
Individualized treatment of type A intramural hematoma—upfront surgery is not always necessary
Highlight box
Key findings
• Survival of watchful waiting in patients with uncomplicated type A intramural hematoma (IMH) was 98.2% at 30 days, 96.3% at 1 year; 95.2% at 5 years; eventual intervention rate was 45.6%.
• Watchful waiting is safe and feasible in selected patients with uncomplicated type A IMH.
What is known and what is new?
• Conventionally, type A IMH is managed with upfront aortic surgery as survival outcomes are superior to medical treatment. Favorable outcomes with initial medical therapy for type A IMH have been reported in Asian studies. The discrepancy in survival outcomes between the West and the East have suggested that watchful waiting may be considered for type A IMH.
• This retrospective analysis has demonstrated that watchful waiting was safe and feasible in selected patients with type A IMH.
What is the implication, and what should change now?
• Watchful waiting with stringent surveillance was justified in selected patients with type A IMH with favorable clinical outcomes.
• There is room for individualized management of type A IMH. Upfront surgery is not always necessary.
Introduction
The conventional management of type A aortic dissection (TAAD) is emergency surgery. Medical therapy alone was associated with poor prognosis (1-3). Type A intramural hematoma (IMH) represents around 5–23% of acute aortic syndrome (4). Compared to classical aortic dissection, the understanding about the pathogenesis of IMH is less certain and studies have suggested a likelihood of a less aggressive clinical course for patients with type A IMH (5,6). IMH differs from classical dissection in that the false lumen (FL) is thrombosed, with no discernible contrast flow observed on computed tomography (CT) scans. Krukenberg first described IMH in 1920 as “dissection without a tear” and it was postulated that IMH originated from ruptured vasa vasorum or ulcerative atherosclerotic plaques rather than intimal tears (7). Indeed, the natural history of IMH was reported to be different from classical aortic dissections in that IMH was associated with fewer occurrences of malperfuson syndrome or acute aortic regurgitation (8). There is considerable discrepancy between approaches in management of type A IMH between the West and the East. Findings in the Asian population have underlined the feasibility of a watchful waiting approach with close medical surveillance and blood pressure control at the initial stage of disease in patients with type A IMH (9-11). Reports from the West however suggested a similar approach of managing IMH as classical TAAD with urgent surgery, as type A IMH was found to be associated with higher risks of cardiac tamponade and rupture (12-14). In our center, we have been adopting a selective watchful waiting approach for patients with type A IMH since 2013. In this single center retrospective study, we aimed to evaluate the short and mid-term survival of all patients with type A IMH. Through this analysis, we aimed to demonstrate the feasibility of watchful waiting for selected type A IMH patients and assess subsequent clinical course of type A IMH patients managed conservatively. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1837/rc).
Methods
Study design
We retrospectively reviewed 95 patients who were admitted to the Prince of Wales Hospital, Hong Kong, from December 2012 to February 2023, for TAAD with IMH or thrombosed FL in the ascending aorta. This made up around 20% of all admissions for acute aortic syndrome in our unit, two patients were excluded from analysis as they were considered prohibitive risk for aortic surgery. Inpatient and outpatient records were retrospectively reviewed, and data were collected from procedural records and images.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the clinical research ethics committee of Prince of Wales Hospital (16 August 2023, CRE 2023.335) and individual consent for this retrospective analysis was waived. Patient anonymity was preserved in this study and all data had been kept secured and confidential.
Definitions
All diagnoses were confirmed by contrast enhanced CT scans. Type A IMH was defined as presence of high attenuation crescent sign on non-contrast CT in the ascending aorta proximal to the brachiocephalic trunk, with lack of contrast enhancement in the FL during arterial or delayed phase. Patients with retrograde type A IMH, with contrast enhancement in the FL of the descending aortic and thrombosed FL in the ascending were also characterized as type A IMH. Penetrating aortic ulcers (PAU) or ulcer like projections (ULP) were defined as areas of blood-filled ulceration or punched out intimal lesions extending into the IMH. The maximal aortic diameter (MAD) of the ascending aorta was measured from external wall to external wall and thickness of IMH was defined as the longest transverse diameter of IMH in the mid ascending aorta (Figure 1).

