
Etiological diagnostic performance of probe capture-based targeted next-generation sequencing in bloodstream infection
Highlight box
Key findings
• Blood targeted next-generation sequencing (tNGS) may be a promising tool for detecting potential pathogens in patients with bloodstream infection (BSI).
What is known and what is new?
• BSI is a life-threatening disease. Rapid and precise etiological diagnosis is crucial for the treatment of BSI.
• Blood tNGS had a higher sensitivity and faster turnaround time than culture and metagenomic next-generation sequencing.
What is the implication, and what should change now?
• The application of blood tNGS for BSI could guide anti-infectious treatment strategies and might improve clinical outcomes.
Introduction
Bloodstream infection (BSI) is a life-threatening disease associated with high mortality rates (1). Appropriate antibiotic treatment is recommended for BSI at the earliest opportunity (2,3). Delayed diagnosis and ineffective treatment may lead to septic shock, multiple organ failure, disseminated intravascular coagulation (DIC), and even death. Thus, rapid and precise etiological diagnosis is crucial for the optimal treatment and prognosis of BSI. Blood culture is the most widely used conventional etiological diagnostic method (4). However, the sensitivity of blood cultures is far from ideal. Just over one-third of the patients with sepsis have positive blood cultures, mainly because of inadequate sampling volumes and prior administration of antibiotics (5). Blood culture is also time-consuming, usually taking 3–7 days or longer to identify potential pathogens, which is not feasible in clinical practice.
In recent years, culture-independent methods such as polymerase chain reaction (PCR) and metagenomic next-generation sequencing (mNGS) have been used for the etiological diagnosis of BSI (6-8). Regarding PCR, a presumptive diagnosis is necessary and misdiagnosis is common. The sensitivity of mNGS varies depending on the specimen type and disease severity (6-8). On account of the large amount of host human DNA and low biomass of pathogens, the positive rate of mNGS in serum samples is still not ideal. In addition, mNGS is expensive, limiting its clinical application. In Shanghai, the cost of a single mNGS test is around 3,500 RMB. If mNGS covers DNA and RNA dual processes, the price will increase to 4,500–6,500 RMB. Therefore, a more sensitive, rapid, and cost-efficient method for diagnosing BSI is urgently needed.
Target enrichment strategies are commonly used in NGS workflows to eliminate genomic DNA regions that are not of interest (9-11). Probe capture-based targeted next-generation sequencing (tNGS) has been applied for the molecular diagnosis of human infectious diseases (12,13). However, the application of probe capture-based tNGS for the etiological diagnosis of BSI has not yet been explored. Our study innovatively applied probe capture-based tNGS in patients with BSI with the aim of evaluating its etiological diagnostic performance. We present this article in accordance with the STARD reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-400/rc).
Methods
Study design
This prospective study was performed at Zhongshan Hospital, Shanghai, China, from 24 November 2023 to 30 December 2023. A total of 88 patients with suspected BSI were screened and 80 were enrolled in the present study, as shown in Figure 1. The inclusion criteria were as follows: (I) age ≥14 years; (II) matching the diagnostic criteria of systemic inflammatory response syndrome (SIRS); (III) febrile, with temperature ≥38.5 °C; (IV) at least one biomarker increased among white blood cell count (WBC), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and procalcitonin (PCT); and (V) severe infection clinically suspicious of BSI which could not be explained by focal infection. As Zhongshan Hospital does not have the Pediatric Department, all the patients admitted must be ≥14 years of age. The exclusion criteria were as follows: (I) the initial diagnosis of fever on admission was a non-infectious disease, such as tumors and autoimmune diseases; (II) unclear clinical diagnosis at discharge/death; and (III) refusal to undergo blood culture, mNGS, or tNGS. All patients enrolled in the study underwent simultaneous blood culture, blood mNGS, and blood tNGS during fever after admission. Other specimens such as pus, pleural fluid, peritoneal fluid, bile, cerebrospinal fluid, urine, and sputum were also collected from some patients for culture and mNGS. This study was registered with the Chinese Clinical Trial Registry at www.chictr.org.cn, with registration number ChiCTR2300077934. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Review Committee of Zhongshan Hospital, Fudan University, Shanghai, China (No. B2023-356R) and informed consent was taken from all the patients.

A clinical diagnosis of BSI or noninfectious fever was made at discharge/death by a clinician team consisting of at least two senior clinicians/professors and four attending doctors, according to the patient’s medical history, signs and symptoms, laboratory and imaging examinations, and response to antibiotic treatment.
