
Early and mid-term outcomes of tricuspid valve surgery in patients with functional tricuspid regurgitation induced by atrial fibrillation
Highlight box
Key findings
• Tricuspid valve (TV) surgery for tricuspid regurgitation (TR) induced by atrial fibrillation (AF) showed low surgical mortality and favorable midterm outcomes.
What is known and what is new?
• AF-induced severe TR was a significant predictor of mortality in the patients who suffered from AF. Although isolated functional tricuspid regurgitation (FTR) and its prognosis had recently drawn more attention from physicians, surgical treatment is uncommon and there is still a lack of study regarding surgical outcomes of AF-induced TR.
• TV surgery for AF-induced TR showed not only favorable surgical morbidity and mortality but also acceptable midterm outcomes in terms of overall survival and tricuspid valve-related events (TVRE).
What is the implication, and what should change now?
• Researchers should investigate the clinical benefits of surgical treatment and find the adequate surgical timing for AF-induced TR.
Introduction
Background
Functional tricuspid regurgitation (FTR) can originate from various etiologies, in the absence of morphological defects in the tricuspid valve (TV) apparatus. According to etiology, FTR can be categorized into two types: ventricular FTR and atrial FTR. Left-sided valvular or myocardial diseases, along with intrinsic pulmonary vascular diseases, result in pressure overload on the right ventricle (RV), leading to ventricular remodeling. This remodeling includes ventricular enlargement, displacement of papillary muscles, leaflet tethering, and annular flattening and dilation, ultimately contributing to tricuspid regurgitation (TR), now defined as ventricular FTR (1,2). On the contrary, atrial fibrillation (AF) can induce remodeling of the right atrium (RA) without concurrent RV remodeling, subsequently enlarging the tricuspid annulus and resulting in the development of FTR, now classified as atrial FTR (3). Since several studies have demonstrated the efficacy of surgery for ventricular FTR (4,5), current guidelines suggest concurrent surgical intervention for significant TR in left-sided valve surgery (6,7). However, the clinical importance of surgical treatment of atrial FTR has been underestimated.
Rationale and knowledge gap
Although the mechanism by which remodeling the RA contributes to FTR is not yet proven precisely, AF basically deteriorates atrial and TV annular contraction, leading to RA enlargement and TV annular dilatation (8). The pathophysiology of TR induced by AF strengthens the plausibility of surgical correction, such as TV annuloplasty. However, research on TV surgery for AF-induced TR is insufficient.
Objective
This study aimed to report early and mid-term clinical outcomes of TV surgery in patients with AF-induced TR. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1776/rc).
Methods
The study protocol was reviewed by the Institutional Review Board (IRB) of Seoul National University Hospital and approved as a minimal-risk retrospective study that did not require individual consent (IRB approval No. 2208-151-1353). The investigation was conducted in accordance with the principles articulated in the Declaration of Helsinki (as revised in 2013), emphasizing a commitment to ethical research practices.
In this study, atrial FTR was defined as (I) isolated TV annular dilatation with RA enlargement and AF and (II) no other reasons to cause FTR, such as significant left ventricular systolic dysfunction [left ventricular ejection fraction (LVEF) <50%], left-sided valvular heart disease (≥ moderate degree), RV failure [right ventricle ejection fraction (RVEF) <35%], significant pulmonary hypertension (pulmonary arterial systolic pressure above 50 mmHg) (9), and atrial or ventricular septal defect.
From January 2000 to December 2021, 1,301 patients underwent TV surgery. In a total of 987 patients who underwent concomitant left-sided valve surgery, coronary bypass grafting, or aortic surgery, TR could be attributed to causes other than AF, those patients were excluded from the study. Among 314 patients who underwent isolated TV surgery, 48 patients who had morphological defects at TV apparatus and 212 patients with other origins of FTR than AF were also excluded. After excluding patients with missing data, 43 patients were finally enrolled in this study and those patients were reviewed by 2 physicians to confirm the presence of AF preceding TR and the absence of any other etiology besides AF (Figure 1). Forty-one patients (95.3%) underwent preoperative cardiac magnetic resonance imaging (CMR). CMR was performed using a 3-T system (Magnetome Skyra; Siemens Healthcare, Erlangen, Germany) with a 60-channel phased-array body surface coil. The CMR protocols have been described previously (10). The RV end-diastolic volume (RVEDV), RV end-systolic volume (RVESV), and RVEF were measured using a software program (Argus; Siemens Healthcare). Preoperative AF was classified as paroxysmal, persistent, or long-standing persistent (11), and all patients had long-standing persistent AF. The rhythm status was evaluated based on an electrocardiogram (ECG) performed 3 months after surgery. Postoperative AF was defined as AF, atrial flutter, atrial tachycardia, or pacing rhythm. Sinus rhythm included normal sinus and junctional rhythms.

