Lung ossification: an orphan disease
Introduction
Diffuse pulmonary ossification (DPO), is a rare usually asymptomatic disease, which most of the time is diagnosed when sectioning the lungs at autopsy (1). Pulmonary ossification occurs with a number of systemic and pulmonary conditions. Idiopathic diffuse pulmonary ossification is even more rare entity, usually occurs in men aged 40-60 years (2). Specific symptoms are often lacking, nevertheless mild symptoms such as fatigue, restrictive pulmonary physiology and impaired transfer factor have been described. It is apparent radiographically only when it is extensive. Chest radiograph and CT findings may be underappreciated, for even experienced radiologists (3). High resolution CT (HRCT) scanning is preferable to use for the decisive diagnosis. There is recent renewed interest in diagnosing and potential treatment of this rare disease (4).
Case report
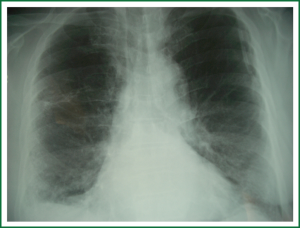
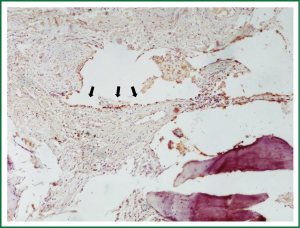
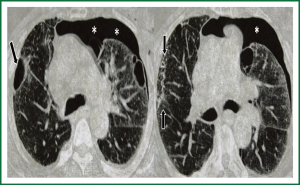
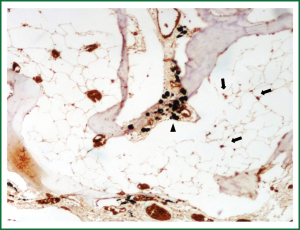
A 68 years old Caucasian patient presented at our hospital due to sudden onset of breathlessness. He was ex-smoker with personal medical history of arterial hypertension without treatment and chronic bronchitis. His blood pressure was 120/70 mmHg with a pulse of 125 beats/min and his saturation was 87% at pulse oximeter. Breath sounds was not heard on auscultation on the left hemithorax while cardiovascular examination was normal except for tachycardia. On the initial chest radiograph total left pneumothorax was revealed, signs of lung emphysema and reticular opacities bilateraly mainly in the lower lobes (Figures 1,2). The pneumothorax necessitated closed - tube thoracostomy and a 28 French sized chest tube with an underwater seal drain were inserted into the chest wall. The left lung was not totally re-expanded by the tube drainage. There was a remaining less than 20% pneumothorax on the upper left side. The patient received oxygen inhalation (3 L/min) and was stable. Seven days later the new X-ray showed persistent pneumothorax even though he had the tube drainage. The patient remained asymptomatic with normal saturation of oxygen on room air. A high resolution computer tomography (HRCT) was revealed to investigate the cause of the persistent pneumothorax and the reticular opacities that have been seen. The findings were paraseptal emphysema bilaterally, persistent pneumothorax on the left and subpleural micronodular lesions mainly in the lower lobes. Routine laboratory examination and bronchoscopy failed to give a definite diagnosis, therefore surgical lung biopsy was performed (VATS). The histological findings were compatible with DPO (Figures 3,4,5,6,7). There was presence of bone tissue in a branching pattern randomly located within the alveolar spaces. Bone marrow was identified within some fragments. On higher magnification images, haemopoietic elements including neutrophils and megakaryocytes were identified. There was no iron deposition and no evidence of granulomas or malignancy. The patient was extubated on the 10th day and the oxygen saturation was persistently greater than SpO2: 96% on room air. He remained asymptomatic, recovered completely and discharged from the hospital.
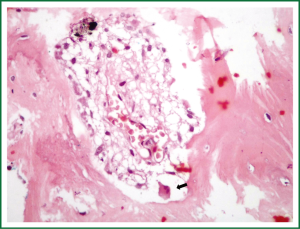
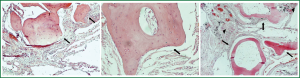
Discussion
DPO is an uncommon and rarely diagnosed disease during life. Usually it is discovered at autopsy in the setting of other pulmonary injury. There are no significant symptoms and can be easily misdiagnosed as other interstitial lung diseases, due to diffuse pulmonary lesions. It is characterized by diffuse small bone fragments in the lung tissue. Sometimes restrictive pulmonary physiology has been described (2). Two types of diffuse or disseminated ossification are described: “dendriform”, with its characteristic branching along terminal airways and occasional islands of marrow; and “nodular”, which tends to be more circumscribed and situated in alveolar spaces. Each is composed of mature lamellar bone with little osteoblastic or osteoclastic activity and no separate ossification. They appear mostly in the lower lobes of patients who are male, in their fifth to sixth decade, and have a history of repeated pulmonary insults such as recurrent bronchopneumonia, anthracosis, interstitial fibrosis, and pulmonary edema, particularly with mitral stenosis.
