
Clinical characteristics and prognostic implications of immune-related hepatitis in patients with advanced non-small cell lung cancer treated with immune checkpoint inhibitors: a retrospective study
Highlight box
Key findings
• Longer progression-free survival (PFS) was observed in patients who developed early or mild ir-hepatitis. And ir-hepatitis timing (development within two treatment cycles) and higher severity grades were risk factors associated with PFS.
What is known and what is new?
• The incidence of immune-related hepatitis (ir-hepatitis), which has been reported to range from 2% to 30%, may be associated with mildly abnormal laboratory findings, hepatobiliary disorders, or liver failure. However, the studies on the clinical characteristics and prognosis outcomes of advanced non-small cell lung cancer (NSCLC) patients with ir-hepatitis are lacking.
• Our study represents one of the largest studies conducted on patients with advanced NSCLC who experienced ir-hepatitis. Our objective was to analyze the clinical characteristics and prognosis of ir-hepatitis across different grades, as well as to assess the timing of ir-hepatitis onset and its impact on patient survival following immunotherapy.
What is the implication, and what should change now?
• It is advisable to maintain vigilant monitoring of immune-related adverse events to ensure the appropriate utilization of immune checkpoint inhibitors. More based researches and prospective studies are required in the future.
Introduction
Immunotherapy has shown promising, effective and durable responses. Immune checkpoint inhibitors (ICIs) have led to a paradigm shift in the oncological treatment of several advanced cancers through exploiting immune mediated mechanisms blocking inhibitory molecules, such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), or its ligand, programmed cell death protein ligand 1 (PD-L1), thus reactivating antitumor T cells.
Despite the achievements of cancer therapies, the administration of immunotherapy carries risks of toxic effects, which are denoted as immune-related adverse events (irAEs). irAEs result from excessive immune system activation, and their pleiotropic manifestations can affect almost any organs, most commonly the gastrointestinal tract, skin, endocrine glands and liver (1,2). Generally, irAEs in immunotherapy are common, thus limiting the application of immunotherapy. Discontinuation of immunotherapy, multidisciplinary management and the introduction of immunosuppression may be required (2,3).
Furthermore, immune-related hepatitis (ir-hepatitis), of which its incidence has been reported to be 2–30%, may be associated with mildly abnormal laboratory findings, hepatobiliary disorders or liver failure (4). Previous studies on irAEs have reported that patients with irAEs show better survival outcomes than those without irAEs (5,6). Several studies have explored the factors associated with prolonged survival (3,7-9). However, more studies on patients with advanced non-small cell lung cancer (NSCLC) with ir-hepatitis are necessary to analyze the prognostic effects associated with survival and time of the occurrence of ir-hepatitis.
This study was conducted on patients with advanced NSCLC to assess the timing of ir-hepatitis and survival in patients with ir-hepatitis, as well as related risk factors, to advance insightful knowledge and support appropriate therapeutic management strategies. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1684/rc).
Methods
Patient characteristics
In this retrospective study, patients with advanced NSCLC diagnosed with ir-hepatitis after receiving at least one dose of immunotherapy were enrolled between June 2016 and December 2022. Ir-hepatitis was preliminarily screened on the basis of the elevated transaminase, the most common presentation, then diagnosed through multidisciplinary discussion. Data including patient characteristics, outcomes of ir-hepatitis and response to immunotherapy were extracted from electronic medical records. Patients with incomplete medical records or a history of other malignancies were excluded. Patients with liver metastases should have the normal liver function at the baseline to ensure adequate liver reserve, whereas those with viral hepatitis type B (HBV) infection received antiviral therapy to ensure adequate viral suppression.