Surgical procedures
All patients underwent full median sternotomy under moderate hypothermia and cold cardioplegic arrest, except for 1 patient with right thoracotomy. TV repair, also known as tricuspid annuloplasty (TAP), was preferred in almost all cases, however, the decision to perform a TV replacement (TVR) or TV repair was made at the discretion of the attending surgeon. Throughout the study period, 3 patients underwent direct TVR due to severely dilated annulus including septal leaflet annulus with or without leaflet thinning while another 3 patients initially underwent TV repair but subsequently required TVR because of residual TR after TAP.
For De Vega annuloplasty, annular size reduction was performed using commercially available cylindrical valve sizers with labeled sizes of 27 (actual diameter 29.5), 29 (31.5), and 31 (33.5) mm. The plication sutures, made of 3-0 extended polytetrafluoroethylene (e-PTFE), were tied down with the cylindrical size inserted into the TV orifice. For ring annuloplasty, Tri-Ad Adams tricuspid ring (Medtronic, Minneapolis, MN, USA) and MC3 annuloplasty ring (Edwards LifeScience, Irvine, CA, USA) were used during study period and were chosen based on the attending surgeon’s preference. The ring size was determined based on the inter-commissural distance of the septal leaflet and the attached leaflet area originating from the anterior papillary muscle.
Evaluation of echocardiographic outcomes
All the patients underwent preoperative and postoperative echocardiography. The severity was categorized on a scale from 0 to 4, where 0 indicated no or trivial severity, 1 indicated mild, 2 indicated moderate, 3 indicated moderate-to-severe, and 4 indicated severe. Echocardiographic follow-up examinations were carried out at the judgment of the operating surgeon or referring physician, guided by symptoms and auscultatory findings. Out of the 41 patients who survived early postoperative period, 36 underwent at least one echocardiographic examination after being discharged. The median follow-up duration of echocardiographic evaluation was 21.1 months [interquartile range (IQR): 11.8–52.6 months].
Evaluation of clinical outcomes
The study primarily focused on assessing early- and mid-term postoperative outcomes. Operative mortality was characterized by fatalities within a 30-day period following the surgery or while under hospital care. Low cardiac output syndrome (LCOS) was characterized by a cardiac index below 2.0 L/min/m2 or a systolic arterial pressure below 90 mmHg, necessitating continuous infusion of inotropic agents such as dopamine or dobutamine exceeding 5 µg/kg/min, or epinephrine or norepinephrine at any rate. Acute kidney injury (AKI) was defined by the RIFLE definition (an acronym corresponding to the risk of renal dysfunction, injury to the kidney, failure of kidney function, loss of kidney function, and end-stage kidney disease) denoting a rise in serum creatinine (Cr) level of 50% or more from the baseline within 7 days after the procedure (12). Perioperative stroke referred to a stroke that presented within the initial 30 days post-procedure, characterized by a focal neurological deficit of central origin persisting over 24 hours, with or without confirmation through neuroimaging (13). Respiratory complications encompassed postoperative pneumonia and prolonged ventilator support lasting >48 hours.
Patients underwent regular postoperative follow-ups at the outpatient clinic at 3- or 4-month intervals. If they did not visit the last clinic at the scheduled time, they were contacted via telephone to confirm their condition. The clinical follow-up ended on December 31, 2022, and the follow-up completion rate was 95.3% (41/43). The follow-up duration was 42.0 months (IQR, 17.3–152.5 months) and most of the patients (n=36, 83.7%) underwent TV surgery since January 1, 2014. As for mid-term outcomes, overall survival rates and cumulative incidences of TVRE, AF recurrence, or permanent pacemaker (PPM) insertion were analyzed. TVRE included development of moderate or severe TV disease, TV reoperation, congestive heart failure (CHF) requiring readmission, major bleeding or thrombosis, and valve-related mortality (14).