Groups
Following CT diagnosis of type A IMH, the patients were assessed by the on call cardiac surgeons for decision on treatment. Treatment decisions were based on patients’ hemodynamics, presence of complications and local expertise as well as availability of resources. A complicated type A IMH was defined as IMH in the ascending aorta with presence of cardiac tamponade, malperfusion or acute aortic regurgitation. Patients decided for emergent surgery were offered surgery within 24 hours of admission. The type of surgery offered was completely dependent upon the discretion of the operating surgeon. A hemiarch replacement consists of establishment of cardiopulmonary bypass via femoral cannulation and right atrial drainage. Aortic root remodeling was performed with the Bioglue (CryoLife Inc., Kennesaw, GA, USA) (15) remodeling technique. Distal anastomoses were performed under circulatory arrest when the rectal temperature reached 25 degrees Celsius. Selective antegrade cerebral protection was routine in our center. Patients with uncomplicated type A IMH with absence of tamponade, malperfusion and hemodynamically stability were offered watchful waiting strategy after careful assessment by the local team. The watchful waiting strategy consisted of admission to the high dependency unit with intensive blood pressure control and close nursing and medical surveillance. All patients had an intra-arterial line inserted for blood pressure monitoring. Optimal blood pressure was systolic blood pressure ≤120 mmHg and optimal heart rate was 50–70 beats per minute (bpm). Intravenous antihypertensives were administered for anti-impulse therapy and all patients were kept rested in bed until the blood pressure was optimal. All patients received daily bedside V scanTM (GE Healthcare, Milwaukee, Wisconsin, USA) to document increasing or emergence of pericardial effusion and aortic regurgitation (16). The V scanTM is a pocket size portable ultrasound machine that enables point-of-care echocardiogram. Patients who remained pain free on analgesics and hemodynamically stable had a repeat CT aortogram within 2 weeks after admission depending on resource availability. Patients were offered surgery if chest pain worsened or persisted despite best medical therapy or if follow up CT scans showed indications for intervention. In terms of subgrouping, patients offered emergent surgery were classified as Group S and patients offered watchful waiting initially were classified as Group W. Patients purely treated with medical therapy throughout the follow up period were classified as Group C.
Study endpoints
The study’s primary endpoint was overall survival at 30 days, 1 year and 5 years. Secondary outcomes of interest included event free survival and rate of aortic events in the overall cohort, as well as each subgroup. Events were defined as any aortic intervention, aortic events, or death. Aortic events were defined as presence of any indications or complications necessitating aortic intervention determined by the local multidisciplinary aortic team. They included new onset or refractory chest pain despite blood pressure control; increase in size of MAD; increasing thickness of IMH, pericardial effusion; emergence of new PAU in the ascending aorta and frank rupture or fistulation.
Statistical analysis
Statistical analysis was performed with IBM SPSS version 23.0 (Chicago, IL, USA). Descriptive statistics were reported as mean with standard deviation or median with interquartile range for continuous variables, and as frequencies and percentages for categorical variables. Difference between means was compared using Student’s t-test or one-way analysis of variance (ANOVA), after verifying equality of variances with Levene’s test and normality of distribution with Shapiro-Wilk Test. If Levene’s test was violated, Welch and Games-Howell tests will be used. Categorical variables were compared using the χ2 test when the minimum number of observations in a category was greater than five; otherwise, likelihood ratios G-tests were used. The Wilcoxon rank-sum test was used for continuous variables, as appropriate. Survival function was generated using the Kaplan-Meier method. Survival models were developed based on the time to the earliest event model. A two-level multivariate Cox proportional hazards logistic regression model was constructed to calculate the association between clinical and radiological findings and event-free survival using generalized linear mixed models. Demographic covariates were first included in the first level of the multivariate model. Clinical and radiological findings were incorporated in the second layer of the regression model. A P value less than 0.05 defines statistical significance.
Results
Follow up
The median follow-up interval was 40.5 months, ranging from 0.2 to 119.8 months. Follow up rates were 100% as all records of clinic visits and imaging were recorded in an integrated computer local database across public hospitals in Hong Kong.