Demographic information, signs and symptoms of infection, laboratory and imaging examinations, antibiotic treatments, and responses were recorded. Targeted adjustment of anti-infectious therapy was defined as the adjustment of anti-infectious medications by a clinician according to the results of tNGS, mNGS, or culture, including the de-escalation or replacement of anti-infectious medications. Clinical improvement was defined as an improvement in the infectious signs and symptoms or a decrease in inflammatory biomarkers.
Etiological testing
All samples were tested using microbiological culture, mNGS, and tNGS following strict procedures. Culture, mNGS, and tNGS were conducted by different technicians not aware of the results of each other, which ensured adequate blinding between different testing methods. The results were reviewed by at least two clinicians to discriminate between infection and contamination or colonization. If a virus was detected but not related to the disease course and the patient had recovered with antibacterial/antifungal/antiparasitic drugs only and without antiviral drugs, the virus was considered colonized. mNGS or tNGS results were recognized as positive if the following conditions were met: (I) the read number of the potential pathogen identified by mNGS or tNGS was significantly higher than that of any other microbe; and (II) mNGS or tNGS results were consistent with the clinical course of the patient according to the judgment of the clinicians. If more than one potential pathogen was identified by mNGS or tNGS with approximate read number (within one-fold) and all the identified potential pathogens were consistent with the clinical course of the patient, the mNGS or tNGS results were recognized as polymicrobial infection. Details of the microbiological cultures are provided in Appendix 1. The details of mNGS and tNGS testing are as follows.
mNGS workflow
Sample processing and DNA extraction
Blood samples of 3 mL were drawn from patients and stored at room temperature for 3–5 minutes before plasma separation, followed by centrifugation at 4,000 rpm for 10 minutes at 4 °C. The plasma samples were then transferred to new sterile tubes. Three mL of pus, sputum, or body fluids were collected from the patients according to standard procedures. Sputum was liquefied by 0.1% DTT (dithiothreitol) for 30 minutes at room temperature. Pus or body fluids could go directly to the next operation. DNA was extracted from 300 µL of plasma using the TIANamp Micro DNA Kit (DP316, TIANGEN BIOTECH, Beijing, China), following the operational manual of the manufacturer. The extracted DNA samples were used to construct DNA libraries (14).
Construction of DNA libraries and sequencing
DNA libraries were constructed using DNA fragmentation, end repair, adapter ligation, and PCR amplification by MetaCAP Pathogen Capture Metagenomic Assay Kit (KingCreate, Guangzhou, China). Agilent 2100 (Agilent, Santa Clara, CA, USA) was used for quality control of the DNA libraries. Quality-qualified libraries were pooled. Then, a DNA nanoball (DNB) was prepared and sequenced using the MGISEQ-2000 platform (MGI Tech, Shenzhen, China) set to 50-bp single end with average of 100 million reads per sample (15).
tNGS workflow
Sample processing and nucleic acid extraction
Blood samples of 3 mL were centrifuged at 1,600 rcf for 10 minutes at 4 °C. Nucleic acid was extracted from 300 µL of plasma using the MasterPure DNA & RNA Extraction Kit (KingCreate) following the manufacturer’s operational manual and was used for the construction of libraries.
Construction of libraries, enrichment, and sequencing
After complementary DNA (cDNA) was converted from RNA, libraries were constructed through fragmentation, end repair, adapter ligation, and PCR amplification. Then, eight uniquely barcoded libraries were pooled for hybridization and captured using specific biotinylated probes for 2 hours after library generation using the MetaCAP Pathogen Capture Metagenomic Assay Kit (KingCreate), following the manufacturer’s instructions. The enrichment panel targeted >3,000 pathogens, including viruses, bacteria, fungi, and parasites. The pathogen target was screened according to the following rules: (I) the target had at least one completed genome sequence; (II) the target had at least one article related to human infection; (III) the target had research reports. Finally, this capture range was 1,060 bacteria, 1,402 viruses, 388 fungi and 210 parasites. Probe design selected the conserved regions such as ribosomal RNA (rRNA) gene (16S rRNA, 18S rRNA or internal transcribed spacer), house-keeping genes which allowed for pathogen identification down to the genus or species level. Agilent 2100 was used for the quality control of the libraries and showed peaks near 350 bp. The Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific Inc., Waltham, MA, USA) was used to construct quality-qualified libraries, and sequencing was performed on an Illumina MiniSeq platform set to 100-bp single end with average of 1 million reads per sample.