Serum parameters such as aspartate aminotransferase (AST; normal range: 5–40 U/L in men and 5–35 U/L in women), alanine aminotransferase (ALT; normal range: 5–40 U/L in men and 5–35 U/L in women) and total bilirubin (BIL; normal range: 0.0–20.0 µmol/L) were recorded at baseline and in the peak serum. The toxicity of ir-hepatitis was assessed according to Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 (Table S1), and was categorized as severe adverse events (AEs) (grade 3–5) or mild AEs (grade 1–2). The criteria for grade 1 were an increase in AST or ALT less than three times the upper limit of normal (ULN). Grade 2 was defined as an increase in AST or ALT between 3 and 5 times the ULN. Grade 3 was defined as an increase in ALT or AST between 5 and 20 times the ULN, whereas grade 4 was defined as an increase to more than 20 times the ULN. Grade 5 was defined as death (10).
Furthermore, the R value for liver injury, which was derived by dividing the peak serum ALT/ULN by that of ALP/ULN, was used to classify the liver damage as hepatocellular (R >5), mixed (2≤ R ≤5) or cholestatic (R <2) types.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Approval of the study protocol was obtained from Zhejiang Cancer Hospital Institutional Review Board Committee (approval No. IRB-2023-377). Individual consent for this retrospective analysis was waived.
Response
According to clinical trials and National Comprehensive Cancer Network (NCCN) guidelines, patients were administered camrelizumab 200 mg, pembrolizumab 200 mg, atezolizumab 1,200 mg or other anti-PD-(L)1 therapy. Computed demographic scans and laboratory tests were performed every three weeks, and tumor response was assessed every six weeks. The treatment response was classified as complete response (CR), partial response (PR) or stable disease (SD) according to the Response Evaluation Criteria in Solid Tumors version 1.1 criteria (RECIST 1.1). The objective response rate (ORR) was defined as the sum of CR and PR, and the disease control rate (DCR) was defined as the sum of CR, PR and SD. Progression-free survival (PFS) was calculated from the initiation of immunotherapy to all-cause death or progression, whichever occurred first. Overall survival (OS) was calculated as the period from the first day of ICI to all-cause death or the last follow-up. The last follow-up date was March 27, 2023.
Statistical analysis
Medians and interquartile ranges (IQRs) were used to report baseline laboratory parameters. Chi-squared test or Fisher’s exact test was used for categorical variables, whereas Wilcoxon rank-sum test was used for continuous variables. Kaplan-Meier curves and log-rank tests were used to visualize and assess survival differences. Univariate and multivariate analyses were used to analyze independent factors associated with survival. Statistical significance was accepted for two-sided P values <0.05. All analyses were performed in SPSS 22.0 (IBM Corp., Armonk, NY, USA), and results were plotted in GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA).
Results
Patient characteristics
A total of 35 patients treated with at least one dose of immunotherapy and developed ir-hepatitis were diagnosed between June 2016 and December 2022. The specific screening flow chart is showed in Figure S1. The median age was 64 (range, 37–79) years, and 9 (25.7%) patients were women. A total of 20 (57.1%) patients were smokers, 15 (42.9%) patients consumed alcohol, and 5 (14.3%) patients had a history of hepatitis viral infection. All patients had good Eastern Cooperative Oncology Group performance status (ECOG PS), with scores of 0 or 1. The most common histology types were adenocarcinoma (n=20, 57.1%), and most patients were in stage IV (n=30, 85.7%). The numbers of patients with bone, brain and liver metastases were 7 (20.0%), 6 (17.1%) and 3 (8.6%), respectively.
All patients were administered immunotherapy, including immune-monotherapy and immune combination therapy. Only 8 (22.9%) patients received monotherapy with anti-PD-(L)1 therapy. Patient characteristics are detailed in Table 1.