Baseline demographics and groupings
Among the 93 patients with type A IMH, 36 patients underwent emergency surgery within 24 hours of admission (Group S) and 57 patients were put under watchful waiting (Group W) (Figure 2). The mean age of all patients was 63.9±8.7 years, with female predominance. Majority (75.3%) of the patients had hypertension, and 40.9% had concomitant hyperlipidemia. The mean MAD was 45.7±4.9 mm and the mean IMH thickness in the ascending aorta was 11.3±5.7 mm. PAU or ULPs were found in the thoracic aorta in 36.6% of patients and 21.5% of patients had pericardial effusion, with 7.5% presenting with cardiac tamponade clinically. Comparison of baseline characteristics between Group S and Group W showed statistically significant differences in ascending MAD, thickness of ascending IMH, incidence of pericardial effusion and tamponade. Patients who were offered emergent surgery had larger MAD (47.8±5.3 vs. 44.4±4.2 mm; P=0.001); thicker ascending IMH (15.8±5.9 vs. 8.5±3.2 mm; P<0.0001); and higher incidence of pericardial effusion (38.9% vs. 10.5%; P=0.001) as well as cardiac tamponade (16.7% vs. 1.8%; P=0.008) (Table 1). The sole case of cardiac tamponade in Group W was based on echocardiogram finding of early right ventricular diastolic collapse with no hemodynamic changes compatible with clinical tamponade.
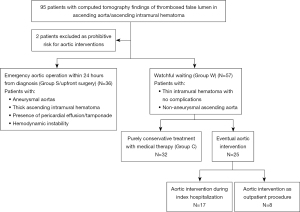
Table 1
| Variable | Overall | Group S (emergency surgery), N=36 |
Group W (watchful waiting), N=57 |
P |
|---|---|---|---|---|
| Age (years) | 63.9±8.7 | 63.6±10.0 | 64.1±7.9 | 0.80 |
| Male sex | 36 (38.7) | 13 (36.1) | 23 (40.4) | 0.68 |
| Hypertension | 70 (75.3) | 27 (75.0) | 43 (75.4) | 0.96 |
| Diabetes mellitus | 7 (7.5) | 3 (8.3) | 4 (7.0) | 0.81 |
| Dyslipidemia | 38 (40.9) | 14 (38.9) | 24 (42.1) | 0.75 |
| End-stage renal failure | 1 (1.1) | 0 | 1 (1.8) | 0.42 |
| History of myocardial infarction | 4 (4.3) | 0 | 4 (7.0) | 0.10 |
| History of cerebrovascular accident | 1 (1.1) | 0 | 1 (1.8) | 0.42 |
| Ascending aortic calibre (mm) | 45.7±4.9 | 47.8±5.3 | 44.4±4.2 | 0.001 |
| Hematoma thickness (mm) | 11.3±5.7 | 15.8±5.9 | 8.5±3.2 | <0.0001 |
| Pleural effusion | 4 (4.3) | 2 (5.6) | 2 (3.5) | 0.63 |
| Pericardial effusion | 20 (21.5) | 14 (38.9) | 6 (10.5) | 0.001 |
| Cardiac tamponade | 7 (7.5) | 6 (16.7) | 1 (1.8) | 0.008 |
| Penetrating aortic ulcer or ulcer-like projection in distal aorta | 34 (36.6) | 17 (47.2) | 17 (29.8) | 0.09 |
Data are presented as n (%) or mean ± standard deviation.
Survival
The overall mortality rate was 4.3% (4/93) in the whole cohort in a median follow up of 40.5 months. Overall 30-day survival was 98.9% at 30 days, 97.7% at 1 year and 93.7% at 5 years (Figure 3). None of the patents in Group S suffered from operative deaths and overall survival was 100% at 30 days and 1 year, and 96.2% at 5 years. One patient died from suspected graft infection 14.4 months after the initial surgery. Patients in Group W had a 30-day survival of 98.2%, 1 year survival of 96.3% and a 5-year survival of 95.2%. One patient died from acute brain hemorrhage 49 months after discharge from hospital, two patients suffered operative deaths during delayed aortic interventions after initial watchful waiting. Causes of death were intraoperative massive myocardial infarction and ruptured heart. Comparison in overall survival between patients in Group S and Group W did not show a statistically significant difference (P=0.64).