Bioinformatic analysis
Bioinformatic analyses of both mNGS and tNGS data were conducted according to the following process. Clean reads were obtained by removing sequencing adapters, reads of low quality, excessive N bases, or reads with length below 35 bp using fastp (version 0.23.1) (16). The remaining reads were aligned to the human reference (hg38) using Burrows-Wheeler aligner (BWA; version 0.7.17-r1188; Linux, San Francisco, CA, USA), and human reads were filtered (17). Subsequently, the reads were compared to the classification reference genome database. The scope of the database contained all targeted pathogens and other species in their genus. In addition, it also included species outside the genus with genome average nucleotide identity >80%. Database contained 11,958 bacteria, 7,373 viruses, 1,714 fungi and 343 parasites. The species genomes were collected and downloaded from the National Center for Biotechnology Information (NCBI) genome (ftp://ftp.ncbi.nlm.nih.gov/genomes/). Genome assemble level at complete or scaffold was selected and shielded contamination introduced by assembly, such as host sequences. Self-developed software was used to calculate the number of reads per million (RPM) at the species and genus levels.
Statistical analysis
Continuous data were expressed as the mean ± standard deviation or median with interquartile range (IQR, 25th–75th percentiles), depending on the normality of the data distribution. The final clinical diagnosis at discharge or death was the gold standard. The sensitivity and specificity of blood culture, blood mNGS, and blood tNGS, determined based on whether the test reflected the clinical diagnosis of BSI, were compared using the Chi-square test. Data analysis was performed using SPSS software (version 21.0; IBM Corp., Armonk, NY, USA). All tests were two-tailed, and statistical significance was set at P<0.05.
Results
Characteristics of the study participants
The demographic and clinical characteristics of the patients are provided in Table S1. A total of 80 patients were enrolled in this study, of whom 11 were diagnosed with noninfectious fever and 69 were diagnosed with BSI. Among the 69 patients with BSI, 50 (72.5%) were male, and the mean age was 59.4±15.8 years. A total of 19 BSIs (27.5%) were nosocomial infections, and 59 patients with BSI (85.5%) received empirical antibiotic treatment within 4 weeks before admission. Nosocomial infections referred to infections acquired during hospitalization. Infections occurred after discharge but acquired during hospitalization still belonged to nosocomial infections. The underlying infectious syndromes of the 69 patients with BSI were classified into 11 types: abdominal infections (26 cases, 37.7%), infective endocarditis (9 cases, 13.0%), catheter/device related infections (8 cases, 11.6%), urinary tract infections (7 cases, 10.1%), skin and soft tissue infections (7 cases, 10.1%), respiratory infections (6 cases, 8.7%), infectious aneurysm (2 cases, 2.9%), osteoarticular infections (1 case, 1.4%), septic thrombophlebitis (1 case, 1.4%), central nervous system infections (1 case, 1.4%), and other/unknown infections (1 case, 1.4%). The underlying risk factors in the 69 patients with BSI were as follows: active malignancy in 13 cases (18.8%), diabetes mellitus in 12 cases (17.4%), and long-term use of immunosuppressive therapy in 7 cases (10.1%).
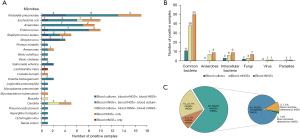
Table S2 lists the results of blood culture, mNGS, and tNGS for the 80 participants. For the 69 patients with BSI, the numbers of different types of microbes detected by blood culture, blood mNGS, and blood tNGS are shown in Figure 2A,2B. The consistency between blood mNGS and blood tNGS is described in Figure 2C.
Diagnostic performance of blood culture, blood mNGS, and blood tNGS
Blood tNGS had a higher sensitivity for the diagnosis of BSI than blood culture (91.3% vs. 23.2%, P<0.001). Additionally, blood tNGS had a higher sensitivity than blood mNGS (91.3% vs. 69.6%, P=0.001), as shown in Table 1. The specificities of blood culture, blood mNGS, and blood tNGS for the diagnosis of BSI were 100.0%, 81.8%, and 100.0%, respectively. There was no significant difference in the specificity between blood mNGS and tNGS (P=0.13).
Table 1
| Methods | Positive number | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|
| Noninfectious fever (n=11) | BSI (n=69) | |||
| Blood culture | 0 | 16 | 23.2 | 100.0 |
| Blood mNGS | 2 | 48 | 69.6 | 81.8 |
| Blood tNGS | 0 | 63 | 91.3 | 100.0 |
mNGS, metagenomic next-generation sequencing; tNGS, targeted next-generation sequencing; BSI, bloodstream infection.
