Sarcopenia of thoracic muscle mass is not a risk factor for survival in lung transplant recipients
Introduction
Lung transplantation (LTx) is the only treatment option for patients with end stage lung disease whose respiratory function is declining. Many candidates for LTx suffer from chronic disease and two thirds of them have reduced muscle mass (1). Maintaining skeletal muscle mass and strength seems to be essential for patients to recover after LTx (2-4).
Sarcopenia, which can be defined as low muscle mass and associated decreased muscle strength or function (5), leads to physical disabilities, poor quality of life, and death in older patients. Sarcopenia has also been reported to be associated with a poor survival outcome following liver (6) and renal (7) transplantation. In chronic lung disease patients, quadriceps strength (8), body composition (9), and mid-thigh cross sectional area (CSA) (10) have been reported to be related to mortality.
Several modalities have been used to assess sarcopenia. Computed tomography (CT) (10), magnetic resonance imaging (MRI) (11), bioelectrical impedance (BIA) (12), anthropometry (13), and dual-energy X-ray absorptiometry (DXA) (14) have been used to assess muscle mass, while handheld and computerized dynamometry have been used to measure hand grip and quadriceps strength (3,15). Short Physical Performance Battery and usual gait speed can assess functional status (16-18). The 6-minute walk test is the most commonly used modality to assess functional status but it cannot index isolated muscle function as part of the sarcopenia definition (5).
Some studies of LTx patients have focused on low muscle mass and the importance of rehabilitation after LTx (3,4), but the clinical effect of sarcopenia on LTx outcome has not been studied until now. In this study, we hypothesized that thoracic skeletal muscle mass measured by analyzing the CSA from a chest CT image could be a predictor of early outcomes and survival after LTx, and can also be used to assess the suitability of lung transplant candidates.
Methods
Patients
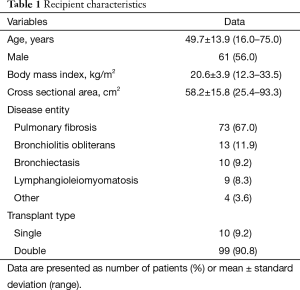
This study was approved by the Severance Hospital Institutional Review Board (4-2016-0129). We retrospectively reviewed the medical records of 111 patients who underwent LTx at our institution between January 2010 and July 2015. Age, sex, underlying diseases, height and weight at the time of the operation, post-operative course and mortality data were collected for all patients; data on thoracic muscle CSA were available in 109 of the patients.
Measurement of thoracic skeletal muscle CSA
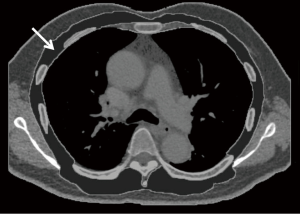
The thoracic muscle CSA at the level of the carina was determined based on a study performed by Rozenberg et al. (19). The first single slice identifying at carina level on each patient’s chest CT scan was selected. We then outlined the borders of the thoracic skeletal muscle (pectoralis, intercostal and paraspinal muscles) in the CSA, and the area was measured (Figure 1). These steps were completed semi-automatically using Aquarius iNtuition Viewer (ver. 4.4.11, TeraRecon Inc., San Mateo, CA, USA). A radiology technician performed these steps without access to patient information.
Statistical analysis
Statistical analysis was performed using Student’s t-test or ANOVA (two-tailed) and the chi-square test. The correlation between the CSA and body mass index (BMI) was analyzed using Pearson’s correlation coefficient. Survival was analyzed with the Kaplan-Meier method. The Log-rank test was used to determine statistical significance. Multivariate analysis was performed using the Cox proportional hazards regression model to investigate the effects of several variables on survival. The criterion for statistical significance was P<0.05. SPSS software (ver. 20.0; SPSS Inc., Chicago, IL, USA) was used to perform the analyses.
Results
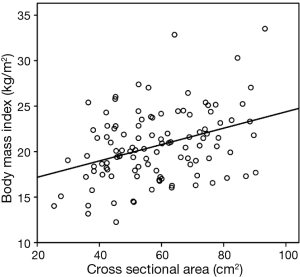
There were 48 female and 61 male patients, with a mean age of 49.7±13.9 years (range, 16–75 years). Patient characteristics are summarized in Table 1. The mean CSA was 58.2±15.8 cm2 (range, 25.4–93.3 cm2). The median interval between the date of the CT scan and the date of LTx was 2 months (range, 0–20 months). The scatter diagram in Figure 2 shows that the CSA and BMI were positively correlated (r=0.367, P<0.001).