Table 1
| Characteristics | Values |
|---|---|
| Age (years), median [range] | 64 [37–79] |
| Sex, n (%) | |
| Male | 26 (74.3) |
| Female | 9 (25.7) |
| Smoking history, n (%) | |
| Never | 15 (42.9) |
| Ever | 20 (57.1) |
| ECOG PS, n (%) | |
| 0 | 3 (8.6) |
| 1 | 32 (91.4) |
| Histology, n (%) | |
| Adenocarcinoma | 20 (57.1) |
| Squamous carcinoma | 15 (42.9) |
| TNM staging, n (%) | |
| III | 5 (14.3) |
| IV | 30 (85.7) |
| Previous radiotherapy, n (%) | |
| Yes | 6 (17.1) |
| No | 29 (82.9) |
| Previous surgery, n (%) | |
| Yes | 10 (28.6) |
| No | 25 (71.4) |
| PD-L1 status, n (%) | |
| Positive | 9 (25.7) |
| Negative | 1 (2.9) |
| Unknown | 25 (71.4) |
| Metastasis, n (%) | |
| Liver | 3 (8.6) |
| Bone | 7 (20.0) |
| Brain | 6 (17.1) |
| Line of ICI treatment, n (%) | |
| 1 | 19 (54.3) |
| ≥2 | 16 (45.7) |
| ICI regimen, n (%) | |
| Monotherapy | 8 (22.9) |
| Combination treatment | 27 (77.1) |
ECOG PS, Eastern Cooperative Oncology Group performance status; TNM, tumor-node-metastasis; PD-L1, programmed cell death protein ligand 1; ICI, immune checkpoint inhibitor.
Incidence of ir-hepatitis
The patients’ ir-hepatitis was graded according to CTCAE 4.03. No patients with grade 5 ir-hepatitis were enrolled in this study. The incidence of mild ir-hepatitis (below grade 3) was 37.1%; 3 (8.6%) patients with grade 1, and 10 (28.6%) patients with grade 2. Seventeen (48.6%) patients had grade 3, and 5 (14.3%) patients had grade 4 ir-hepatitis.
The median number of treatment cycles from the start of immunotherapy to the diagnosis of ir-hepatitis was 2 (range, 1–31), and the median time from the initiation of immunotherapy to the onset of ir-hepatitis was 1.6 months (Figure S2A). The median time from initiation of ICI to the occurrence of mild and severe ir-hepatitis was 2.4 [95% confidence interval (CI): 0.5–4.2] and 1.4 (95% CI: 0.7–2.1) months, respectively. The courses of immunotherapy and the occurrences of ir-hepatitis are presented in a swimming plot (Figure 1).
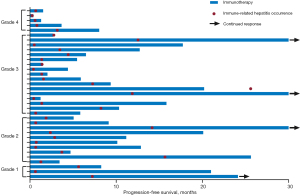
No statistical differences in the timing of ir-hepatitis development were found according to the line of treatment (first-line vs. further-line treatment: 1.4 vs. 1.6 months, P=0.155), immunotherapy treatment regimen (monotherapy vs. combination: 1.4 vs. 1.9 months, P=0.533) and severity of hepatitis AEs (mild vs. severe: 2.4 vs. 1.4 months, P=0.701) (Figure S2B-S2D). Further univariable and multivariable analyses indicated that the clinical characteristics, including a history of alcohol consumption (P=0.837), hepatitis viral infection (P=0.073) or liver metastases (P=0.795), were not significantly associated with earlier occurrence of ir-hepatitis (Table 2).