Statistical analysis
Statistical analyses were performed using R ver. 4.2.1 and IBM SPSS Statistics ver. 26. The data are expressed as mean ± standard deviation, medians with IQRs or proportions. Overall survival was analyzed using the Kaplan-Meier method. The cumulative incidence was estimated by considering death as a competing risk. The overall survival and TVRE were compared between groups using log-rank test and Fine-Gray’s test. The risk factors for time-related events were analyzed using the Cox proportional hazards model. The minimal P value approach was used to estimate a cutoff value of time interval that associated with the occurrence of TVRE. Statistical significance was set at P<0.05 univariate analysis.
Results
Patient characteristics
Preoperative and operative characteristics are summarized in Tables 1,2, respectively. Thirty-nine (90.7%) patients had severe TR and 4 (9.3%) had moderate-to-severe TR. As for concurrent functional mitral regurgitation (FMR), 2 (4.7%) patients had mild-to-moderate MR, 16 (37.2%) had trivial or mild MR while 25 (58.1%) patients had no MR.
Table 1
| Preoperative data | Values (n=43) |
|---|---|
| Age (years) | 69.3±8.6 |
| Gender (female) | 22 (51.2) |
| Body surface area (mm2) | 1.67±0.18 |
| Body mass index >25 kg/m2 | 15 (34.9) |
| Functional class ≥3 | 12 (27.9) |
| Diabetes mellitus | 15 (34.9) |
| Hypertension | 19 (44.2) |
| History of stroke | 6 (14.0) |
| Dyslipidemia | 9 (20.9) |
| Chronic renal failure (eGFR <60 mL/min/1.73 m2) | 19 (44.2) |
| Coronary artery disease | 4 (9.3) |
| Liver disease | 4 (9.3) |
| Preoperative TR grade | |
| Moderate-to-severe | 4 (9.3) |
| Severe | 39 (90.7) |
| Echocardiographic dimensional data (n=41) | |
| Tricuspid valve annulus (mm) | 45.5±6.5 |
| RA area at end-systole (cm2) | 39.2±11.4 |
| RA volume (mL) | 169.1 (105.1–227.9) |
| Left atrium dimension (mm) | 56.1±8.8 |
| RV end-systolic area (cm2) | 16.4±4.0 |
| RV end-diastolic area (cm2) | 27.7±8.2 |
| Cardiac magnetic resonance data (n=41) | |
| RV ejection fraction (%) | 48.4±8.1 |
| RV end-systolic volume (mL) | 132.3±47.4 |
| RV end-diastolic volume (mL) | 256.3±82.4 |
| Interval from AF to significant TR (months) | 61.2 (6.4–107.1) |
| Interval from severe TR to surgery (n=39, months) | 2.4 (0.8–20.9) |
Values are presented as median (interquartile range), number (percentage) or mean ± standard deviation. eGFR, estimated glomerular filtration rate; TR, tricuspid regurgitation; RA, right atrium; RV, right ventricle; AF, atrial fibrillation.
Table 2
| Variables | Values (n=43) |
|---|---|
| Type of tricuspid valve surgery | |
| Repair | 37 (86.0) |
| De-Vega annuloplasty | 4 (9.3) |
| Ring annuloplasty | 33 (76.7) |
| Replacement | 6 (14.0) |
| Concomitant Cox-maze III procedure | 39 (90.7) |
| CPB time (min) | 160.6±50.7 |
| ACC time (min) | 100.4±27.5 |
Values are presented as number (percentage) or mean ± standard deviation. CPB, cardiopulmonary bypass; ACC, aortic cross-clamp.
The interval from initial diagnosis of AF to significant TR (moderate-to-severe or greater TR) was 61.2 months (IQR, 6.4–107.1 months), and the interval from initial diagnosis of severe TR to surgery was 2.4 months (IQR, 0.8–20.9 months), respectively. Preoperative echocardiographic dimensional data and CMR data were also stated in Table 1.