Blood tNGS versus blood culture
Of the 16 blood culture-positive BSIs, microbes were detected by tNGS in 16 cases (100.0%), including a single microbe detected by tNGS in 13 cases and multiple microbes in three cases. The most common microbes identified using both methods were Enterobacteriaceae, including Klebsiella pneumoniae (K. pneumoniae) and Escherichia coli (E. coli). The results of tNGS were concordant with the blood culture in 13 cases (81.3%), with additional potential pathogens detected in three cases (18.8%), including Enterococcus in two cases, and Staphylococcus aureus (S. aureus) and Candida each in one case, as shown in Table 2.
Table 2
| Case classification | Number of samples | Identical findings | New microbes detected by tNGS | Microbes not identified by tNGS |
|---|---|---|---|---|
| Blood tNGS versus blood culture | ||||
| Noninfectious fever | 11 | 11 (100.0) | NA | NA |
| Blood culture-positive BSI | 16 | 13 (81.3) | 3 (18.8)a | 0 |
| Blood culture-negative BSI | 53 | 6 (11.3) | 47 (88.7)b | NA |
| Blood tNGS versus blood mNGS | ||||
| Noninfectious fever | 11 | 9 (81.8) | NA | NA |
| Blood mNGS-positive BSI | 48 | 42 (87.5) | 5 (10.4)c | 1 (2.1)d |
| Blood mNGS-negative BSI | 21 | 6 (28.6) | 15 (71.4)e | NA |
Data shown are the n (%) of samples in which there are identical or discrepant findings between blood culture, blood mNGS and blood tNGS. a, 5 new microbes were detected by tNGS in 16 blood culture-positive BSIs; b, 56 new microbes were detected by tNGS in 53 blood culture-negative BSIs; c, 5 new microbes were detected by tNGS in 48 blood mNGS-positive BSIs; d, 1 microbe was missed by tNGS in 48 blood mNGS-positive BSIs; e, 17 new microbes were detected by tNGS in 21 blood mNGS-negative BSIs. mNGS, metagenomic next-generation sequencing; tNGS, targeted next-generation sequencing; NA, not applicable; BSI, bloodstream infection.
Of the 53 patients with blood culture-negative BSIs, 6 (11.3%) had negative tNGS results, whereas 56 additional microbes were detected by tNGS among the remaining 47 (88.7%) patients. A total of eight participants presented with polymicrobial infections. Additional microbes detected by tNGS included K. pneumoniae (13 cases), Anaerobes (9 cases), E. coli (8 cases), Enterococcus (6 cases), S. aureus (2 cases), Aeromonas (2 cases), Orientia tsutsugamushi (O. tsutsugamushi) (2 cases), Vibrio vulnificus (1 case), Vibrio cholerae (1 case), Streptococcus (1 case), Coxiella burnetii (C. burnetii) (1 case), Mycobacterium tuberculosis (M. tuberculosis) (1 case), Legionella pneumophila (L. pneumophila) (1 case), Mycoplasma pneumoniae (M. pneumoniae) (1 case), Proteus mirabilis (1 case), Candida (2 cases), Aspergillus fumigatus (1 case), Pneumocystis jirovecii (1 case), Cytomegalovirus (1 case), and Taenia solium (1 case).
Blood tNGS versus blood mNGS
Among the 48 patients with blood mNGS-positive BSIs, the blood tNGS results were also all positive, which were concordant with the blood mNGS results in 42 cases (87.5%), with additional potential pathogens detected in 5 (10.4%) cases, including Candida in two cases and S. aureus, K. pneumoniae, and Enterococcus in one case each, as shown in Table 2. In one case (2.1%), Lactobacillus iners was detected by mNGS but missed by tNGS. The most common microbe identified using both methods was K. pneumoniae, followed by Enterococcus.
Of the 21 patients with blood mNGS-negative BSIs, 6 (28.6%) had negative tNGS results, whereas 17 additional microbes were detected by tNGS in the remaining 15 cases (71.4%). Polymicrobial infections were detected in two participants. New microbes detected by tNGS included E. coli (4 cases), K. pneumoniae (3 cases), Anaerobes (2 cases), Candida (2 cases), Enterococcus (1 case), S. aureus (1 case), Aeromonas (1 case), C. burnetii (1 case), M. tuberculosis (1 case), and Brucella (1 case).