Table 2
| Characteristics | Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Sex (male vs. female) | 0.587 (0.250–1.375) | 0.220 | 0.591 (0.155–2.254) | 0.441 | |
| Age (>65 vs. ≤65 years) | 0.986 (0.499–1.947) | 0.968 | 1.631 (0.655–4.061) | 0.294 | |
| Smoking history (ever vs. never) | 1.306 (0.647–2.634) | 0.456 | 0.710 (0.201–2.512) | 0.595 | |
| Drinking history (ever vs. never) | 1.291 (0.639–2.609) | 0.477 | 0.901 (0.332–2.442) | 0.837 | |
| Histology (adenocarcinoma vs. squamous carcinoma) | 1.295 (0.656–2.555) | 0.457 | 2.218 (0.779–6.314) | 0.136 | |
| PS (0 vs. 1) | 0.612 (0.181–2.065) | 0.429 | 0.503 (0.114–2.211) | 0.363 | |
| Hepatitis virus infection (yes vs. no) | 0.705 (0.265–1.876) | 0.483 | 0.265 (0.062–1.129) | 0.073 | |
| Liver metastases (yes vs. no) | 1.078 (0.325–3.571) | 0.903 | 1.222 (0.270–5.537) | 0.795 | |
| Line of immunotherapy (1 vs. ≥2) | 0.598 (0.290–1.234) | 0.164 | 0.419 (0.140–1.255) | 0.120 | |
| Treatment pattern (monotherapy vs. combination therapy) | 0.777 (0.348–1.737) | 0.539 | 1.211 (0.337–4.349) | 0.769 | |
Ir-hepatitis, immune-related hepatitis; HR, hazard ratio; CI, confidence interval; PS, performance status.
Of the 35 total patients, 14 (14/35, 40.0%) developed at least one other type of irAE, (range: one to three irAEs). The most common concurrent AEs were pneumonitis (n=5), dermatological reactions (n=4) and nephritis (n=3).
Clinical parameters of ir-hepatitis
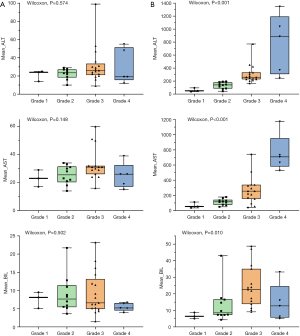
The mean values of ALT, AST and BIL at baseline for the entire cohort were 25.0 (IQR: 8.0) U/L, 29.0 (IQR: 9.0) U/L and 6.6 (IQR: 6.4) µmol/L, respectively. No statistical differences were observed in the mean values of inflammatory and liver function parameters, such as ALT (P=0.574), AST (P=0.148) and BIL (P=0.502), in any severity grade before immunotherapy. The values of AST and ALT increased with increasing grade according to the diagnostic criteria. However, the mean value of BIL was higher in grade 3 (22.6 µmol/L) than that of grade 4 (12.7 µmol/L) ir-hepatitis in the study. Baseline and peak parameters for the different grades are summarized in Table S2 and depicted in Figure 2.
Efficacy of immunotherapy and survival outcomes in patients with ir-hepatitis
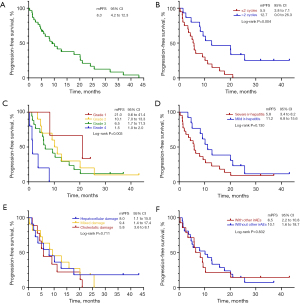
The median progression-free survival (mPFS) of all patients who developed ir-hepatitis was 8.3 (95% CI: 4.2–12.3) months, whereas the median OS was not reached for 15 patients who still benefited from subsequent treatments. No patients achieved CR, whereas 9 (25.7%) patients achieved PR, and 22 (62.9%) achieved SD. Thus, the ORR and DCR were 25.7% and 88.6%, respectively.
The PFS differed among patients who developed hepatitis within two treatment cycles rather than within more than two treatment cycles, thus indicating that early hepatitis occurrence was associated with poorer survival (5.5 vs. 12.7 months, respectively, P=0.004). Additionally, survival differences were observed among grades of ir-hepatitis (grade 1 vs. grade 2 vs. grade 3 vs. grade 4: 21.0 vs. 10.1 vs. 6.5 vs. 1.5 months, P=0.003). Although no significant difference was observed in PFS in patients with mild vs. severe ir-hepatitis, the tendency was still notable (11.2 vs. 5.8 months, P=0.130): patients with mild rather than severe irAEs were more likely to have longer PFS. However, the differences according to liver damage status were not significant for mPFS (hepatocellular vs. mixed vs. cholestatic damage: 8.0 vs. 9.4 vs. 5.8 months, P=0.711). Furthermore, no statistical difference was observed between patients with or without other concurrent irAEs (without other irAEs vs. with other irAEs: 10.1 vs. 6.5 months, P=0.832) (Figure 3).