The concomitant Cox-maze III procedure was performed in 39 patients (90.7%). For 3 patients, the Maze procedure was not indicated due to the factors such as severe pericardial adhesion, a significantly enlarged and thin RA, concurrent RV dysfunction, and various co-morbidities including old age above 80. Among the entire cohort, 34 patients underwent the left atrial appendage (LAA) treatment through various technique including internal obliteration (n=7), external exclusion (n=12), or excision (n=15). Another five patients did not undergo LAA treatment because their LAAs had severe adhesions and a broad base that was not suitable for both internal and external procedures. Most patients underwent TV repair (n=37, 86.0%), while 6 patients (14.0%) underwent TVR. The mean cardiopulmonary bypass (CPB) and aortic cross-clamp (ACC) times in the whole cohort were 160.6±50.7 and 100.4±27.5 min, respectively (Table 2).
Anticoagulation strategy during follow-up
As for anti-coagulation during follow-up, 5 patients (11.6%) without mechanical TV and free from AF were prescribed no anti-coagulation. Fifteen patients (34.9%) were on novel oral anticoagulants (NOAC) while 10 (23.3%) on anti-platelet agents such as aspirin or clopidogrel, and 7 (16.3%) on warfarin. Additionally, 5 patients (11.6%) with concurrent cerebral or coronary arterial disease received a combination of anti-platelet therapy with NOAC or warfarin.
Early clinical outcomes
Operative mortality occurred in 1 patient (2.3%). The patient had been diagnosed with severe TR 9 years preoperatively and had RV dysfunction (RVEF, 33.1% on CMR) before surgery. The patient died of ventricular fibrillation arrest-associated bradycardia and electrolyte imbalance 145 days after surgery during the same hospitalization. The median length of hospital stay was 15 (IQR, 11–20) days. Two (4.7%) patients experienced low postoperative cardiac output syndrome. Reoperation for postoperative bleeding was performed in 2 patients (4.7%). Nine patients (21.0%) developed AKI, and renal replacement therapy was required in 5 patients. Perioperative stroke occurred in 2 patients (4.7%), while respiratory complications occurred in 7 patients (16.3%). No patient required PPM implantation after surgery (Table 3). The TR grade was significantly improved after surgery from 3.8±0.4 to 0.6±0.8 (P<0.001). Two patients had moderate or greater postoperative residual TR. One patient with severe TR improved to trivial TR 11 months after surgery, and the other patient with moderate TR improved to mild TR 66 months after surgery, without additional TV procedures.
Table 3
| Early outcomes | Values (n=43) |
|---|---|
| Operative mortality | 1 (2.3) |
| Hospital course (days) | 15 [6–145] |
| Low cardiac output syndrome | 2 (4.7) |
| Bleeding reoperation | 2 (4.7) |
| AKI | 9 (20.9) |
| AKI requiring RRT | 5 (11.6) |
| Permanent pacemaker implantation | 0 |
| Stroke | 2 (4.6) |
| Respiratory complication | 7 (16.3) |
Values are presented as number (%) or median [range]. AKI, acute kidney injury; RRT, renal replacement therapy.
Mid-term clinical outcomes
Late death occurred in seven patients. The 1- and 5-year overall survival rates were 90.6% and 79.3%, respectively (Figure 2). The cumulative incidences of TVRE at 1- and 5-year were 16.3% and 26.5%, respectively (Figure 3A). Four patients who underwent TAP developed moderate (n=3, 7.0%) or moderate-to-severe (n=1, 2.3%) TR, and two of these patients were readmitted due to CHF. One patient in the TVR group underwent reoperation because of structural bioprosthetic valve degeneration, approximately 40 months after the first surgery while 2 patients in the TAP group underwent reoperation of TVR with concomitant mitral valve repair due to recurred TR along with newly developed significant MR. During follow-up, bleeding events occurred in two patients, but there were no embolic events, including thromboembolic stroke (Table 4). The cumulative incidences of AF recurrence at 1- and 3-year in patients who underwent concurrent Cox maze III procedures (n=39) were 29.7% and 67.6%, respectively (Figure 3B). The cumulative incidence of PPM insertion at 1- and 3-year were 4.7% and 17.1%, respectively.