Group S
The great majority of patients in Group S underwent hemi-arch replacement (34/36; 94%) and 2 patients had emergency total arch replacement (TAR). None of the patients had endovascular treatment. Eight patients out of 36 (22.2%) had repeat interventions post operatively. The indications and nature of repeat intervention included 2 cases of zone 2 thoracic endovascular aortic repair (TEVAR) for arch PAU and distal stent induced new entry tear (SINE) post frozen elephant trunk (FET); 2 cases of redo TAR for arch aneurysm; 2 cases of open descending aortic replacement for descending aortic aneurysm and 2 cases of re-sternotomy for mediastinitis.
Group W
Among the 57 patients treated initially with watchful waiting, 25 patients (43.9%) eventually had aortic intervention. Event-free survival was 82.8% at 30 days, 73.3% at 1 year and 63.6% at 5 years (Figure 4). Twenty patients (35.1%) underwent aortic intervention within 3 months of diagnosis and 5 patients 8.8%) required aortic intervention beyond 3 months of index hospitalization. Amongst the 25 patients with eventual aortic intervention, 17 had initial open aortic surgery and 8 underwent initial endovascular intervention. In the patients with subsequent open aortic surgeries, ten had hemi- aortic arch replacements and seven had TARs. Seventeen out of 25 patients had aortic intervention during the same hospitalization (range, 3 to 35 days after admission). Thirteen patients required next-day early surgery. Reasons for early in-patient aortic intervention were CT findings of progressive IMH/conversion to classical type A dissection/increased pericardial effusion (11/17) and refractory chest pain despite good blood pressure control (2/17). Four patients had elective aortic intervention within same admission for increased prominence of arch PAU (3/4) and ascending aortic size >5 cm (1/4). None of the surgeries performed during index hospitalization were emergency surgeries for hemodynamic instability. Eight patients had aortic interventions after discharge from hospital, 7 patients had elective aortic stenting and 1 had emergency TAR done for progression to classical TAAD with hemodynamic instability. Overall operative mortality for patients with delayed aortic intervention was 8%, 2 patients died from intraoperative complications. Six out of 25 patients (24%) required repeat aortic interventions after initial aortic intervention. 5 out of the 6 cases were endovascular interventions for distal SINE post FET (2/5), arch PAU (1/5), descending and abdominal aneurysms (2/5). One patient suffered from retrograde TAAD post TEVAR which required emergency TAR (Table 2).

Table 2
| Group S (emergency surgery), N=36 | Group W (watchful waiting), N=25 | |
|---|---|---|
| Intervention | ||
| Open surgery | 36 (100.0) | 17 (68.0) |
| Hemiarch replacement | 34 (94.4) | 10 (40.0) |
| Total arch replacement +/− frozen elephant trunk | 2 (5.6) | 7 (28.0) |
| Descending aortic replacement | 0 (0) | 0 (0) |
| TEVAR | 0 (0) | 8 (32.0) |
| Presence of entry tear in theatre or pre intervention computed tomography for TEVAR | 16 (44.4) | 4 (16.0) |
| Reinterventions | 8 (22.2) | 6 (24.0) |
| Redo total arch replacement | 2 (25.0) | 1 (16.7) |
| Open descending aorta | 2 (25.0) | 0 (0) |
| Re-sternotomy for other reasons | 2 (25.0) | 0 (0) |
| TEVAR | 2 (25.0) | 5 (83.3) |
Data are presented as n (%). TEVAR, transcatheter endovascular aortic repair.