In the univariate analysis for PFS, the occurrence time (more than two cycles vs. two or fewer cycles) (P=0.005) and severity grades (P=0.016) of ir-hepatitis were independent factors associated with survival. Multivariable analysis revealed that occurrence of ir-hepatitis after two cycles immunotherapy (P=0.002) and lower (P=0.005) severity grades were significant factors independently associated with longer PFS (Table 3).
Table 3
| Characteristics | Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Sex (male vs. female) | 0.979 (0.449–2.134) | 0.957 | – | – | |
| Age (>65 vs. ≤65 years) | 0.683 (0.334–1.393) | 0.294 | – | – | |
| Smoking history (ever vs. never) | 1.114 (0.546–2.272) | 0.767 | – | – | |
| Histology (adenocarcinoma vs. squamous carcinoma) | 1.696 (0.829–3.470) | 0.148 | – | – | |
| Hepatitis virus infection (yes vs. no) | 0.475 (0.163–1.387) | 0.173 | – | – | |
| Liver metastases (yes vs. no) | 0.509 (0.120–2.153) | 0.359 | – | – | |
| Line of immunotherapy (1 vs. ≥2) | 0.665 (0.327–1.352) | 0.260 | – | – | |
| Treatment pattern (monotherapy vs. combination therapy) | 0.829 (0.355–1.934) | 0.664 | – | – | |
| Liver damage (hepatocellular vs. mixed vs. cholestatic) | 1.190 (0.735–1.928) | 0.480 | – | – | |
| irAE occurrence time (>2 vs. ≤2 cycles) | 0.325 (0.147–0.717) | 0.005 | 0.263 (0.114–0.609) | 0.002 | |
| AE grade (1 vs. 2 vs. 3 vs. 4) | 1.912 (1.130–3.237) | 0.016 | 2.212 (1.272–3.848) | 0.005 | |
PFS, progression-free survival; ir-hepatitis, immune-related hepatitis; HR, hazard ratio; CI, confidence interval; ir-AE, immune-related adverse event; AE, adverse event.
Treatment outcomes of ir-hepatitis
A total of 19 (54.3%) patients were treated with steroids, and 28 (80.0%) received liver protection treatments. After exclusion of patients lost to follow-up, the laboratory parameters of 24 (68.6%) patients with complete electronic medical records were found to have returned to normal levels. The median time from the occurrence of ir-hepatitis to the return to normal status was 2.0 (95% CI: 1.5–2.6) months. A total of 14 (40.0%) patients had interrupted ICI for ir-hepatitis, and 8 (22.9%) patients for progression. Only 1 (2.9%) patient developed delayed ir-hepatitis. Moreover, 7 (20.0%) patients rechallenged the immunotherapy in the further line.
Discussion
Our study aimed at analyzing the clinical characteristics and the development of different grades of ir-hepatitis, and further evaluating the timing of ir-hepatitis occurrence and patient survival after immunotherapy. The median values of AST, ALT and BIL significantly differed among grades in the peak serum. The median occurrence time of ir-hepatitis was 1.6 months (nearly two cycles) after the start of immunotherapy. However, no clinical characteristics and treatment regimens were associated with the timing of ir-hepatitis occurrence. Interestingly, outcomes of early ir-hepatitis developing within two treatment cycles significantly differed from late ir-hepatitis (developing after more than two treatment cycles). Earlier ir-hepatitis occurrence was associated with poorer survival. Additionally, patients with mild ir-hepatitis tended to achieve longer survival benefits than those with severe ir-hepatitis. However, the type of liver damage had limited effects on survival.