Full table
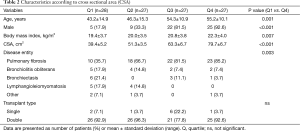
We divided the patients into four groups according to CSA to visualize the effects of both the highest and lowest quartile CSA (Table 2). Patients in the highest CSA quartile (Q4; 79.7±6.7) were more likely to be male (92.6% vs. 17.9%, P<0.001) and older (55.2±10.1 vs. 43.2±14.9 years, P=0.001), to have a higher BMI (22.3±4.0 vs. 19.4±3.7 kg/m2, P=0.007) and to have more pulmonary fibrosis (85.2% vs. 35.7%, P=0.003) compared with the lowest CSA quartile (Q1; 39.4±5.2) (Table 2).
Full table
Early post-operative recovery times including ventilator support duration (32.9±49.1 vs. 24.5±39.5 days, P=0.488), intensive care unit (ICU) stay duration (28.4±43.7 vs. 24.4±35.9 days, P=0.714) and hospital stay duration (61.4±48.2 vs. 50.8±37.2 days, P=0.367) showed longer trends in Q1 compared with Q4, but the difference was not significant. Early (≤30 days) mortality (7.1% vs. 11.1%, P=0.669) and 90 days mortality (28.6% vs. 29.6%, P= ns) were not different between Q1 and Q4 (Table 3).
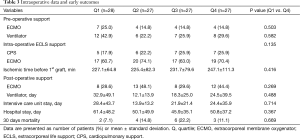
Full table
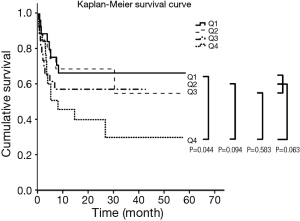
Survival was analyzed according to CSA quartile. The highest CSA (Q4) showed poorer survival compared to sarcopenia (Q1; P=0.044), Q2 (P=0.094) and Q3 (P=0.583). When Q4 was compared to the other groups simultaneously (Q1, Q2, Q3), poorer survival times were noted, but the difference was not statistically significant (P=0.063) (Figure 3). Sarcopenia (Q1) was not a risk factor for survival.
Discussion
The first human LTx was performed in 1963 (20), and the first successful LTx was reported in 1983 (21). Thereafter, the quantity of LTx operations has increased significantly, and 3,893 adult LTx procedures were performed worldwide in 2013 (22). LTx is the only therapeutic option available to patients with end-stage lung diseases of various nonmalignant etiologies in whom medical therapy has failed. Survival following LTx has steadily increased over time, and risk factors for early and late outcomes have been studied to improve survival.
Many transplant recipient factors, such as old age, type of disease, increased severity of illness (e.g., ICU stay, ventilator support), lower cardiac output and higher creatinine levels have been identified as risk factors for increased mortality in the International Society for Heart and Lung Transplantation (ISHLT) Registry (22).
BMI is considered a risk factor for death after LTx. Obesity and decreased body weight, which are defined using BMI, have been considered relative contraindications for LTx (23,24). In previous studies, obesity (BMI greater than 30 kg/m2) was considered a relative contraindication to LTx (25), and these patients had a marked decrease in post-transplantation survival rate (26). A recent study suggested that higher levels of plasma leptin and adipose tissue were detected in patients with obesity, factors that may be associated with primary graft dysfunction (27). Therefore, obesity was associated with an increased mortality rate after LTx. However, another study suggested that obesity is not associated with 1-year mortality after LTx, and a BMI greater than 30 kg/m2 may no longer represent a contraindication (28). Overall, controversy surrounds interpretation the relationship between obesity and survival rates in LTx patients.
In addition, the relationship between low body mass and a poor outcome after LTx has not been fully elucidated. In order to explain this problem, sarcopenia has emerged as a potential risk factor (2,23), but it is not clear whether sarcopenia is an independent risk factor for survival.
Sarcopenia has been defined as decreased muscle mass and peripheral muscle strength or function (5). However, exact definition of sarcopenia and standardized measurement techniques have not yet been established in LTx patients (2). Several methods of measuring sarcopenia have been used to assess muscle mass (quadriceps, triceps), muscle strength (quadriceps strength, hand-grip strength) and muscle function in patients with lung disease (5,29). In this study, sarcopenia of thoracic skeletal muscles was assessed by analyzing a CSA from chest CT images and defined as the lowest quartile of CSA (Q1).
Sarcopenia (quadriceps strength) is a risk factor for survival in patients with chronic obstructive pulmonary disease (COPD) (8), and patients treated with LTx showed similar changes in muscle mass and strength to patients with COPD (11). Furthermore, sarcopenia has been associated with poor post-transplantation outcomes in liver and renal transplant recipients (6,7), therefore, we hypothesize that it may negatively impact survival following LTx.
