0.024). Further data on perioperative complications are displayed in Table 4.
Table 4
| Complication | Atrial fibrillation | Sinus rhythm | P value |
|---|---|---|---|
| Total complications | 4.3% (30/704) | 2.8% (111/3,982) | 0.38 |
| Death | 0.6% (6/946) | 0.2% (6/3,981) | 0.016 |
| Stroke | 0.1% (1/704) | 0.0% (1/3,086) | 0.34 |
| Myocardial infarction | 0.0% (0/704) | 0.0% (1/3,986) | >0.99 |
| MACCE | 0.8% (6/709) | 0.2% (6/3,090) | 0.014 |
| Major complications requiring interventional/surgical treatment | 2.3% (22/939) | 1.7% (67/3,968) | 0.22 |
| Pericardial effusion | 0.0% (0/939) | 0.2% (7/3,968) | 0.36 |
| Pneumothorax | 0.4% (4/939) | 0.4% (17/3,968) | >0.99 |
| Haemothorax | 0.0% (0/939) | 0.1% (5/3,968) | 0.59 |
| Pocket haematoma | 1.9% (18/939) | 1.0% (38/3,967) | 0.024 |
| Need for device revision before discharge | 2.3% (16/688) | 2.0% (61/2,992) | 0.66 |
MACCE, major adverse cardiovascular and cerebrovascular events.
Follow-up
The median follow-up duration was 15.6 (IQR, 12.8–20.0) months in the AF vs. 16.1 (IQR, 12.9–20.7) months in the SR patients (P=0.14). 20.0% of patients from the AF group died during this follow-up period, compared to 11.0% from the SR group (P<0.001). During follow-up, AF patients experienced a significantly higher rate of defibrillator shocks (25% vs. 15.3%; P<0.001). One-year estimated mortality (Kaplan-Meier) was 10.8% in the AF and 5.9% in the SR group (P<0.001) (Table 5). A corresponding Kaplan-Meier estimate representing survival over the first 365 days after implantation is displayed in Figure 1. Estimated 1-year MACCE rate was 11.2% vs. 7.0% (P<0.001). The impact of AF on ICD shocks and mortality persisted even after adjusting for age, sex, advanced NYHA class, severely impaired LVEF, CAD, COPD, DM, CRF, QRS duration, and type of indication for electronic device implantation. In particular, the risk for ICD shocks is doubled in patients that undergo ICD implantation, and is increased by 52% in those submitted to CRT-D implantation. Furthermore, 1-year mortality risk is 67% higher in AF patients undergoing CRT-D implantation and 23% higher in those receiving ICD, when compared to patients in SR (Figure 2). In particular, AF is a greater risk factor for shock delivery in patients with ICDs than secondary prophylaxis (odds ratio 2.05 vs. 1.49). Detailed data on follow-up mortality and shock delivery are depicted in Tables 6,7.
Table 5
| Follow-up data | Atrial fibrillation | Sinus rhythm | P value |
|---|---|---|---|
| Complete documented follow-up | 96.1% (909/946) | 96.9% (3,859/3,982) | 0.21 |
| Follow-up duration (months), median (IQR) | 15.6 (12.8–20.0) | 16.1 (12.9–20.7) | 0.14 |
| Death during follow-up | 20.0% (189/946) | 11.0% (438/3,983) | <0.001 |
| Survival-time (months), median (IQR) | 11.1 (5.0–28.2) | 11.5 (4.3–28.1) | 0.89 |
| 1-year mortality | 10.8% | 5.9% | <0.001 |
| 1-year MACCE | 11.2% | 7.0% | <0.001 |
IQR, interquartile range; MACCE, major adverse cardiovascular and cerebrovascular events.