Concordance between blood tNGS results and microbes detected by culture or mNGS in other specimens
Among the 69 patients with BSIs, 23 patients underwent culture or mNGS of other specimens such as pus, sputum, pleural fluid, peritoneal fluid, bile, cerebrospinal fluid, or urine, as shown in Table S3. Among the 23 patients, 21 (91.3%) had blood tNGS results consistent with the microbes detected by culture or mNGS in other specimens. In one patient (P56), blood tNGS identified Enterococcus faecalis (E. faecalis), Enterococcus faecium (E. faecium), and K. pneumoniae, whereas the bile culture grew E. faecalis and bile mNGS identified E. faecalis and K. pneumoniae. In another patient (P73), blood tNGS identified Bacteroides thetaiotaomicron (B. thetaiotaomicron) and E. faecium, whereas culture of the peritoneal fluid grew E. faecium and E. coli, and mNGS of the peritoneal fluid identified B. thetaiotaomicron, E. faecium, K. pneumoniae, and Candida albicans.
Analysis of cases with noninfectious fever
Of the 11 patients with noninfectious fever, microbes were detected by blood culture in 0 patients, mNGS in two cases, and tNGS in 0 cases. The two microbes detected by mNGS were Streptococcus mitis and Haemophilus parainfluenzae, which were suspected to be contaminant. These two patients were respectively diagnosed with Takayasu arteritis and polyarteritis nodosa. After transferred to the Rheumatology Department, they received glucocorticoid and immunosuppressant treatment and clinically improved.
Turnaround time (TAT) of blood culture, mNGS, and tNGS
TAT was defined as the time from sample reception to identification of potential pathogens. Among the 12 patients who were simultaneously positive by blood culture, blood tNGS, and blood mNGS, TAT of blood culture, blood mNGS, and blood tNGS were 50.1±9.9, 30.5, and 16 hours, respectively. Blood tNGS had a faster TAT than blood culture and blood mNGS.
Targeted adjustment of anti-infectious therapy
Among the 69 patients with BSI, 22 (31.9%) underwent targeted adjustment of anti-infectious therapy according to the blood tNGS results and showed clinical improvement, as shown in Table 3. Among these 22 patients, 6 (27.3%) were blood culture positive, 17 (77.3%) were blood mNGS-positive, and 22 (100.0%) were blood tNGS-positive. Due to having a faster TAT compared to that of culture and mNGS, blood tNGS guided the clinical anti-infectious treatment in the first place. Through the rapid and broad detection of potential pathogens, blood tNGS minimizes the time required for empirical treatment and allows for precise anti-infectious therapy. For the other 47 patients with BSI, tNGS results were concordant with the clinician’s initial evaluation of the patient and the initial anti-infectious therapy continued.
Table 3
| Patient number | Anti-infectious therapy before tNGS testing | Anti-infectious therapy after adjustment | Microbes detected by tNGS | Underlying infectious syndromes |
|---|---|---|---|---|
| P15 | Ceftriaxone + levofloxacin | Piperacillin/tazobactam | Treponema denticola, Fusobacterium vincentii | Catheter/device related infectionsa |
| P19 | Ceftazidime/avibactam | Ceftazidime/avibactam + daptomycin | Klebsiella pneumoniae, Staphylococcus aureus | Infective endocarditisb (PVE) |
| P24 | Meropenem | Ceftriaxone + levofloxacin | Aeromonas veronii | Skin and soft tissue infections |
| P26 | Meropenem + vancomycin | Ceftriaxone + levofloxacin | Vibrio vulnificus | Skin and soft tissue infections |
| P33 | Vancomycin | Ceftriaxone + amikacin | Streptococcus sanguinis | Infective endocarditis (NVE) |
| P35 | Meropenem | Piperacillin/tazobactam+ caspofungin | Escherichia coli, Candida parapsilosis | Urinary tract infections |
| P39 | Ertapenem | Caspofungin | Candida parapsilosis | Other/unknown infections |
| P42 | Meropenem + levofloxacin | Sulfamethoxazole and trimethoprim, ganciclovir | Pneumocystis jirovecii, Cytomegalo virus | Respiratory infections |
| P44 | Piperacillin/tazobactam | Doxycycline | Orientia tsutsugamushi | Skin and soft tissue infections |
| P45 | Cefoperazone/sulbactam | Cefoperazone/sulbactam + metronidazole | Bacteroides fragilis | Abdominal infections |
| P50 | Levofloxacin | Isoniazid + rifampin + ethambutol + pyrazinamide |
Mycobacterium tuberculosis | Respiratory infections |
| P52 | Ceftriaxone | Doxycycline | Orientia tsutsugamushi | Skin and soft tissue infections |
| P53 | Meropenem | Ceftriaxone + levofloxacin | Salmonella enterica | Infectious aneurysm |
| P55 | Piperacillin/tazobactam | Doxycycline | Mycoplasma pneumoniae | Respiratory infections |
| P57 | Meropenem + vancomycin | Caspofungin | Candida metapsilosis | Infective endocarditis (PVE) |