Previous studies on sarcopenia have focused on physical activity and rehabilitation in LTx patients, and the quadriceps muscle is mainly used in the context of skeletal muscle assessment (3,30-32). Thoracic muscles and major respiratory muscles have been surgically dissected; nevertheless, sarcopenia of thoracic muscles in LTx patients has not been fully evaluated. Recently, Rozenberg et al. (19) suggested that the thoracic muscle CSA is related to physical activity, quadriceps volume, and health-related quality of life after LTx, but survival was not assessed. Therefore, the present study was designed to investigate the relationship between thoracic sarcopenia and survival after LTx.
When we began our study, we assumed that thoracic sarcopenia was related to BMI, which would negatively affect the early outcomes (ventilator support, ICU stay, and hospital stay) and early survival. Like other muscles (quadriceps, triceps) (33), respiratory muscles have been correlated with BMI, as shown in Figure 2. Early survival tended to be longer in sarcopenia (Q1), but the difference was not statistically significant (Table 3). Also, the survival of LTx patients was not affected by sarcopenia (Q1); by contrast, Q1 was associated with better survival than Q4 (Figure 3).
The increased survival of Q1 compared with Q4 after LTx may be explained as follows. The first possible explanation is that sarcopenia of the thoracic muscles may not be a modifiable risk factor. Quadriceps muscle atrophy can be caused by medications (corticosteroids or immunosuppressants) or inactivity after LTx (11,34), and rehabilitation leads to recovery of skeletal muscle function (3). Pinet et al. (34) reported that the diaphragm and abdominal respiratory muscle volume are preserved, but the quadriceps can atrophy, after transplantation (12 patients; 5 of whom underwent heart-LTx and 7 who underwent LTx). Thoracic muscles are constantly exercised by respiratory movements even on mechanical ventilation, and thus thoracic muscles may not atrophy similarly like the diaphragm or abdominal respiratory muscles. Therefore, we suggest that it may be more accurate to assess the quadriceps rather than the thoracic muscle to analyze survival in LTx patients.
Second, Q4 may be related to a high BMI. The thoracic muscle CSA showed a linear correlation with BMI (Figure 2), and a high BMI was a risk factor for LTx, although reports were controversial. Third, these results may be due to the heterogeneity of the patient population used in this study. In Q4, patients tended to be older males with pulmonary fibrosis (Table 2). However, these factors (age, sex, and diagnosis) were not independent risk factors for survival in subgroup multivariate analysis (data not shown). Lastly, there is no standard definition of sarcopenia, although in this study we defined it as Q1. Considering these possible causes, more studies are required to better understand sarcopenia of thoracic muscles in patients undergoing LTx.
The limitations of this study include its retrospective, single-center design and clinically heterogeneous patient population. These limitations notwithstanding, it is important to note that the present study attempted to analyze the association between thoracic sarcopenia and outcome after LTx.
In conclusion, sarcopenia of thoracic muscle mass did not compromise either the early outcome or 1-year survival in LTx patients. More studies are needed to clarify the relationship between thoracic sarcopenia and outcomes in LTx patients.
Acknowledgements
JG Lee received funding from a 2015 The Korean Society for Transplantation Young Research Fund.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Severance Hospital Institutional Review Board (4-2016-0129).
References
- Kyle UG, Nicod L, Raguso C, et al. Prevalence of low fat-free mass index and high and very high body fat mass index following lung transplantation. Acta Diabetol 2003;40 Suppl 1:S258-60. [Crossref] [PubMed]
- Rozenberg D, Wickerson L, Singer LG, et al. Sarcopenia in lung transplantation: a systematic review. J Heart Lung Transplant 2014;33:1203-12. [Crossref] [PubMed]
- Langer D, Burtin C, Schepers L, et al. Exercise training after lung transplantation improves participation in daily activity: a randomized controlled trial. Am J Transplant 2012;12:1584-92. [Crossref] [PubMed]
- Vivodtzev I, Pison C, Guerrero K, et al. Benefits of home-based endurance training in lung transplant recipients. Respir Physiol Neurobiol 2011;177:189-98. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412-23. [Crossref] [PubMed]
- Englesbe MJ, Patel SP, He K, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg 2010;211:271-8. [Crossref] [PubMed]
- Streja E, Molnar MZ, Kovesdy CP, et al. Associations of pretransplant weight and muscle mass with mortality in renal transplant recipients. Clin J Am Soc Nephrol 2011;6:1463-73. [Crossref] [PubMed]