Group C
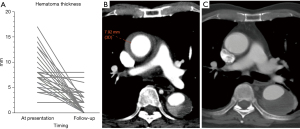
Thirty-two patients in Group W did not require any aortic intervention. This represents 34.4% of the whole IMH cohort. Event free survival amongst patients in Group C was 100% at 30 days, 100% at 1 year and 90.9% at 5 years (Figure 5). One patient died from brain hemorrhage 49 months post presentation. In purely conservatively managed patients, the mean thickness of IMH was 8.29±3.5 mm on presentation. Follow up CT scans at a median of 17.2 months (range, 4 days to 106 months) showed statistically significant reduction in IMH thickness to 1.9±2.37 mm, difference in IMH thickness =7.703 mm, P<0.001; using paired samples t-test, the difference is statistically significant [t(Jeny30) =7.703, P<0.001] with a difference of 6.39 [95% confidence interval (CI): 4.69 to 8.08 mm] (Figure 6A-6C). There was no statistically significant change in MAD on follow up scans. In all patients initially offered watchful waiting, a greater ascending aortic diameter [hazard ratio (HR) 1.262 (1.123–1.418), P<0.0001] and the presence of PAU or ULP in the arch or descending aorta [HR 3.445 (1.473–8.058), P=0.004] were independent predictors of aortic events (Table S1).

Discussion
Type A IMH constitutes about 20–25% of admissions for acute aortic syndrome in our center. While there has been little variation in the diagnosis and emergent management of classical TAAD with contrast enhancement in the FL, we have found, through our own cohort, that TAADs with thrombosed FL or IMH may run a different clinical course, allowing room for a more nuanced approach in management during the initial phase of the disease. These findings are consistent with other Asian studies on type A IMH (17-20). We have adopted a selective watchful waiting approach for patients with uncomplicated type A IMH since 2013 with comparable short and mid-term survival as upfront surgery. The original intent for close medical monitoring was to avoid unnecessary emergency surgery, and to allow time for better planning and introduction of varied and definitive aortic interventions at a delayed date. Our institution is a high-volume center in aortic surgery with more than 100 cases per year and we have a quality aortic program as evidenced by our excellent survival post emergency aortic repair. Nonetheless avoidance of unnecessary open aortic surgery carries equal importance, if not more, than good surgical outcomes, especially from a patient’s perspective. Indeed, out of the 93 patients in our cohort, 32 patients did not require any aortic intervention with reduction in ascending IMH. The significance of this finding suggests that 34.4% of type A IMH patients would have had unnecessary emergent open aortic surgery if we had a blanket policy of emergency aortic surgery for all type A IMH patients. These patients had thinner IMH of around 8 mm and smaller aortic sizes with an average MAD of 42 mm. One-year overall survival was 100% and 1 patient died from non-aortic cause 49 months post admission. Upfront surgery was not necessary for this group of patients.
Another advantage of watchful waiting is the potential to offer a more definitive or less invasive intervention at an elective setting. Hemiarch replacement is the most performed emergency surgery for type A pathologies. Emergency TAR is associated with high risk of mortality and morbidity (21). TEVAR in acute TAAD is experimental (22). In Group S, 94% of the emergent procedures were hemiarch replacements. Among the 57 patients in the watchful waiting group, 45.6% eventually had aortic intervention. In this group, 40% of the patients had hemiarch replacement, 28% had TAR and 32% had endovascular stenting. This demonstrates the variability of treatment options available to patients after initial watchful waiting and stabilization. Reintervention rates were comparable between Group S and Group W (22% vs. 24%), but the types of aortic reinterventions for Group W were predominantly endovascular procedures, while 50% of reinterventions in Group S included redo arch surgeries and open descending procedures, which are complex procedures with higher risks. Unlike classical aortic dissections where entry tears are usually evident on CT scans, the radiological appearances of IMH may not accurately reflect the underlying pathology. Appearances of thrombosed FL in the ascending can be a result of the following: (I) a true classical dissection with a small intimal tear and rapid thrombosis of the FL; (II) a retrograde dissection with entry tear distally and thrombosis of the FL in the ascending aorta; (III) IMH originating from PAU or ULP in the arch or descending aorta; (IV) a true IMH as a result of ruptured vasa vasorum in the aortic wall. Sixteen out of the 36 (44.4%) patients in Group S had an entry tear found in the ascending aorta or proximal arch during emergency surgery, while only 4 out of the 25 (16%) patients with delayed aortic intervention had tears identifiable in ascending aorta either during open surgery or on follow up CT scans before intervention. Initial CT features provide important information regarding the underlying cause of IMH. Patients with thicker IMH, larger aortic sizes and pericardial effusion on CT scans are more likely to have genuine intimal tears in the ascending and warrant upfront surgery, while for patients with thinner IMH and smaller ascending aortas, pathologies are more likely distal in the arch or descending and may benefit from watchful waiting and delayed definitive aortic intervention. PAU/ULP were found in the thoracic aorta in 36.6% of patients, and were found to be associated with delayed aortic intervention in Group W. The presence of PAU/ULP with ascending IMH usually warrants definitive treatment. Watchful waiting and serial CT scans allow time for the ascending IMH to resolve, which may unravel favorable disease-free landing zones for TEVAR.