| P59 | Isoniazid + rifampin + ethambutol + pyrazinamide | Albendazole + praziquantel | Taenia solium | Central nervous system infections |
| P61 | Ceftriaxone | Doxycycline + rifampin | Brucella | Osteoarticular infection |
| P62 | Vancomycin | Doxycycline + moxifloxacin | Coxiella burnetii | Catheter/device related infections |
| P70 | Piperacillin/tazobactam | Moxifloxacin | Legionella pneumophila | Respiratory infections |
| P72 | Piperacillin/tazobactam | Vancomycin + caspofungin | Enterococcus faecalis, Candida glabrata | Abdominal infections |
| P73 | Tigecycline | Vancomycin + piperacillin/tazobactam | Enterococcus faecium, Bacteroides thetaiotaomicron | Abdominal infections |
| P75 | Meropenem | Voriconazole | Aspergillus fumigatus | Septic thrombophlebitis |
a, including infections related to central line catheters, artificial blood vessels, and stent implantation; b, including NVE, PVE, and pacemaker-related endocarditis. tNGS, targeted next-generation sequencing; BSI, bloodstream infection; NVE, native valve endocarditis; PVE, prosthetic valve endocarditis.
Discussion
Despite the great advances in medical science in the past century, BSI remains a growing public health concern, with an incidence ranging between 113 and 204 per 100,000 people, including nosocomial BSIs that account for approximately 30–50% of the cases in high-income countries (18). Overall, E. coli was the most common pathogen causing BSI, followed by Klebsiella spp. and S. aureus. Other key pathogens of BSI include coagulase-negative staphylococci, Pseudomonas aeruginosa, Acinetobacter baumanni, Salmonella enterica, Streptococcus spp., and Enterococcus spp., the first three of which are almost always healthcare-associated (18). The short-term case fatality rate following BSI ranges from 13.5% to 20.6%, whereas the 1-year mortality ranges from 8% to 48% (1,19). Prompt etiological diagnosis and anti-infectious therapy are essential for the effective treatment of BSI.
Blood cultures remain an imperfect diagnostic tool for deadly BSIs; 30–50% of positive blood cultures are contaminated, whereas false-negative blood cultures occur in 40–60% of cases with suspected sepsis, leading to a diagnostic conundrum (6). Novel molecular diagnostics, mainly nucleic acid amplification-based testing, have dramatically changed the clinical approach to the diagnosis of BSI, with significant improvements in sensitivity and speed. mNGS has been used to diagnose BSI. In a previous study conducted by Sun et al., the positivity rate of blood mNGS was higher than that of blood culture (67.74% vs. 19.35%, P<0.001) in intensive unit care patients with sepsis (20). However, due to the interference of host DNA and low biomass of circulating pathogens, mNGS in serum samples is still not sufficiently sensitive. Furthermore, owing to the complexity of data processing and analysis, high cost, and difficulty to reduce TAT, the expansion of mNGS applications for BSI has encountered limitations.
Various target enrichment strategies have been used for the sequencing and analysis of a number of targets of interest (9-11). Hernández-Neuta et al. adopted molecular inversion probes as a cost-effective target-enrichment approach to identify pathogens and antimicrobial resistance markers in blood samples using short-read sequencing (13). Li et al. applied tNGS for target detection of 153 pathogens in adults with pneumonia, and observed that tNGS was effective and comparable to mNGS in detecting respiratory pathogens (21). Fida et al. prospectively investigated 16S rRNA gene-based tNGS in 60 adults with sepsis and compared tNGS results with blood cultures. They concluded that 16S rRNA gene-based tNGS may be useful for detecting bacteria in the plasma of patients with sepsis (22). In a study conducted by Hong et al., 16S rRNA gene-based tNGS was found to be a potential diagnostic tool for the identification of periprosthetic joint infection pathogens. However, there was no difference in the positive percent agreement between 16S rRNA gene-based tNGS and shotgun metagenomic sequencing (sNGS) (72.1% vs. 73.1%, P=0.83) for detecting microorganisms in the sonicate fluid of periprosthetic joint infection (23). We assume that this result was attributable to the retrospective study design in which sonicate fluid sNGS had been conducted between 2011 and 2016, whereas −80 °C frozen clinical samples were collected for tNGS testing in 2022. Samples stored for several years may have affected the detection efficiency of tNGS. The results of this study may also be affected by the inherent limitations of the 16S rRNA gene-based tNGS methodology.