- Swallow EB, Reyes D, Hopkinson NS, et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax 2007;62:115-20. [Crossref] [PubMed]
- Slinde F, Grönberg A, Engström CP, et al. Body composition by bioelectrical impedance predicts mortality in chronic obstructive pulmonary disease patients. Respir Med 2005;99:1004-9. [Crossref] [PubMed]
- Marquis K, Debigaré R, Lacasse Y, et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002;166:809-13. [Crossref] [PubMed]
- Mathur S, Levy RD, Reid WD. Skeletal muscle strength and endurance in recipients of lung transplants. Cardiopulm Phys Ther J 2008;19:84-93. [PubMed]
- Kyle UG, Nicod L, Romand JA, et al. Four-year follow-up of body compostion in lung transplant patients. Transplantation 2003;75:821-8. [Crossref] [PubMed]
- Soler-Cataluña JJ, Sánchez-Sánchez L, Martínez-García MA, et al. Mid-arm muscle area is a better predictor of mortality than body mass index in COPD. Chest 2005;128:2108-15. [Crossref] [PubMed]
- Engelen MP, Schols AM, Does JD, et al. Skeletal muscle weakness is associated with wasting of extremity fat-free mass but not with airflow obstruction in patients with chronic obstructive pulmonary disease. Am J Clin Nutr 2000;71:733-8. [PubMed]
- Langer D, Cebrià i Iranzo MA, Burtin C, et al. Determinants of physical activity in daily life in candidates for lung transplantation. Respir Med 2012;106:747-54. [Crossref] [PubMed]
- Working Group on Functional Outcome Measures for Clinical Trials. Functional outcomes for clinical trials in frail older persons: time to be moving. J Gerontol A Biol Sci Med Sci 2008;63:160-4. [Crossref] [PubMed]
- Cesari M, Kritchevsky SB, Newman AB, et al. Added value of physical performance measures in predicting adverse health-related events: results from the Health, Aging And Body Composition Study. J Am Geriatr Soc 2009;57:251-9. [Crossref] [PubMed]
- Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol (1985) 2003;95:1851-60. [Crossref] [PubMed]
- Rozenberg D, Singer LG, Mendes P, et al. Association of Thoracic Muscle Cross-Sectional Area and Clinical Outcomes in Lung Transplant Candidates. J Heart Lung Transplant 2015;34:S15-6. [Crossref]
- Hardy JD, Webb WR, Dalton ML Jr, et al. Lung homotransplantation in man. JAMA 1963;186:1065-74. [Crossref] [PubMed]
- Unilateral lung transplantation for pulmonary fibrosis. Toronto Lung Transplant Group. N Engl J Med 1986;314:1140-5. [Crossref] [PubMed]
- Yusen RD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-second Official Adult Lung and Heart-Lung Transplantation Report--2015; Focus Theme: Early Graft Failure. J Heart Lung Transplant 2015;34:1264-77. [Crossref] [PubMed]
- Hook JL, Lederer DJ. Selecting lung transplant candidates: where do current guidelines fall short? Expert Rev Respir Med 2012;6:51-61. [Crossref] [PubMed]
- Lederer DJ, Wilt JS, D'Ovidio F, et al. Obesity and underweight are associated with an increased risk of death after lung transplantation. Am J Respir Crit Care Med 2009;180:887-95. [Crossref] [PubMed]
- Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update--a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006;25:745-55. [Crossref] [PubMed]
- Kanasky WF Jr, Anton SD, Rodrigue JR, et al. Impact of body weight on long-term survival after lung transplantation. Chest 2002;121:401-6. [Crossref] [PubMed]
- Lederer DJ, Kawut SM, Wickersham N, et al. Obesity and primary graft dysfunction after lung transplantation: the Lung Transplant Outcomes Group Obesity Study. Am J Respir Crit Care Med 2011;184:1055-61. [Crossref] [PubMed]
- Singer JP, Peterson ER, Snyder ME, et al. Body composition and mortality after adult lung transplantation in the United States. Am J Respir Crit Care Med 2014;190:1012-21. [Crossref] [PubMed]
- Robles PG, Mathur S, Janaudis-Fereira T, et al. Measurement of peripheral muscle strength in individuals with chronic obstructive pulmonary disease: a systematic review. J Cardiopulm Rehabil Prev 2011;31:11-24. [Crossref] [PubMed]
- Wickerson L, Mathur S, Helm D, et al. Physical activity profile of lung transplant candidates with interstitial lung disease. J Cardiopulm Rehabil Prev 2013;33:106-12. [Crossref] [PubMed]
- Walsh JR, Chambers DC, Davis RJ, et al. Impaired exercise capacity after lung transplantation is related to delayed recovery of muscle strength. Clin Transplant 2013;27:E504-11. [Crossref] [PubMed]
- Bossenbroek L, den Ouden ME, de Greef MH, et al. Determinants of overweight and obesity in lung transplant recipients. Respiration 2011;82:28-35. [Crossref] [PubMed]
- Van Der Woude BT, Kropmans TJ, Douma KW, et al. Peripheral muscle force and exercise capacity in lung transplant candidates. Int J Rehabil Res 2002;25:351-5. [Crossref] [PubMed]
- Pinet C, Scillia P, Cassart M, et al. Preferential reduction of quadriceps over respiratory muscle strength and bulk after lung transplantation for cystic fibrosis. Thorax 2004;59:783-9. [Crossref] [PubMed]