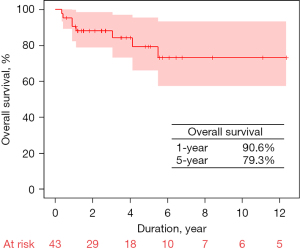

Table 4
| Midterm outcomes | Values (n=42) |
|---|---|
| Late mortality | 7 (16.7) |
| Cardiac death | 2 (4.8) |
| PPM insertion | 6 (14.3) |
| Recurrence of AF after Cox-maze III procedure (n=39) | 20 (51.3) |
| Recovery to sinus rhythm, free from PPM | 15 (38.5) |
| TV re-operation | 3 (7.1) |
| Development of moderate or severe TV disease | 4 (9.5) |
| Congestive heart failure requiring re-admission | 2 (4.8) |
| Major bleeding or thrombotic events | 2 (4.8) |
Values are presented as number (%). AF, atrial fibrillation; PPM, permanent pacemaker; TV, tricuspid valve.
The interval from the diagnosis of severe TR to surgery was not associated with overall survival (P=0.48), but was significantly associated with TVRE (hazard ratio: 1.023, 95% confidence interval: 1.005–1.042). The cutoff value of interval from the diagnosis of severe TR to surgery for predicting the TVRE was 23.1 months.
Outcomes in patients with sinus rhythm
During follow-up, sinus rhythm was maintained in 20 patients. There was no long-term mortality in patients with sinus rhythm, and the cumulative incidence of TVRE was 10% at 3 years. While there was a significant difference in overall survival between patients with sinus rhythm and those without sinus rhythm (P=0.01, Figure S1A), there was no significant difference in the incidence of TVRE between patients with sinus rhythm and those without sinus rhythm (P=0.45, Figure S1B).
Discussion
Key findings
The present study demonstrated that TV surgery for AF-induced TR showed favorable surgical morbidity and mortality, as well as acceptable mid-term outcomes in terms of overall survival and TVRE. AF-induced TR usually originates from a dilated and posteriorly displaced TV annulus, accompanied by RA enlargement and RV eccentric dilatation (15,16). Several studies have shown that severe AF-induced TR is a significant predictor of mortality in patients with AF (17-20). Although more attention has recently been drawn to isolated FTR and its prognosis, surgical treatment is uncommon, and there is still a paucity of research on the surgical outcomes of AF-induced TR (21).
Strengths and limitations
Previous studies on isolated TV surgery have reported an operative mortality rate of approximately 10% (22-24); however, this study showed a very low mortality rate. Early surgical referral can affect these favorable early clinical outcomes by physicians. In our study, the median interval from severe TR to surgery was just 2.4 months. In the follow-up results, a longer duration from diagnosis of severe TR to surgery was also associated with a higher incidence of TVRE, although this analysis was only performed using univariate analysis. Further large-sample studies are required to elucidate the clinical effect of surgical referral timing on AF-induced TR.
Comparison with similar research
The risk factors attributed to recurrent AF or significant TR were not analyzed in the present study because of the small sample size. Wang et al. reported that a high tethering height >6 mm and RV sphericity index are independent risk factors for the recurrence of AF or TR in AF-induced TR (25). Because RV dilatation can result in displacement of the papillary muscles and leaflet tethering (26), it is important to consider intervention before the RV is severely dilated in patients with AF-induced TR.
Explanations of findings
Despite the significance of surgery for isolated FTR, the reluctance of physicians towards surgery might be due to the critical morbidity and mortality associated with TV surgery, based on previous studies. However, previous studies on TV surgery included patients with heterogeneous etiologies and inadequate timing of surgery (22,27).
The AF recurrence rate was very high in this population, compared with that in other studies that reported the results of the Cox-maze III procedure. This high recurrence rate can be attributed to the long duration of AF. Although the median interval from AF to significant TR was 61.8 months in this study, this value could have been underestimated because of the missing records of the first diagnosis of AF. Although the recurrence rate of AF was high, there was no thromboembolic stroke during follow-up. Decreasing the AF burden using the Cox maze procedure and LAA treatment can lead to favorable clinical outcomes (28).