However, the application of probe capture-based tNGS for the etiological diagnosis of BSI has not been explored. Compared with mNGS, probe capture-based tNGS has fewer data burdens and simplified data interpretation, facilitating a lower cost and faster TAT (12). In this study, we demonstrated that blood tNGS has significantly higher sensitivity and faster TAT than blood mNGS for the diagnosis of BSI. However, the specificities of blood tNGS and mNGS were not statistically different. tNGS especially outperformed mNGS in the detection of fungi and intracellular bacteria, including M. pneumoniae, L. pneumophila, O. tsutsugamushi, Salmonella enterica, Brucella, C. burnetii, and M. tuberculosis. The biomass of fungi and intracellular bacteria in the serum is relatively low, which may explain the false negative results of mNGS to some extent. Probe capture and enrichment in tNGS increases the ability to detect fungi and intracellular bacteria.
Furthermore, mNGS can generally detect either DNA or RNA pathogens, but not both. If mNGS covers DNA and RNA dual processes, testing will become more complicated and double in cost, increasing the economic burden on patients. In contrast, tNGS inherently covers both DNA and RNA pathogens and is much more convenient and cost-effective than mNGS.
Compared with tNGS, mNGS has much more data burdens and the data interpretation process is more complex. The data size is approximately 100–120 M per sample for mNGS. Fifty bp is recommended by BGI to balance the diagnosis performance and clinical TAT. If 50 bp increases to 100 bp, time spent on sequencing will double, consuming almost 30 hours on the sequencing platform. TAT of 100 bp in mNGS cannot meet clinical needs. However, in tNGS, target capture and enrichment can reduce data burdens. The data size is approximately 1 M per sample for tNGS and sequencing needs only 6 hours on Illumina Miniseq platfrom. One hundred bp is more suitable for tNGS because specific target for pathogen identification requires certain length. These are the inherent differences between mNGS and tNGS.
In this study, 23 patients with BSI underwent culture or mNGS of specimens other than blood, such as pus, sputum, or body fluids. Among these, 21 (91.3%) had blood tNGS results consistent with those of microbes detected by culture or mNGS of other specimens, demonstrating the accuracy of blood tNGS for the etiological diagnosis of BSI. Compared with the culture or mNGS of other specimens, blood tNGS is much more convenient as blood samples can be easily collected at admission. In contrast, the collection of other specimens is more complicated, requiring procedures such as percutaneous puncture and drainage, which are more time consuming.
Among the 69 patients with BSI, 22 underwent targeted adjustment of anti-infectious therapy according to the tNGS results and clinically improved. tNGS may help clinicians to downsize antibiotic regimens and decrease the empirical usage of broad-spectrum antibiotics, which might reduce antibiotic abuse and affect the clinical prognosis.
Despite these findings, this study has some limitations. As this was a single-center study, a patient selection bias may have existed. The gold standard was the clinical evaluation at death/discharge which would follow the testing and thus further introduce bias as evaluations would be done retrospectively. The number of patients enrolled was relatively small, and we could not divide the patients into subgroups according to the underlying infectious syndromes. Additionally, no RNA microbes were detected in this study and no patient with viremia was diagnosed, perhaps because of the small sample size.
Conclusions
In conclusion, blood tNGS may be a promising tool for the etiological diagnosis of BSI. Owing to its higher sensitivity and faster TAT than blood mNGS and blood culture, blood tNGS is a valuable tool for identifying the pathogens responsible for BSI and guiding anti-infectious treatments.
Acknowledgments
The authors thank all the clinicians and microbiologists who assisted with this study.
