Treatment decision based on the biological behavior of pulmonary benign metastasizing leiomyoma
Introduction
Martin classified leiomyomatous lung lesions into three categories: (I) benign metastasizing leiomyoma (BML) in women; (II) metastatic leiomyoma in men and children; and (III) multiple pulmonary fibroleiomyomatous hamartoma (1).
BML is a rare disease in women occurring several years after an initial gynecological procedure (hysterectomy, or myomectomy), involving distant locations of metastases, such as lung, retroperitoneum, lymph nodes, bones, muscular tissue, skin, scars or central nervous system. The lung is the most common site of metastases. Sekine et al. report that leiomyomas represent 0.085% of pulmonary benign tumors (2).
Metastases can appear as solitary or multiple lesions. Features of nodules include a specific smooth muscle phenotype, low proliferation, and slow growth (3). Lesions are also positive for estrogen receptors (ER) and progesterone receptors (PR), revealing the origin of the disease (4). The treatment of BML is, in most cases, quite controversial. Non-surgical treatment offers various options. Hormonal therapy (gonadotropin-releasing hormone analogues, selective ER modulators, or progesterone and aromatase inhibitors) with or without oophorectomy has been suggested in non-resectable cases (5). Nevertheless, non-surgical treatments still lack significant results. Primary treatment of BML, with the highest success rate, is surgery.
Case presentation
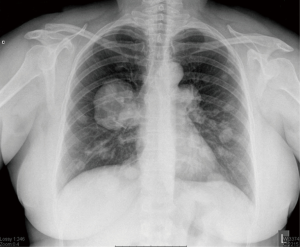
The patient was a 36-year-old asymptomatic, non-smoking woman who had a hysterectomy for myoma of the uterus seven years earlier. Routine chest radiography and CT revealed 73 nodules on both sides of the lungs (Figure 1).
Core biopsy with hematoxylin-eosin and immunohistochemical staining revealed a mesenchymal tumor with smooth muscle cell differentiation of tumor cells. The sample was strongly positive for smooth muscle actin (SMA), which is indicative of BML.
From the diagnosis of BML until the last metastasectomy, continuous oncological treatment (VIP protocol: etoposide, ifosfamide, cisplatin) was administered. Since this was unsuccessful, the same oncology team decided to proceed with metastasectomies. Although preoperative chest CT revealed lesions in both lungs, the procedures were carried out through mini-thoracotomy (MT), instead of sternotomy.
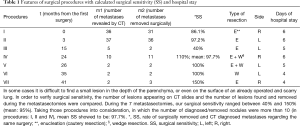
Surgical procedures are detailed in Table 1. During the metastasectomies, lesions were removed by parenchyma-sparing cautery resection and wedge resection (procedures IV, V and VI). One chest drain was inserted after the metastasectomies. Throughout the seven hospitalizations, every postoperative phase was uneventful, and hospital stay ranged between four and six days (mean, 5.14 days). During the first operation (I), we removed 31 lesions from the right lung, and three months later, 36 lesions from the left lung (II) (see Figure 2). Despite oncological treatment, bilateral multiple recurrences were revealed on CT scan, and repeat metastasectomies were performed to remove most of these lesions. During each procedure, we measured surgical sensitivity (SS), and later calculated mean surgical sensitivity (mSS). SS was defined as the rate of surgically removed and CT-diagnosed metastases in each individual surgery, and mSS was the mean of the SS results of the seven procedures.
Full table

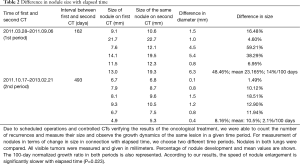
We could count the number of recurrences of lesions, measure their size, and observe their growth dynamics in a given time period owing to scheduled operations and controlled CTs verifying the results of oncological treatment. To measure the growth of the nodules, in relation to elapsed time, we chose two different time periods. Nodules in both lungs were compared. All visible tumors were measured in millimeters (mm). The percentages of nodule size development and mean values are shown in Table 2, and the 100-day normalized growth ratio is also represented.
Full table
We used two time phases (162 and 493 elapsed days from first appearance of lung nodules) and measured the size of the same nodules on chest CT in each phase (Table 2).