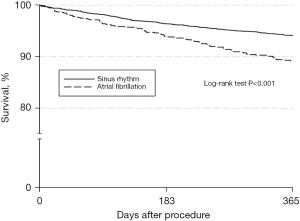
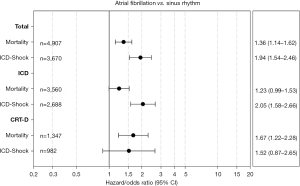
Table 6
| Risk factor | Adjusted hazard ratio | 95% CI | P value |
|---|---|---|---|
| AF vs. SR | 1.36 | 1.14–1.62 | 0.0006 |
| Age (linearly every 10 years) | 1.63 | 1.48–1.79 | <0.0001 |
| Male | 1.39 | 1.09–1.72 | 0.0071 |
| CAD | 1.30 | 1.08–1.56 | 0.0055 |
| NYHA III/IV | 1.47 | 1.23–1.76 | <0.0001 |
| EF ≤30% | 1.27 | 1.05–1.53 | 0.012 |
| QRS >120 ms | 1.12 | 0.94–1.33 | 0.21 |
| Secondary vs. primary prevention | 1.41 | 1.19–1.68 | <0.0001 |
| Renal failure | 1.91 | 1.60–2.28 | <0.0001 |
| Diabetes | 1.29 | 1.09–1.52 | 0.0032 |
| COPD | 1.62 | 1.20–2.19 | 0.0018 |
CI, confidence interval; AF, atrial fibrillation; SR, sinus rhythm; CAD, coronary artery disease; NYHA, New York Heart Association; EF, ejection fraction; COPD, chronic obstructive pulmonary disease.
Table 7
| Risk factor | Adjusted hazard ratio | 95% CI | P value |
|---|---|---|---|
| AF vs. SR | 1.94 | 1.54–2.46 | <0.0001 |
| Age (linearly every 10 years) | 0.93 | 0.86–1.01 | 0.083 |
| Male | 1.30 | 0.99–1.70 | 0.055 |
| EF ≤30% | 1.30 | 1.04–1.62 | 0.019 |
| NYHA III/IV | 0.86 | 0.68–1.08 | 0.18 |
| QRS >120 ms | 0.73 | 0.58–0.91 | 0.0047 |
| Secondary vs. primary prevention | 1.62 | 1.31–2.00 | <0.0001 |
| CAD | 1.00 | 0.81–1.24 | 0.99 |
ICD, implantable cardioverter defibrillator; CRT-D, cardiac resynchronization therapy with defibrillator; CI, confidence interval; AF, atrial fibrillation; SR, sinus rhythm; EF, ejection fraction; NYHA, New York Heart Association; CAD, coronary artery disease.
Discussion
This study reports on the impact of AF compared to SR in a large patient cohort undergoing ICD or CRT implantation or replacement from the German DEVICE registry. In this analysis of clinical data, including data from implantation and mid-term follow-up, results show that: (I) AF is frequently present in patients referred for ICD/CRT implantation, and, for this reason, its potential impact should be better defined and considered for perioperative and follow-up risk stratification. (II) AF patients are often older and suffer from a significantly higher rate of comorbidities, especially peripheral artery disease, diabetes, arterial hypertension, and chronic renal failure (CRF). (III) AF patients have a higher rate of reduced LVEF and more severe HF symptoms/signs. (IV) Presence of AF at time of device implantation will impact upon follow-up outcome. AF patients have a significantly higher 1-year estimated mortality rate and 1-year MACCE rate and experience significantly more electrical shocks from the cardiac rhythm device. (V) The impact of AF on electrical shocks persists even after adjusting for age, sex, advanced NYHA class, severely impaired LVEF, CAD, COPD, DM, CRF, QRS duration, and type of indication for electronic device implantation. In particular, the risk is doubled in patients that undergo ICD implantation, and it is increased of 50% in those submitted to CRT-D implantation. (VI) Furthermore, the impact of AF on 1-year mortality persists after adjusting for the conditions mentioned above. One-year mortality risk is 67% higher in AF patients undergoing CRT-D implantation and 23% higher in those receiving ICD, when compared to patients in SR. (VII) Finally, presence of AF in candidates for ICD and CRT-D will imply a heavier medical management burden. In fact, patients in AF are significantly more prone to re-hospitalization and require a more intensive pharmacological management, including diuretics, beta-blockers, antiarrhythmics, anticoagulants and platelets’ inhibitors.