It can be identified using high resolution computer tomography and thoracoscopic biopsy. Sometimes bone scintigraphy can also be used in addition to definite a diagnosis. Linear and round shadows are often revealed on chest radiographs. Dendriform type shows branching shadows of calcific density extending along the bronchovascular distribution commonly interpreted as scarring fibrosis or bronchiectasis. In many cases there is another type, the nodular, where multiple subpleural calcified nodules less than 1cm can be found that may be confused with healed infectious disease (3).
Pulmonary ossification can be idiopathic or can result from a variety of pulmonary, cardiac, or extracardiopulmonary disorders. Pathophysiologic states predisposing to pulmonary calcification and ossification include hypercalcemia, a local alkaline environment and previous lung injury (5). Scar tissue injury is the responsible factor that induces an alkaline environment, initiates precipitation of calcium salts, enables alkaline phosphatase activity and activates profibrogenic cytokines. Moreover, alveolar bleeding is responsible for interstitial metallic deposition that attracts calcium salts and multinucleated giant cells (4).
Other factors that may also contribute to the pathogenesis of the disease are enhanced alkaline phosphatase activity, active angiogenesis and mitogenic effects of growth factors (5). Transforming growth factor-β(TGF-β) elaborated by inflammatory cells plays a role in embryonal organogenesis, tissue regeneration, fibrosis, and the formation of extracellular matrix TMTGF-β strongly stimulates the biosynthesis of type 1 collagen (6), fibronectin, proteoglycans, and protease inhibitors while inhibiting the expression of proteases (7). The gene for TGF-β shares sequence homology with that for bone morphogenic protein (BMP) (8). TGF43 and BMP genes expressed at a site of inflammation would promote fibrosis and metaplastic bone formation, instead of healing.
To determine the incidence of diffuse pulmonary ossification (DPO) and its subtypes at autopsy and correlation with clinical and histological features was previously performed in an Australian institution. Over a 64‐month period these reports were reviewed for findings of DPO. The pattern of ossification, demographics and clinical data were obtained from both paraffin sections and final autopsy reports. The results were that seventeen histologically confirmed cases of DPO were found in 10 out of 426 autopsy cases, representing an incidence of 1.63 cases/1,000 autopsies. There was a correlation with pulmonary underlying disease and male sex. In contrast to previously published studies, dendriform DPO was more common than the nodular type in our patient (1).
Conclusions
DPO is so rare disease that may be confused with other entities. It is rarely diagnosed during life but can be identified often during autopsy. It is often without significant symptoms despite radiologically diffuse pulmonary lesions. A timely diagnosis will enable a better understanding of pathogenesis, natural course of the disease and finding new more effective treatments. Reports on the efficacy of biophosphonates and warfarin in the management of heterotopic ossification encourage further investigation (4).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Tseung J, Duflou J. Diffuse pulmonary ossification: an uncommon incidental autopsy finding. Pathology 2006;38:45-8. [PubMed]
- Ryan CF, Flint JD, Müller NL. Idiopathic diffuse pulmonary ossification. Thorax 2004;59:1004. [PubMed]
- Fraser RG. Diagnosis of diseases of the chest. 3rd ed. Philadelphia: Saunders; 1988.
- Peros-Golubicić T, Tekavec-Trkanjec J. Diffuse pulmonary ossification: an unusual interstitial lung disease. Curr Opin Pulm Med 2008;14:488-92. [PubMed]
- Chan ED, Morales DV, Welsh CH, et al. Calcium deposition with or without bone formation in the lung. Am J Respir Crit Care Med 2002;165:1654-69. [PubMed]
- McDonald JA. Idiopathic pulmonary fibrosis. A paradigm for lung injury and repair. Chest 1991;99:87S-93S. [PubMed]
- Raghow R. Role of transforming growth factor-beta in repair and fibrosis. Chest 1991;99:61S-65S. [PubMed]
- Roberts AB, Flanders KC, Kondaiah P, et al. Transforming growth factor beta: biochemistry and roles in embryogenesis, tissue repair and remodeling, and carcinogenesis. Recent Prog Horm Res 1988;44:157-97. [PubMed]