Seven months after the last procedure, spirometry results of the patient were as follows: FVC 77%; FEV1 64%; FEV1/FVC 0.83. Mean hospital stay was 5.14 days (range, 4–6 days).
Histological examination confirmed all nodules were benign, with smooth muscle characteristics, originating from the uterine leiomyoma. Fluorescent in situ hybridization confirmed the presence of a 19q 22q terminal deletion, which is pathognomonic for BML.
Chest CT performed 1.5 years after the last procedure showed an unverified 5-mm solitary nodule in the right lower lobe.
Results
The mSS during the seven procedures was 95% (40–150%). During procedures in which over ten nodules were present on chest CT or removed surgically (procedures I, II and IV, see Table 1), mSS was 97.7%.
During the first period (elapsed days: 162), the mean change in nodule size was 23.165%, whereas during the second period (elapsed days: 493) the mean value decreased to 10.5%. The 100-day normalized growth ratio in the two periods was 14% versus 2.1%. According to our results, the speed of nodule enlargement was significantly slower with elapsed time (P=0.023).
Statistical analysis
For comparison, a t-test and one-way analysis of variance were used. Categorical data were analyzed by using Fisher’s exact test. SPSS version 15.0 (© 2007 SPSS Inc.) was used for statistical analysis.
Discussion
The pathogenesis of BML is not yet clear. Theories include the following: (I) hormone-sensitive in situ proliferation of smooth muscle bundles; (II) benign smooth muscle cells transported from a uterine leiomyoma and colonized in the lung or metastasis of a low-grade uterine leiomyosarcoma to the lung; and (III) surgically-induced mechanical displacement from a preexisting uterine tumor.
In our unusual case, 87 nodules were removed either by cautery resection (n=83; 95%) or wedge resection (n=4; 5%), in seven procedures. After these surgeries, the patient remained asymptomatic, continued with her job, and had a near-normal FEV1 (64%). Her physical status and the excellent postoperative results were achieved only with parenchyma-sparing metastasectomies.
A challenge of a repeat metastasectomy is finding smaller lesions in the lung parenchyma. SS results show that repeat metastasectomy is a feasible and effective procedure in cases of BML. Regarding the growth dynamics of recurrent lesions, we found that tumors grew faster initially, and the number of recurrent lesions decreased with elapsed time (P=0.023).
Effectiveness of oncological treatment was assessed based on whether necrosis occurred in the tumor after chemotherapy. In our case, pathological examination of the removed nodules showed no signs of necrosis, thrombosis, or fibrosis. Thus, it can be stated that in our case, chemotherapy did not have a significant influence on the course of the disease. This unsuccessful oncological treatment resulted in the decision to surgically remove as many lesions as possible, even with repeated metastasectomies.
Based on the reduced number of recurrent lesions with elapsed time and their decreasing enlargement tendency, our results support the theory that metastases in BML are surgically induced mechanical displacements of a preexisting uterine tumor and not newly formed lesions. Based on our results, it can be concluded that surgery is still the most effective choice of treatment of BML, and repeated parenchyma-sparing cautery resection is a safe and effective method with excellent patient tolerance—even in cases with an extreme number of BML nodules (n=87).
Acknowledgements
For payment of APC, I received support from the University of Szeged.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Martin E. Leiomyomatous lung lesions: a proposed classification. AJR Am J Roentgenol 1983;141:269-72. [Crossref] [PubMed]
- Sekine I, Kodama T, Yokose T, et al. Rare pulmonary tumors - a review of 32 cases. Oncology 1998;55:431-4. [Crossref] [PubMed]
- Chen S, Liu RM, Li T. Pulmonary benign metastasizing leiomyoma: a case report and literature review. J Thorac Dis 2014;6:E92-8. [PubMed]
- Nuovo GJ, Schmittgen TD. Benign metastasizing leiomyoma of the lung: clinicopathologic, immunohistochemical, and micro-RNA analyses. Diagn Mol Pathol 2008;17:145-50. [Crossref] [PubMed]
- Taftaf R, Starnes S, Wang J, et al. Benign metastasizing leiomyoma: a rare type of lung metastases-two case reports and review of the literature. Case Rep Oncol Med 2014;2014:842801.