In this investigated cohort from the DEVICE registry, 19.3% of the patients were in AF at the time of device implantation or revision. These findings align with rates reported from large studies on patients undergoing ICD implantation (4,8,9) and previous data reported from the German DEVICE registry on this topic in a smaller cohort in 2013 (10). In this context, the AF prevalence has remained stable through the years, at least within the German premises, despite the optimization of medical treatment of congestive HF. It could be because the referral pattern for ICD/CRT has slightly changed in the last 10 years. Patients with chronic diseases have more prolonged survival, so we are now treating older patients with a more complex comorbidity burden (10). We should consider AF an epiphenomenon of a more complex risk profile, especially in HF patients referred for ICD/CRT. Our observation of an older cohort, as well as a more severe comorbid profile within the AF group, is expected since older age and comorbidities, especially HF, diabetes, arterial hypertension, peripheral vascular disease, and renal impairment are known risk factors for the development of AF (11-15). At the same time, AF can worsen HF and its symptoms, harming renal performance and leading to further morbidities and all-cause mortality (14). The presence of AF at the time of referral for cardiac rhythm device implant should be therefore regarded as an expression of the patient’s frailty which, per se, results in an essential factor impacting upon periprocedural and follow-up outcomes. Although it should not be a surprise that the sicker patients have worse outcomes, adjustment for major comorbidities and demographic data confirms the independent and malignant effect of pre-procedural AF. In particular, because LVEF is known to have an independent effect upon mortality and malignant ventricular arrythmias, we have confirmed that even after adjusting for severely impaired LVEF (<30%), the negative impact of AF persists.
Our observed mid-term mortality rate falls in line with those reported from other large registries (16), where mortality was not separately investigated and compared between AF and SR patients. Our contemporary analysis of the DEVICE registry reveals a follow-up mortality rate of 19.2% in the AF group. Bunch et al. have previously reported a mortality rate of 8.9% in device patients with new onset of AF (17). However, it can be assumed that patients with newly diagnosed AF do not have the same risk profile and comorbidities as those with consolidated AF history, which leads to long-term consequences. Moreover, previous results from the German DEVICE registry have documented a 1-year mortality rate ranging from 10.7% to 12% for ICD and CRT patients respectively (10). As already elucidated, although the contemporary mortality rates are higher than those reported in historical cohorts with ICD/CRT and AF, the referral pattern for cardiac rhythm device implantation has changed and there are differences between the reported groups’ comorbid profiles that make direct comparison difficult.
Current literature continues to show a tendency towards an increased incidence of inappropriate shocks in AF patients, identifying AF as an independent predictor for inadequate ICD shocks (18). In our experience, AF patients experienced a significantly higher rate of ICD shocks during follow-up than the SR group. As already said, differences persisted in both ICD and CRT-D patients, even after adjusting for major comorbidities, including ventricular function. Although in the present database information concerning the rate of inappropriate shocks was not available, we have documented that patients in AF have a twice as much risk of experiencing VT storms. This consistent finding should be pondered because shocks are associated with greater healthcare resource utilization, poorer quality of life, and higher mortality (19). Because initial device detection of ventricular tachycardia or ventricular fibrillation is based predominately on ventricular rate, AF patients will incur a higher rate of shocks, including inappropriate shocks. Although we do not have specific information concerning the device programming strategy in every single center involved with the Device registry, we should stress that our findings confirm the importance, particularly in AF patients, of adopting programming enhanced supraventricular tachycardia discrimination algorithms (morphology discrimination, rate stability, and sudden or chamber onset) for reducing inappropriate ICD therapies rates (19). While in the CRT-D group the probability of a shock delivery was increased by 52% in patients with AF, the adjusted odds ratio for a shock in patients with an ICD and AF was 2.05, making AF the strongest of all risk factors for shock delivery in this patient group. A possible explanation for this could be the fact that an atrial lead is almost always implanted in the CRT-D group, which can contribute to better discrimination of arrhythmias. In comparison, ICDs are partially implanted only with an RV lead. Finally, we should comment upon the risk of periprocedural bleeding complications in AF patients undergoing ICD/CRT implantation. Although the technique for cardiac rhythm device implantation and the designs of the devices have been, at this stage, and after many years of experience, optimized, periprocedural complications are still reported. In this context, even though trials demonstrate safe implantation under continued oral anticoagulation, some recent literature confirms oral anticoagulation to be associated with an increase in complications (20), suggesting that real-world clinical observations could differ from randomized prospective trial data. In particular, while in our experience, significant complications requiring surgical intervention did not differ between both groups, pocket hematoma occurred significantly more often within the AF group. Rates are comparable to those presented by Cheng et al. in a prospective randomized trial comparing patients with and without warfarin interruption and heparin bridging before ICD implantation (21). Moreover, anticoagulation of AF patients included in our experience herein summarized has been performed according to contemporary standards and, in this context, we still need evidence on the safest strategy to adopt for the majority of patients that are, nowadays, treated with the newer direct oral anticoagulants, before ICD/CRT implantation (22).