Implications and actions needed
Current guidelines recommend surgical intervention for ventricular FTR originating from left valvular heart disease at the time of left-sided valve surgery or in symptomatic patients (6,7). However, there is no consensus regarding the optimal surgical timing for AF-induced TR. As patients with AF-induced TR are less likely to have RV dysfunction or a history of cardiac surgery than patients requiring TV surgery, more favorable operative outcomes are expected, as in this study. Therefore, further studies should be performed to evaluate the clinical benefits of surgical treatment and to determine the appropriate surgical timing for AF-induced TR.
Limitations
The present study has several limitations. First, this was a single-center, retrospective, non-comparative observational study. Therefore, the number of patients included in the study is not sufficient to draw comprehensive conclusions, particularly in terms of conducting detailed research on surgical techniques, such as a comparison between TV repair and replacement. The further comparative studies according to surgical techniques or origin of TR should be performed. Second, the study period was long because isolated TV surgery for AF-induced TR was not prevalent. Although the surgical technique did not change during the study period, changes in the medical system may have affected the results. Third, although isolated TV surgery can be performed safely through minimally invasive procedures (29), most surgeries in the present study were conducted via median sternotomy because the concomitant Maze procedure can increase the complexity of surgery for patients with atrial FTR. Further studies regarding surgical approaches for these patients are needed to assess more effective strategies.
Conclusions
TV surgery for TR induced by AF has shown low surgical mortality and favorable mid-term outcomes. In addition, this study showed the possibility of improving surgical outcomes in these patients through early surgery after progression to severe TR. Further studies are needed to improve the clinical outcomes of surgery in patients with AF-induced TR.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1776/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1776/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1776/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1776/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB approval No. 2208-151-1353), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hahn RT, Waxman AB, Denti P, et al. Anatomic Relationship of the Complex Tricuspid Valve, Right Ventricle, and Pulmonary Vasculature: A Review. JAMA Cardiol 2019;4:478-87. [Crossref] [PubMed]
- Fender EA, Zack CJ, Nishimura RA. Isolated tricuspid regurgitation: outcomes and therapeutic interventions. Heart 2018;104:798-806. [Crossref] [PubMed]
- Guta AC, Badano LP, Tomaselli M, et al. The Pathophysiological Link between Right Atrial Remodeling and Functional Tricuspid Regurgitation in Patients with Atrial Fibrillation: A Three-Dimensional Echocardiography Study. J Am Soc Echocardiogr 2021;34:585-94.e1. [Crossref] [PubMed]
- Benedetto U, Melina G, Angeloni E, et al. Prophylactic tricuspid annuloplasty in patients with dilated tricuspid annulus undergoing mitral valve surgery. J Thorac Cardiovasc Surg 2012;143:632-8. [Crossref] [PubMed]
- Badhwar V, Rankin JS, He M, et al. Performing Concomitant Tricuspid Valve Repair at the Time of Mitral Valve Operations Is Not Associated With Increased Operative Mortality. Ann Thorac Surg 2017;103:587-93. [Crossref] [PubMed]
- Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;143:e35-71. Erratum in: Circulation 2021;143:e228 Erratum in: Circulation 2021;143:e784. [Crossref] [PubMed]
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2022;43:561-632. Erratum in: Eur Heart J 2022;43:2022. [Crossref] [PubMed]
- Utsunomiya H, Itabashi Y, Mihara H, et al. Functional Tricuspid Regurgitation Caused by Chronic Atrial Fibrillation: A Real-Time 3-Dimensional Transesophageal Echocardiography Study. Circ Cardiovasc Imaging 2017;10:e004897. [Crossref] [PubMed]