With the advent of the immunotherapy era, the introduction of ICIs in past decades has demonstrated promising outcomes and durable responses, thus providing evidence supporting the standard first-line treatment regimen in guidelines for patients with advanced NSCLC without gene positivity. However, most patients show AEs. Ir-hepatitis, an immune-related hepatotoxicity, varies in incidence from 3% to 9% for anti-CTLA-4, and 0.7% to 1.8% for anti-PD-(L)1 (11). Most patients with ir-hepatitis are asymptomatic; the observed abnormalities are predominantly elevated liver enzymes, and liver injury can manifest as fatigue, fever, nausea and jaundice (12-14).
The mechanisms of ir-hepatitis remain unclear. One mechanism is the direct cytotoxicity of the administered antibodies via complement activation, which may fail to explain the targeted specificity of the liver (10,11). Additionally, Hercun et al. (15) have described the development of ir-hepatitis, including the adhesion of activated T cells to hepatic sinusoids, and apoptosis of T cells and hepatocytes. Ir-hepatitis features include pre-existing anti-nuclear antibodies and characteristic liver histology, including CD8+ cell dominance and granulomatous hepatitis (16,17). In addition, ICI-induced hepatotoxicity may derive from either a hepatocellular or a cholestatic injury pattern (4).
The incidence of ir-hepatitis has been reported to be associated with many risk factors (8): a retrospective study (18) has demonstrated that male sex is an independent risk factor for ir-hepatitis incidence. However, another retrospective study (19) with Japanese patients has indicated that women are significantly more likely than men to have higher grade ir-hepatitis, although further studies are required. A retrospective study in China (7) has found that younger age is a predictive factor for ir-hepatitis. Moreover, liver metastasis (20) and liver disease [e.g., autoimmune disorder (21) and combined alcoholic liver disease (22) are also associated with elevated risk of ir-hepatitis]. Thus, previous studies have focused primarily on independent factors associated with the incidence of hepatitis, but few studies have assessed the characteristics associated with the timing of ir-hepatitis. Cho et al. (23) have confirmed that male patients and patients younger than 65 years have an approximately 1.5-fold increased hazard of time to reach ir-hepatitis. Chen et al. (18) have also found that irAEs manifest later in men than women. However, in this study, according to the outcomes of multivariable analysis, we discovered no risk factors affecting the time of occurrence of ir-hepatitis. More larger scale prospective studies are needed to identify appropriate biomarkers.
In terms of the survival, prior studies by Daniello et al. (5) and Hata et al. (6) have reported that patients with AEs have longer survival times than those without AEs. Moreover, previous studies have demonstrated poorer survival in patients with severe than mild AEs (13,23,24). Meta-analyses conducted by Miah et al. (8) have indicated that grade 3 or higher irAEs tend to be associated with shorter survival. Similarly, the outcomes in this study indicated that patients with higher grade ir-hepatitis severity had poorer survival, and those with earlier ir-hepatitis, developing within two treatment cycles, had poorer survival. The possible reasons may be that (I) patients with severe AEs often require discontinuation of immunotherapy; (II) patients with serious ir-hepatitis are recommended to undergo treatments with high-dose corticosteroid shock and/or immunosuppression (24-27), thus inevitably compromising their immune systems and resulting in serious complications (e.g., opportunistic infection of the lungs, thrombi and hypokalemia); and (III) severe ir-hepatitis may lead to liver function impairment or even liver failure, thereby affecting performance status and ultimately the efficacy of subsequent treatments.
Furthermore, this study considered liver injury types to explore the characteristics associated with survival. Although no difference in survival outcomes was observed among damage types, further studies are needed.
This study has several limitations. First, the small sample, single-center, retrospective study design, inevitably introduced potential bias and confounding risk factors. Second, the lack of complete information on the treatments and overturn of ir-hepatitis for partial patients prevented further analysis of the features associated with the time to remission. Third, the diagnosis of ir-hepatitis is often performed through liver biopsy. Despite the diagnosis through multidisciplinary discussions, some errors would have been unavoidable.