Limitations
The data presented in this study originate from a prospective registry and have typical limitations associated with this. Participation in the registry was voluntary and therefore enrolled patients do not represent every consecutive patient implanted. There is also a risk that particularly disabled or frail individuals do not take part for fear of an additional burden and thus distort the overall distribution of the cohort. Furthermore, the information and parameters collected were limited to keep its acquisition realizable, feasible, and in context. All information included was provided by the concerned investigator without coherent approaches or independent verification. Classification into AF and SR groups was made exclusively on the ECG performed at enrolment. Additional information such as initial diagnoses, duration of AF, and AF status (paroxysmal, persistent, etc.) is lost, making it difficult to assess patients and their risk more precisely. At the same time, patients with paroxysmal AF may have erroneously entered the SR group. All follow-up information was gathered by telephone contact only. Therefore, the assessment of individual events can be biased by the patient’s subjective experience, and essential information might be remembered incorrectly or even forgotten.
Conclusions
The present referral pattern for CRT-ICD implantation has includes patients with increasingly more complex clinical profiles. Consistent conclusions about the independent impact of AF in this very comorbid cohort could be drawn if we had the possibility to actively control for the many recognized and unrecognized comorbid conditions, or if we had the chance to perform adequately sized prospective randomized trials to test the benefits of AF burden reduction. The German DEVICE I–II registry is a nationwide prospective multicentre database of ICD/CRTs with clinical follow-up data. Our findings in this real-world scenario are hypothesis generating and support the fact that AF in patients requiring ICD and CRT therapy may negatively impact clinical follow-up outcomes, even after adjusting for major comorbidities, including severely impaired heart function.
Acknowledgments
Funding: This study was supported by the institutional research grant from Boston Scientific.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-274/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-274/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-274/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-274/coif). G.D. serves as an unpaid editorial board member of Journal of Thoracic Disease from February 2023 to January 2025. H.I. received an institutional research grant from Boston Scientific. J.K. received honoraria for lectures and travel grants from Abbott, Boston Scientific and Biotronik. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Patients included in the registry all supplied written informed consent. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of the State Medical Association of Rhineland-Palatinate approved the registry [No. 837.279.15 (10047)].