The uncertainty underlying the disease process in type A IMH has been cited as a reason for upfront surgery for all patients. Another point of contention in watchful waiting approach is the high rate of subsequent aortic interventions. The subsequent aortic intervention rate was 43.9% in Group W. However, only 1 out of 25 patients had emergency conversion from medical therapy to surgery for progression to classical acute TAAD, otherwise all cases were performed as in-hospital early surgery or outpatient elective procedures. Planned aortic reinterventions are usually of lower risks than emergent operations, and we should not misinterpret delayed aortic interventions which are non-emergency as failure of watchful waiting strategy. On the contrary, watchful waiting allows more time for procedural planning and patient optimization before definitive aortic treatment, especially in patients with arch or descending pathologies.
In addition, the relevance of watchful waiting, while safe and feasible in selected patients across all risk profiles, is particularly useful in patients at elevated risk of operation or in institutions with lower volumes in aortic surgery. In lower volume centers, mortality rates are higher after emergency proximal aortic repair in acute dissections with demonstration of outcome-volume relationships (23). A judicious approach to managing patients with type A IMH can allow time for risk assessment, stabilization or if necessary, transfer of the patient to a higher volume aortic center with more expertise for more definitive treatment.
This was a retrospective, non-randomized single center study reporting on results of individualized management of type A IMH. The sample size was small, and the potential of data and measurement error as well as selection bias impacted the scientific value of the conclusion. Treatment decisions were based on local expert opinions with no preceding randomization. The study did not draw meaningful comparisons between surgery and watchful waiting and was not designed to show superiority or non-inferiority. Nonetheless, this cohort adds to the body of evidence that not all type A IMH requires upfront surgery and there is room for a more individualized approach with watchful waiting.
Conclusions
Not all type A IMH patients require upfront surgery. A watchful waiting approach has been shown to be safe, and an individualized approach can be considered in selected patients with uncomplicated type A IMH with thin ascending IMH & small aortic diameter with no deleterious effects on survival.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1837/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1837/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1837/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1837/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the clinical research ethics committee of Prince of Wales Hospital (16 August 2023, CRE 2023.335) and individual consent for this retrospective analysis was waived. Patient anonymity was preserved in this study and all data had been kept secured and confidential.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kitamura T, Torii S, Horai T, et al. Outcomes of patients who declined surgery for acute Stanford type A aortic dissection with patent false lumen of the ascending aorta. Interact Cardiovasc Thorac Surg 2017;25:47-51. [Crossref] [PubMed]
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897-903. [Crossref] [PubMed]
- Writing Committee Members. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022;80:e223-393. [PubMed]
- Harris KM, Braverman AC, Gutierrez FR, et al. Transesophageal echocardiographic and clinical features of aortic intramural hematoma. J Thorac Cardiovasc Surg 1997;114:619-26. [Crossref] [PubMed]
- Chou AS, Ziganshin BA, Charilaou P, et al. Long-term behavior of aortic intramural hematomas and penetrating ulcers. J Thorac Cardiovasc Surg 2016;151:361-72, 373.e1.
- Evangelista A, Mukherjee D, Mehta RH, et al. Acute intramural hematoma of the aorta: a mystery in evolution. Circulation 2005;111:1063-70. [Crossref] [PubMed]
- Krukenberg E. Beitrage zur Frage des Aneurysma dissecans. Beitr Pathol Anat Allg Pathol 1920;67:329-51.