Conclusions
The median values of AST and ALT were the basis for grading of ir-hepatitis. No independent risk factors were associated with the timing of ir-hepatitis development. However, poorer PFS was observed in patients who developed early ir-hepatitis within two treatment cycles. Moreover, mild hepatitis was associated with longer survival. Hepatitis timing (development within two treatment cycles) and higher severity grades were risk factors associated with PFS.
Acknowledgments
The authors would like to thank all patients for their cooperation and participation. In addition, we are thankful to all research staffs and co-investigators involved in this study.
Funding: The study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1684/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1684/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1684/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1684/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Approval of the study protocol was obtained from Zhejiang Cancer Hospital Institutional Review Board Committee (approval number: IRB-2023-377). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fujiwara Y, Horita N, Harrington M, et al. Incidence of hepatotoxicity associated with addition of immune checkpoint blockade to systemic solid tumor therapy: a meta-analysis of phase 3 randomized controlled trials. Cancer Immunol Immunother 2022;71:2837-48. [Crossref] [PubMed]
- Kotha S, Zen Y, Berry P. Diagnostic, therapeutic and prognostic challenges in a jaundiced patient treated with a checkpoint inhibitor. Clin J Gastroenterol 2022;15:446-50. [Crossref] [PubMed]
- Schilling HL, Hutchinson JA, Haferkamp S. Prediction of immune checkpoint blockade-related hepatitis in metastatic melanoma patients. J Dtsch Dermatol Ges 2022;20:773-5. [Crossref] [PubMed]
- Fu J, Li WZ, McGrath NA, et al. Immune Checkpoint Inhibitor Associated Hepatotoxicity in Primary Liver Cancer Versus Other Cancers: A Systematic Review and Meta-Analysis. Front Oncol 2021;11:650292. [Crossref] [PubMed]
- Daniello L, Elshiaty M, Bozorgmehr F, et al. Therapeutic and Prognostic Implications of Immune-Related Adverse Events in Advanced Non-Small-Cell Lung Cancer. Front Oncol 2021;11:703893. [Crossref] [PubMed]
- Hata H, Matsumura C, Chisaki Y, et al. A Retrospective Cohort Study of Multiple Immune-Related Adverse Events and Clinical Outcomes Among Patients With Cancer Receiving Immune Checkpoint Inhibitors. Cancer Control 2022;29:10732748221130576. [Crossref] [PubMed]
- Zhang Z, Rafei-Shamsabadi D, Lehr S, et al. Incidence and severity of immune-related hepatitis after dual checkpoint therapy is linked to younger age independent of herpes virus immunity. J Transl Med 2022;20:582. [Crossref] [PubMed]
- Miah A, Tinoco G, Zhao S, et al. Immune checkpoint inhibitor-induced hepatitis injury: risk factors, outcomes, and impact on survival. J Cancer Res Clin Oncol 2023;149:2235-42. [Crossref] [PubMed]
- Liu Z, Zhu Y, Xie H, et al. Immune-mediated hepatitis induced by immune checkpoint inhibitors: Current updates and future perspectives. Front Pharmacol 2022;13:1077468. [Crossref] [PubMed]
- Personeni N, Pressiani T, D’Alessio A, et al. Hepatotoxicity in Patients with Hepatocellular Carcinoma on Treatment with Immune Checkpoint Inhibitors. Cancers (Basel) 2021;13:5665. [Crossref] [PubMed]
- Poto R, Troiani T, Criscuolo G, et al. Holistic Approach to Immune Checkpoint Inhibitor-Related Adverse Events. Front Immunol 2022;13:804597. [Crossref] [PubMed]