- Haddad F, Doyle R, Murphy DJ, et al. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation 2008;117:1717-31. [Crossref] [PubMed]
- Choi JW, Kim JS, Kang Y, et al. Results after Tricuspid Valve Surgery for Preserved and Dysfunctional Right Ventricle. Thorac Cardiovasc Surg 2023; Epub ahead of print. [Crossref] [PubMed]
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019;140:e125-51. Erratum in: Circulation 2019;140:e285. [Crossref] [PubMed]
- Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8:R204-12. [Crossref] [PubMed]
- Head SJ, Milojevic M, Daemen J, et al. Stroke Rates Following Surgical Versus Percutaneous Coronary Revascularization. J Am Coll Cardiol 2018;72:386-98. [Crossref] [PubMed]
- Akins CW, Miller DC, Turina MI, et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. Eur J Cardiothorac Surg 2008;33:523-8. [Crossref] [PubMed]
- Utsunomiya H, Harada Y, Susawa H, et al. Tricuspid valve geometry and right heart remodelling: insights into the mechanism of atrial functional tricuspid regurgitation. Eur Heart J Cardiovasc Imaging 2020;21:1068-78. [Crossref] [PubMed]
- Park JH, Shin SH, Lee MJ, et al. Clinical and Echocardiographic Factors Affecting Tricuspid Regurgitation Severity in the Patients with Lone Atrial Fibrillation. J Cardiovasc Ultrasound 2015;23:136-42. [Crossref] [PubMed]
- Vîjan AE, Daha IC, Delcea C, et al. Prognostic Impact of Severe Atrial Functional Tricuspid Regurgitation in Atrial Fibrillation Patients. J Clin Med 2022;11:7145. [Crossref] [PubMed]
- Najib MQ, Vinales KL, Vittala SS, et al. Predictors for the development of severe tricuspid regurgitation with anatomically normal valve in patients with atrial fibrillation. Echocardiography 2012;29:140-6. [Crossref] [PubMed]
- Dietz MF, Goedemans L, Vo NM, et al. Prognostic Implications of Significant Isolated Tricuspid Regurgitation in Patients With Atrial Fibrillation Without Left-Sided Heart Disease or Pulmonary Hypertension. Am J Cardiol 2020;135:84-90. [Crossref] [PubMed]
- Kwak S, Lim J, Yang S, et al. Atrial Functional Tricuspid Regurgitation: Importance of Atrial Fibrillation and Right Atrial Remodeling and Prognostic Significance. JACC Cardiovasc Imaging 2023;16:575-87. [Crossref] [PubMed]
- Topilsky Y, Nkomo VT, Vatury O, et al. Clinical outcome of isolated tricuspid regurgitation. JACC Cardiovasc Imaging 2014;7:1185-94. [Crossref] [PubMed]
- Zack CJ, Fender EA, Chandrashekar P, et al. National Trends and Outcomes in Isolated Tricuspid Valve Surgery. J Am Coll Cardiol 2017;70:2953-60. [Crossref] [PubMed]
- Wang TKM, Griffin BP, Miyasaka R, et al. Isolated surgical tricuspid repair versus replacement: meta-analysis of 15 069 patients. Open Heart 2020;7:e001227. [Crossref] [PubMed]
- Dreyfus J, Flagiello M, Bazire B, et al. Isolated tricuspid valve surgery: impact of aetiology and clinical presentation on outcomes. Eur Heart J 2020;41:4304-17. [Crossref] [PubMed]
- Wang J, Li S, Ye Q, et al. Catheter ablation or surgical therapy in moderate-severe tricuspid regurgitation caused by long-standing persistent atrial fibrillation. Propensity score analysis. J Cardiothorac Surg 2020;15:277. [Crossref] [PubMed]
- Spinner EM, Lerakis S, Higginson J, et al. Correlates of tricuspid regurgitation as determined by 3D echocardiography: pulmonary arterial pressure, ventricle geometry, annular dilatation, and papillary muscle displacement. Circ Cardiovasc Imaging 2012;5:43-50. Erratum in: Circ Cardiovasc Imaging 2012;5:e54. [Crossref] [PubMed]
- Mangieri A, Montalto C, Pagnesi M, et al. Mechanism and Implications of the Tricuspid Regurgitation: From the Pathophysiology to the Current and Future Therapeutic Options. Circ Cardiovasc Interv 2017;10:e005043. [Crossref] [PubMed]
- Whitlock RP, Belley-Cote EP, Paparella D, et al. Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke. N Engl J Med 2021;384:2081-91. [Crossref] [PubMed]
- Abdelbar A, Kenawy A, Zacharias J. Minimally invasive tricuspid valve surgery. J Thorac Dis 2021;13:1982-92. [Crossref] [PubMed]