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599-726. [Crossref] [PubMed]
- Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2022;43:3997-4126. [Crossref] [PubMed]
- Gopinathannair R, Chen LY, Chung MK, et al. Managing Atrial Fibrillation in Patients With Heart Failure and Reduced Ejection Fraction: A Scientific Statement From the American Heart Association. Circ Arrhythm Electrophysiol 2021;14:HAE0000000000000078. [Crossref] [PubMed]
- Borleffs CJ, van Rees JB, van Welsenes GH, et al. Prognostic importance of atrial fibrillation in implantable cardioverter-defibrillator patients. J Am Coll Cardiol 2010;55:879-85. [Crossref] [PubMed]
- Elliott MK, Mehta VS, Martic D, et al. Atrial fibrillation in cardiac resynchronization therapy. Heart Rhythm O2 2021;2:784-95. [Crossref] [PubMed]
- Singh JP, Hall WJ, McNitt S, et al. Factors influencing appropriate firing of the implanted defibrillator for ventricular tachycardia/fibrillation: findings from the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II). J Am Coll Cardiol 2005;46:1712-20. [Crossref] [PubMed]
- Haines DE, Wang Y, Curtis J. Implantable cardioverter-defibrillator registry risk score models for acute procedural complications or death after implantable cardioverter-defibrillator implantation. Circulation 2011;123:2069-76. [Crossref] [PubMed]
- van Gelder IC, Phan HM, Wilkoff BL, et al. Prognostic significance of atrial arrhythmias in a primary prevention ICD population. Pacing Clin Electrophysiol 2011;34:1070-9. [Crossref] [PubMed]
- Kattih B, Operhalski F, Boeckling F, et al. Clinical outcomes of subcutaneous vs. transvenous implantable defibrillator therapy in a polymorbid patient cohort. Front Cardiovasc Med 2022;9:1008311. [Crossref] [PubMed]
- Köbe J, Wasmer K, Andresen D, et al. Impact of atrial fibrillation on early complications and one year-survival after cardioverter defibrillator implantation: results from the German DEVICE registry. Int J Cardiol 2013;168:4184-90. [Crossref] [PubMed]
- Aune D, Feng T, Schlesinger S, et al. Diabetes mellitus, blood glucose and the risk of atrial fibrillation: A systematic review and meta-analysis of cohort studies. J Diabetes Complications 2018;32:501-11. [Crossref] [PubMed]
- Hobbelt AH, Siland JE, Geelhoed B, et al. Clinical, biomarker, and genetic predictors of specific types of atrial fibrillation in a community-based cohort: data of the PREVEND study. Europace 2017;19:226-32. [Crossref] [PubMed]
- Nalliah CJ, Sanders P, Kalman JM. The Impact of Diet and Lifestyle on Atrial Fibrillation. Curr Cardiol Rep 2018;20:137. [Crossref] [PubMed]
- Lip GYH, Coca A, Kahan T, et al. Hypertension and cardiac arrhythmias: a consensus document from the European Heart Rhythm Association (EHRA) and ESC Council on Hypertension, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE). Europace 2017;19:891-911. [Crossref] [PubMed]
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373-498. Erratum in: Eur Heart J 2021;42:507 Erratum in: Eur Heart J 2021;42:546-7. Erratum in: Eur Heart J 2021;42:4194. [Crossref] [PubMed]
- Saxon LA, Hayes DL, Gilliam FR, et al. Long-term outcome after ICD and CRT implantation and influence of remote device follow-up: the ALTITUDE survival study. Circulation 2010;122:2359-67. [Crossref] [PubMed]
- Bunch TJ, Day JD, Olshansky B, et al. Newly detected atrial fibrillation in patients with an implantable cardioverter-defibrillator is a strong risk marker of increased mortality. Heart Rhythm 2009;6:2-8. [Crossref] [PubMed]
- Oosterwerff E, Adiyaman A, Elvan A, et al. Significantly less inappropriate shocks in ischemic patients compared to non-ischemic patients: The S-ICD experience of a high volume single-center. Pacing Clin Electrophysiol 2021;44:1918-24. [Crossref] [PubMed]
- Geller JC, Wöhrle A, Busch M, et al. Reduction of inappropriate implantable cardioverter-defibrillator therapies using enhanced supraventricular tachycardia discriminators: the ReduceIT study. J Interv Card Electrophysiol 2021;61:339-48. [Crossref] [PubMed]
- Gasperetti A, Schiavone M, Ziacchi M, et al. Long-term complications in patients implanted with subcutaneous implantable cardioverter-defibrillators: Real-world data from the extended ELISIR experience. Heart Rhythm 2021;18:2050-8. [Crossref] [PubMed]
- Cheng A, Nazarian S, Brinker JA, et al. Continuation of warfarin during pacemaker or implantable cardioverter-defibrillator implantation: a randomized clinical trial. Heart Rhythm 2011;8:536-40. [Crossref] [PubMed]
- Stewart MH, Morin DP. Management of Perioperative Anticoagulation for Device Implantation. Card Electrophysiol Clin 2018;10:99-109. [Crossref] [PubMed]