- von Kodolitsch Y, Csösz SK, Koschyk DH, et al. Intramural hematoma of the aorta: predictors of progression to dissection and rupture. Circulation 2003;107:1158-63. [Crossref] [PubMed]
- Song JK, Yim JH, Ahn JM, et al. Outcomes of patients with acute type a aortic intramural hematoma. Circulation 2009;120:2046-52. [Crossref] [PubMed]
- Choi YJ, Son JW, Lee SH, et al. Treatment patterns and their outcomes of acute aortic intramural hematoma in real world: multicenter registry for aortic intramural hematoma. BMC Cardiovasc Disord 2014;14:103. [Crossref] [PubMed]
- Kitamura T, Torii S, Miyamoto T, et al. Watch-and-wait strategy for type A intramural haematoma and acute aortic dissection with thrombosed false lumen of the ascending aorta: a Japanese single-centre experience. Eur J Cardiothorac Surg 2020;58:590-7. [Crossref] [PubMed]
- Harris KM, Braverman AC, Eagle KA, et al. Acute aortic intramural hematoma: an analysis from the International Registry of Acute Aortic Dissection. Circulation 2012;126:S91-6. [Crossref] [PubMed]
- Robbins RC, McManus RP, Mitchell RS, et al. Management of patients with intramural hematoma of the thoracic aorta. Circulation 1993;88:II1-10. [PubMed]
- Sandhu HK, Tanaka A, Charlton-Ouw KM, et al. Outcomes and management of type A intramural hematoma. Ann Cardiothorac Surg 2016;5:317-27. [Crossref] [PubMed]
- Shimamura J, Yamamoto S, Oshima S, et al. Surgical outcomes of aortic repair via transapical cannulation and the adventitial inversion technique for acute Type A aortic dissection. Eur J Cardiothorac Surg 2018;54:369-74. [Crossref] [PubMed]
- Wong RH, Yang F, Fujikawa T, et al. Pocket-Size Mobile Echocardiographic Screening of Thoracic Aortic Aneurysms in Hypertensive Patients. Ann Thorac Surg 2021;111:1554-9. [Crossref] [PubMed]
- Kitai T, Kaji S, Yamamuro A, et al. Clinical outcomes of medical therapy and timely operation in initially diagnosed type a aortic intramural hematoma: a 20-year experience. Circulation 2009;120:S292-8. [Crossref] [PubMed]
- Sadamatsu K, Takase S, Sagara S, et al. Initial medical management in acute type A aortic dissection patients with a thrombosed false lumen in the ascending aorta combining intramural hematoma and retrograde dissection from the descending to the ascending aorta. Eur Heart J Acute Cardiovasc Care 2020;9:S13-20. [Crossref] [PubMed]
- Nakamae K, Oshitomi T, Uesugi H, et al. Noncommunicating acute type A aortic dissection in elderly patients: Surgery versus medical management. Eur J Cardiothorac Surg 2022;62:ezac484. [Crossref] [PubMed]
- Chen YY, Yen HT, Lo CM, et al. Natural courses and long-term results of type A acute aortic intramural haematoma and retrograde thrombosed type A acute aortic dissection: a single-centre experience. Interact Cardiovasc Thorac Surg 2020;30:113-20. [Crossref] [PubMed]
- Poon SS, Theologou T, Harrington D, et al. Hemiarch versus total aortic arch replacement in acute type A dissection: a systematic review and meta-analysis. Ann Cardiothorac Surg 2016;5:156-73. [Crossref] [PubMed]
- Ye C, Chang G, Li S, et al. Endovascular stent-graft treatment for Stanford type A aortic dissection. Eur J Vasc Endovasc Surg 2011;42:787-94. [Crossref] [PubMed]
- Mori M, Shioda K, Wang X, et al. Perioperative Risk Profiles and Volume-Outcome Relationships in Proximal Thoracic Aortic Surgery. Ann Thorac Surg 2018;106:1095-104. [Crossref] [PubMed]