Funding: This work was funded by
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-400/rc
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-400/prf
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-400/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-24-400/coif). J.Y., Y.L., F.G., Z.L., H.L., J.L., and C.H. are from Guangzhou KingCreate Biotechnology Company Limited. P.L. is from Guangzhou KingMed Diagnostics Group Company Limited. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Review Committee of Zhongshan Hospital, Fudan University, Shanghai, China (No. B2023-356R) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 2013;19:501-9. [Crossref] [PubMed]
- Seymour CW, Gesten F, Prescott HC, et al. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N Engl J Med 2017;376:2235-44. [Crossref] [PubMed]
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43:304-77. [Crossref] [PubMed]
- Lamy B, Sundqvist M, Idelevich EA, et al. Bloodstream infections - Standard and progress in pathogen diagnostics. Clin Microbiol Infect 2020;26:142-50. [Crossref] [PubMed]
- Towns ML, Jarvis WR, Hsueh PR. Guidelines on blood cultures. J Microbiol Immunol Infect 2010;43:347-9. [Crossref] [PubMed]
- Samuel L. Direct-from-Blood Detection of Pathogens: a Review of Technology and Challenges. J Clin Microbiol 2023;61:e0023121. [Crossref] [PubMed]
- Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet 2019;20:341-55. [Crossref] [PubMed]
- Gu W, Miller S, Chiu CY. Clinical Metagenomic Next-Generation Sequencing for Pathogen Detection. Annu Rev Pathol 2019;14:319-38. [Crossref] [PubMed]
- Kozarewa I, Armisen J, Gardner AF, et al. Overview of Target Enrichment Strategies. Curr Protoc Mol Biol 2015;112:7.21.1-7.21.23.
- Mamanova L, Coffey AJ, Scott CE, et al. Target-enrichment strategies for next-generation sequencing. Nat Methods 2010;7:111-8. [Crossref] [PubMed]
- Gaudin M, Desnues C. Hybrid Capture-Based Next Generation Sequencing and Its Application to Human Infectious Diseases. Front Microbiol 2018;9:2924. [Crossref] [PubMed]
- Pei XM, Yeung MHY, Wong ANN, et al. Targeted Sequencing Approach and Its Clinical Applications for the Molecular Diagnosis of Human Diseases. Cells 2023;12:493. [Crossref] [PubMed]
- Hernández-Neuta I, Magoulopoulou A, Pineiro F, et al. Highly multiplexed targeted sequencing strategy for infectious disease surveillance. BMC Biotechnol 2023;23:31. [Crossref] [PubMed]
- Long Y, Zhang Y, Gong Y, et al. Diagnosis of Sepsis with Cell-free DNA by Next-Generation Sequencing Technology in ICU Patients. Arch Med Res 2016;47:365-71. [Crossref] [PubMed]
- Jeon YJ, Zhou Y, Li Y, et al. The feasibility study of non-invasive fetal trisomy 18 and 21 detection with semiconductor sequencing platform. PLoS One 2014;9:e110240. [Crossref] [PubMed]
- Chen S, Zhou Y, Chen Y, et al. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018;34:i884-90. [Crossref] [PubMed]
- Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009;25:1754-60. [Crossref] [PubMed]
- Kern WV, Rieg S. Burden of bacterial bloodstream infection-a brief update on epidemiology and significance of multidrug-resistant pathogens. Clin Microbiol Infect 2020;26:151-7. [Crossref] [PubMed]
- McNamara JF, Righi E, Wright H, et al. Long-term morbidity and mortality following bloodstream infection: A systematic literature review. J Infect 2018;77:1-8. [Crossref] [PubMed]
- Sun L, Zhang S, Yang Z, et al. Clinical Application and Influencing Factor Analysis of Metagenomic Next-Generation Sequencing (mNGS) in ICU Patients With Sepsis. Front Cell Infect Microbiol 2022;12:905132. [Crossref] [PubMed]
- Li S, Tong J, Liu Y, et al. Targeted next generation sequencing is comparable with metagenomic next generation sequencing in adults with pneumonia for pathogenic microorganism detection. J Infect 2022;85:e127-9. [Crossref] [PubMed]
- Fida M, Wolf MJ, Hamdi A, et al. Detection of Pathogenic Bacteria From Septic Patients Using 16S Ribosomal RNA Gene-Targeted Metagenomic Sequencing. Clin Infect Dis 2021;73:1165-72. [Crossref] [PubMed]
- Hong HL, Flurin L, Thoendel MJ, et al. Targeted Versus Shotgun Metagenomic Sequencing-based Detection of Microorganisms in Sonicate Fluid for Periprosthetic Joint Infection Diagnosis. Clin Infect Dis 2023;76:e1456-62. [Crossref] [PubMed]