- Seguí E, Zamora-Martínez C, Barreto TD, et al. Severe Immune-Related Adverse Events: A Case Series of Patients Needing Hospital Admission in a Spanish Oncology Referral Center and Review of the Literature. Diagnostics (Basel) 2022;12:2116. [Crossref] [PubMed]
- Vaddepally R, Doddamani R, Sodavarapu S, et al. Review of Immune-Related Adverse Events (irAEs) in Non-Small-Cell Lung Cancer (NSCLC)-Their Incidence, Management, Multiorgan irAEs, and Rechallenge. Biomedicines 2022;10:790. [Crossref] [PubMed]
- Yang H, Zhou C, Yuan F, et al. Case Report: Severe Immune-Related Cholestatic Hepatitis and Subsequent Pneumonia After Pembrolizumab Therapy in a Geriatic Patient With Metastic Gastric Cancer. Front Med (Lausanne) 2021;8:719236. [Crossref] [PubMed]
- Hercun J, Vincent C, Bilodeau M, et al. Immune-Mediated Hepatitis During Immune Checkpoint Inhibitor cancer Immunotherapy: Lessons From Autoimmune Hepatitis and Liver Immunology. Front Immunol 2022;13:907591. [Crossref] [PubMed]
- Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers 2020;6:38. [Crossref] [PubMed]
- Kondo Y, Akahira J, Morosawa T, et al. Anti-nuclear antibody and a granuloma could be biomarkers for iCIs-related hepatitis by anti-PD-1 treatment. Sci Rep 2022;12:3669. [Crossref] [PubMed]
- Chen C, Zhang C, Jin Z, et al. Sex differences in immune-related adverse events with immune checkpoint inhibitors: data mining of the FDA adverse event reporting system. Int J Clin Pharm 2022;44:689-97. [Crossref] [PubMed]
- Kitagataya T, Suda G, Nagashima K, et al. Prevalence, clinical course, and predictive factors of immune checkpoint inhibitor monotherapy-associated hepatitis in Japan. J Gastroenterol Hepatol 2020;35:1782-8. [Crossref] [PubMed]
- Suzman DL, Pelosof L, Rosenberg A, et al. Hepatotoxicity of immune checkpoint inhibitors: An evolving picture of risk associated with a vital class of immunotherapy agents. Liver Int 2018;38:976-87. [Crossref] [PubMed]
- Abdel-Wahab N, Shah M, Lopez-Olivo MA, et al. Use of Immune Checkpoint Inhibitors in the Treatment of Patients With Cancer and Preexisting Autoimmune Disease: A Systematic Review. Ann Intern Med 2018;168:121-30. [Crossref] [PubMed]
- Sawada K, Hayashi H, Nakajima S, et al. Non-alcoholic fatty liver disease is a potential risk factor for liver injury caused by immune checkpoint inhibitor. J Gastroenterol Hepatol 2020;35:1042-8. [Crossref] [PubMed]
- Cho YA, Han JM, Kang SY, et al. Analysis of Risk Factors for Hepatotoxicity Induced by Immune Checkpoint Inhibitors. J Immunother 2021;44:16-21. [Crossref] [PubMed]
- Wang W, Gu X, Wang L, et al. The prognostic impact of mild and severe immune-related adverse events in non-small cell lung cancer treated with immune checkpoint inhibitors: a multicenter retrospective study. Cancer Immunol Immunother 2022;71:1693-703. [Crossref] [PubMed]
- Xu L, Xu M, Sun W, et al. Clinical characteristics and prognostic impact of immune checkpoint inhibitor-associated myocarditis in advanced non-small cell lung cancer. Invest New Drugs 2023;41:816-24. [Crossref] [PubMed]
- Kadokawa Y, Inoue S, Tatsumi A, et al. Efficacy and safety of mycophenolate mofetil in treating immune-related hepatitis induced by immune checkpoint inhibitor use: A retrospective study. JGH Open 2023;7:87-97. [Crossref] [PubMed]
- Macía-Rodríguez C, Santomé Couto L, Fernández Villaverde A, et al. Mycophenolate mofetil as a treatment of severe steroid-resistant immune-related hepatitis. Gastroenterol Hepatol 2022;45:32-6. [Crossref] [PubMed]


